Abstract
Repeated brief seizures, such as those induced by electroconvulsive therapy (ECT), markedly elevate neurotrophic factor levels in the adult rat brain but it is not known whether a similar response to seizures occurs in immature animals. To address this question, we evoked brief seizures with electroconvulsive shock (ECS) in rat pups at different stages of postnatal development and examined basic fibroblast growth factor (FGF-2), nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) proteins in selected brain regions in which these trophic factors are known to increase in the adult rat following ECS-induced seizures.
ECS treatments were administered daily (3 episodes/day) over 7 days to rat pups of three different ages: postnatal day (P)1-7, P7-13, or P14-20. Protein levels were measured 6 h after the last ECS using Western blotting for FGF-2 in rhinal cortex, ELISA for BDNF and NGF in hippocampus, and NGF in frontal cortex.
7 days of repeated ECS-induced seizures during P1-7 did not alter protein levels for BDNF, FGF-2, or NGF. The repeated seizures during P7-13 affected only BDNF protein, causing a significant elevation of 40% in hippocampus over sham-treated controls. In P14-20 pups, the repeated seizures resulted in a significant increase in BDNF in hippocampus (162% over controls) and FGF-2 in rhinal cortex (34% over controls), while NGF protein did not show a significant change in either hippocampus or frontal cortex.
The results suggest that during the first postnatal week there is a resistance to seizure-induced increase in neurotrophic factors, but by the third postnatal week, both BDNF and FGF-2 are elevated substantially in response to repeated seizures. This time-dependent profile suggests that synthesis of these proteins is initially activity-independent, becoming subject to activity-dependent regulation by 3 weeks of age. This maturation of seizure-evoked changes in trophic factors may be important for understanding the impact of ECT and seizures in childhood.
Keywords: trophic factors, electroconvulsive shock (ECS), seizures, postnatal development
Introduction
The therapeutic efficacy of electroconvulsive therapy (ECT) for treating mood disorders is thought to result from ECT-induced neuroprotection and neuroplasticity (Taylor, 2008; Masco et al., 1999; Elfving et al., 2008). These actions may derive from ECT-induced changes in the availability of neurotrophic factors. In the adult rat brain, brief seizure episodes induced by electroshock trigger upregulation of several neurotrophic factors, including BDNF, NGF and FGF-2 [see for example (Gwinn et al., 2002; Jankowsky and Patterson, 2001; Conti et al., 2009; Balu et al., 2008)]. However, surprisingly little is known about the effect of seizures on neurotrophic factor expression in the brains of immature animals. The only published data in this context come from studies in which BDNF expression was evaluated following several hours of prolonged continuous seizures (status epilepticus); the results, which varied across studies, depended upon whether kainic acid (Danzer et al., 2004; Dugich-Djordjevic et al., 1992; Kornblume et al., 1997) or pilocarpine (Kornblum et al., 1997; da Penha Berzaghi et al., 1993) was used to provoke status epilepticus. The effects of repeated brief seizures, such as those induced by electroshock, on neurotrophic factor expression in the brain during postnatal development have not been investigated.
Given the clinical interest in the potential therapeutic value of ECT for the treatment of pre-adolescent children (Russell et al., 2002; Zaw, 2006), it is important to evaluate the impact of electroshock-induced seizures on trophic factors in young animals.
The experiments in the present report were designed to examine the effect of repeated brief seizure episodes induced by electroshock on the expression of BDNF, NGF, and FGF-2 protein in selected brain areas of rat pups between P7 and P21 (corresponding to the period from human infancy through early childhood), in order to identify the earliest age at which an induction of expression can be detected in the brain regions previously documented to show substantial seizure-induced induction in adults.
In adult rats, electroshock seizure (ECS)-induced upregulation of BDNF and NGF proteins was especially pronounced in hippocampus (Altar et al., 2003; Conti et al., 2009; Angelucci et al., 2002), while FGF-2 protein was markedly elevated in rhinal cortex (Gwinn et al., 2002). NGF also exhibited ECS-induced increase in frontal cortex (Conti et al., 2009). Thus, in the present study, hippocampus was used for analysis of BDNF and NGF, rhinal cortex was used for analysis of FGF-2, and frontal cortex was used for analysis of NGF.
Experimental procedures
Animals
Sprague–Dawley male and female rat pups (Harlan) between the ages of postnatal day 1 (P1) to P20 were used. The day of birth was defined as P0. Rats were maintained with a dam in a temperature-controlled (21°C) room with a 12-h light cycle. All experimental protocols used were in compliance with the American Association for Accreditation of Laboratory Animal Care standards and were approved by the Georgetown University Animal Care and Use Committee. All efforts were made to minimize the number of animals used and procedures causing discomfort; there was no illness or mortality caused by the treatments in this study. For all experiments, brain and body weight were recorded in order to examine the changes in the developmental growth after seizure treatment. The data for males and females were combined since there was no significant difference between males and females in our results.
ECS seizure treatment
Minimal ECS was administered in a standard fashion via transorbital electrodes (60 Hz, 200 ms, 20–35 mA) delivered by a Wahlquist stimulator (Wahlquist Instrument Company) as described previously (Gwinn et al., 2002). During the first 2 postnatal weeks, ECS current was 35 mA to evoke a threshold seizure; the current was reduced to 20 mA for treatments during the third week of age, to adjust for the decrease in seizure threshold during this period. Control (sham) animals received the same handling and contact with the electrodes, but no current was passed. Animals were behaviorally observed to ensure that minimal motor seizures lasting 5–60 s occurred after each ECS. One ECS treatment session consisted of three ECS seizures, given at 30-min intervals (i.e., at 0, 30, and 60 min).
Treatment groups
ECS treatment was administered to groups of animals at different ages according to the following regimen:
7 daily ECS treatments
One session of ECS (three ECS seizures at 30 min intervals) was given daily for 7 days during three different developmental periods: a) P1-7, b) P7-13, c) P14-20, respectively.
Sham ECS treatment
The animals were treated as described above, but no current was passed through the electrodes.
Animals were sacrificed 6 h after the last treatment in order to observe the changes in protein levels of neurotrophic factors (Altar et al., 2003; Zetterstrom et al., 1998; Follesa et al., 1994).
Tissue processing
Animals were decapitated and their brains were rapidly removed and placed on ice for immediate dissection of required brain areas. Dissected tissue was frozen and stored at −80°C until analysis. The regions dissected for protein measurements differed for each neurotrophic factor studied: rhinal cortex was used for FGF-2 measurements; hippocampus and frontal cortex were used for NGF measurements; hippocampus was used for BDNF measurements. These areas represent those which exhibit the clear increases in response to repeated seizures in adult rats (Gwinn et al., 2002; Bengzon et al., 1992; Angelucci et al., 2002). The sample referred to as ‘rhinal cortex’ corresponded to a single piece of tissue containing the entorhinal and perirhinal cortex as well as the adjacent lateral periamygdaloid cortex and the posterior piriform cortex.
Enzyme-linked immunosorbent assay (ELISA) for measuring BDNF and NGF
Both BDNF and NGF were determined using the Emax® Immunoassay system (Promega, Madison, WI) according to the manufacturer’s instructions. Regional samples were homogenized in ice-cold lysis buffer (137 mM NaCl, 20 mM Tris–HCl (pH=8.0), 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium vanadate) and then centrifuged at 15,000×g for 20 min. Protein concentrations in the supernatant were measured using the mini-Bradford Coomassie Blue colorimetric assay (Bio-Rad). Samples were acidified and then neutralized in order to measure the total level of trophic factors.
Standard 96-well flat-bottom Nunc ELISA plates were coated with either polyclonal anti-NGF or monoclonal anti-BDNF in carbonate coating buffer and incubated overnight at 4°C. The plates were then blocked with Block and Sample 1 buffer for 1 h. The samples diluted to within the range of the standard curve were applied and incubated for 6 h (NGF) or 2 h (BDNF) at room temperature. Wells were washed and incubated with a secondary monoclonal anti-NGF overnight at 4°C or anti-human BDNF polyclonal antibody for 2 h. After washing, anti-rat IgG (NGF) or anti-IgY (BDNF) horseradish peroxidase conjugate was added for 2.5 h (NGF) or 1 h (BDNF) at room temperature. Then, TMB One solution was used to develop color for 10 min. The reaction was stopped with 1N HCl and the optical density was measured at 450 nm using a plate reader. All samples were measured in triplicate and total protein concentrations were determined from the regression line for the NGF or BDNF standards.
The validity of the ELISA for detecting mature BDNF was verified by Western blotting which was performed on subset of samples taken from the supernatants collected for ELISA (50 μg of protein). For detecting BDNF, membrane was incubated with primary antibody N-20 (Santa Cruz, CA) at a dilution of 1:200 overnight at 4°C and secondary (1:300) antibody for 1 h at room temperature. The rest of the Western blotting procedure was performed as described below for FGF-2. Western documented the presence of a single band corresponding to mature BDNF.
Western blotting for measuring FGF-2
Western blotting was used for measuring levels of FGF-2 (ELISA kits are not available for rat FGF-2). FGF-2 protein was isolated from the rat brain samples as described previously (Gwinn et al., 2002). Regional samples were homogenized in 500 μl ice-cold extraction buffer (20 mM Tris, pH 7.4, 2 mM EDTA, 10% Nonidet P-40, 2 M NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 μg/μl aprotinin, 1 μg/μl leupeptin, and 1 μg/μl pepstatin). Samples were then centrifuged at 15,000×g for 20 min and the supernatant was collected. NaCl concentration was brought to 0.6 M with the addition of 10 mM Tris, 1 mM EDTA (TE) and protease inhibitors. Samples were re-centrifuged at 15,000×g for 20 min. Protein concentrations in the supernatant were measured and aliquots containing 120 μg total protein were then added to an Eppendorf tube containing 12 μl of heparin-Sepharose CL-6B (Pharmacia) slurry (100 mg/ml in TE containing 0.6 M NaCl). Tubes were rocked overnight at 4°C. Samples were then centrifuged at 13,000×g for 5 min and the resulting pellet was washed with 750 μl of 10 mM Tris/0.6 M NaCl and re-centrifuged three times at 13,000×g. The final pellet was resuspended in water and diluted into loading buffer. Samples were then boiled for 5 min.
Samples were run on 15% acrylamide gels and proteins were then transferred to PVDF membrane using a Tris/glycine/methanol buffer. Immunoblotting for FGF-2 was carried out using a mouse monoclonal primary antibody directed against FGF-2 (Clone bFM-2, Upstate Biotechnology, Lake Placid, NY) at a dilution of 1:500. Primary and secondary (1:500) antibody incubations were conducted overnight at 4°C. Horseradish peroxidase-labeled secondary antibody was visualized by autoradiography using ECL chemiluminescence (Amersham, Arlington Heights, IL, USA). Densitometry was performed on autoradiographs using an image analysis system (UVP GDS-8000, Upland, CA, USA) with integrated densities measured by image analysis software (NIH Image 1.62, Bethesda, MD, USA). The concentration of FGF was measured by comparing the density of samples and that of 1 ng recombinant FGF-2 protein, which were run simultaneously on the same gel.
Statistics
Statistical comparisons were based on analysis of unpaired student-t test or one-way analysis of variance (ANOVA) followed by post-hoc Tukey test at a significance level of P<0.05.
Results
Effect of repeated ECS on brain and body weight during postnatal development
7 days of chronic ECS treatment did not significantly affect either body weight gain (Δ body weight) during any of the three treatment periods (P1-7, P7-13, P14-20), or brain weight at the time of sacrifice (Table 1). There was also no significant difference in brain weight/body weight ratio (Table 1).
Table 1.
Brain and body weight after 7-day ECS treatments during postnatal development
| Treatment period |
Group (n) | At time of sacrifice |
Δ body weight for 7 daysa |
Brain/body weight ratio x 100 |
|
|---|---|---|---|---|---|
| Brain weight (g) |
Body weight (g) |
||||
| P1-7 | Sham (8) | 0.70 ± 0.019 | 16.06 ± 0.82 | 9.38 ± 0.76 | 4.41 ± 0.24 |
| ECS (8) | 0.67 ± 0.019 | 13.86 ± 1.42 | 7.08 ± 1.33 | 5.10 ± 0.44 | |
|
| |||||
| P7-13 | Sham (9) | 1.06 ± 0.019 | 25.62 ± 1.21 | 12.88 ± 1.36 | 4.20 ± 0.21 |
| ECS (9) | 1.08 ± 0.021 | 24.09 ± 0.79 | 9.68 ± 1.33 | 4.50 ± 0.17 | |
|
| |||||
| P14-20 | Sham (11) | 1.26 ± 0.031 | 39.32 ± 3.11 | 14.10 ± 1.75 | 3.35 ± 0.20 |
| ECS (11) | 1.27 ± 0.021 | 37.61 ± 2.56 | 9.75 ± 1.62 | 3.51 ± 0.18 | |
Values are expressed as mean ± SEM. There was no significant difference between ECS treated and sham treated animals for any parameters.
Δ Body weight = Body weight at time of sacrifice minus body weight at time of the first ECS (or sham) treatment.
Seizure behavior induced by repeated ECS during postnatal development
ECS-induced threshold behavioral seizures changed in semiology with age, as follows:
-
P1-13: head bobbing, pedaling, forelimb clonus and running movements.
Duration: 10-15 sec (P1-7); 10-40 sec (P7-13);
-
P14-17: forelimb clonus, running, head bobbing and loss of posture.
Duration: 30-60 sec
-
P17+: highly stereotyped movements with facial and forelimb clonus, rearing and loss of balance, followed by jumping and head bobbing.
Duration: 60 sec (by 60 sec, normal activity resumed with no evidence of postictal refractoriness).
The intensity and duration of seizures gradually increased throughout the entire tested developmental period and peaked in the third postnatal week. When compared with adults, animals in the third postnatal week had longer and more intense seizures. This age-dependent shift in seizure susceptibility is consistent with the pattern described for pilocarpine-induced seizures in the developing brain (Cavalheiro et al., 1987).
At P7, seizure behavior in response to the last ECS treatment in the group given ECS from P1 to P7 was comparable to that seen after the first treatment in the group given ECS from P7 to P13. Likewise, seizures at P13 in the group given ECS from P7-P13 were comparable to those obtained at P14 in the group given ECS from P14-P20. This indicates that the age-related changes in the behavioral seizure pattern were unrelated to the repeated exposure to ECS.
Changes in FGF-2 protein in rhinal cortex following chronic ECS in postnatal development
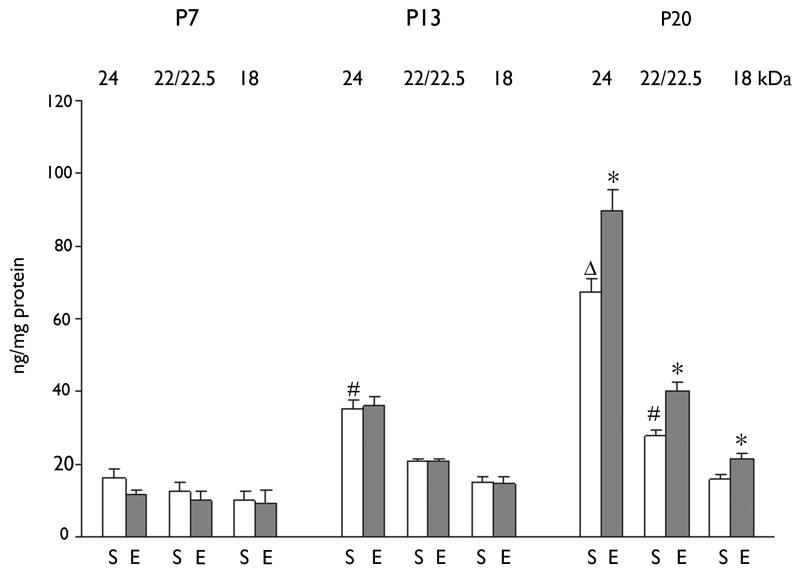
During the first three postnatal weeks, baseline FGF-2 levels increased by almost threefold (from 38.3 ng/mg protein at P7 to 111.1 ng/mg protein at P20; Fig. 1 A,B) in the rhinal cortex. When individual isoforms of FGF-2 were examined, the 24 kDa isoform showed the greatest age-dependent increase, with only a modest increase in the 22/22.5 kDa isoform and a minimal increase in the 18 kDa isoform (Fig. 2). Repeated seizures had no effect on FGF-2 protein levels during the first two postnatal weeks, while a significant increase was observed during the third postnatal week (33.7%; Fig. 1 A,B). As previously observed in adults (Gwinn et al., 2002), the seizure-induced increase seen at P20 was significant for all three isoforms (Fig. 2). However, in contrast to the results in adult brain (Gwinn et al., 2002), seizures did not increase FGF-2 level in frontal cortex during these stages of postnatal development (data not shown).
Figure 1. Effect of brief repeated ECS on FGF-2 expression in rhinal cortex during postnatal development.
(A) Age-associated effect of chronic ECS treatment on level of total FGF-2 protein.
(B) Representative Western blot showing three isoforms of FGF-2 expressed in rhinal cortex at P7, P13 and P20 in rats after chronic (7 day) ECS or sham (control) treatment.
1 Session of ECS consisting of 3 consecutive ECS with 30 minute interval was daily treated for 7days from P1 to P7, from P7 to P13 or from P14 to P20. Rats were sacrificed 6 h after the last ECS treatment. Concentration of FGF-2 protein was calculated based on the optical density of bands for tissue samples and recombinant FGF-2 as standard protein. Data are shown as mean ± SEM (for each group of animals; n=5-6). * p<0.05 compared to sham-treated (control) group at the same age. # p<0.05 compared to sham-treated group at P7. Δ p<0.05 compared to sham-treated group at P13.
Figure 2. The effect of repeated brief ECS on the separate FGF-2 isoforms in rhinal cortex.
1 Session of ECS consisting of 3 consecutive ECS with 30 minute interval was daily treated for 7days from P1 to P7, from P7 to P13 or from P14 to P20. Data are shown as mean ± SEM (for each group of animals; n=5-6). * p<0.05 compared to sham-treated (control) group at the same age. # p<0.05 compared to sham-treated group at P7. Δ p<0.05 compared to sham-treated group at P13. S: Sham-treated group, E: ECS-treated group.
Changes in BDNF protein in hippocampus following chronic ECS in postnatal development
Baseline levels of BDNF in the hippocampus increased significantly between P7 and P13 and then remained steady to P20. Daily repeated seizures during the first postnatal week did not induce an increase in BDNF protein in hippocampus (Fig. 3A). However, protein expression was significantly upregulated by chronic ECS during the second and third postnatal weeks (Fig. 3A). The degree of induction became greater with age (by 42% in week 2 and 162% in week 3), reaching the adult level of induction during the third postnatal week. Western blotting was performed on tissue samples from the same animals to confirm that mature BDNF was increased in response to seizures. Western blot results showed that the mature BDNF protein was increased by repeated seizures to an extent comparable to that demonstrated using ELISA (Fig. 3B).
Figure 3. Effect of brief repeated ECS on BDNF expression in hippocampus during postnatal development.
1 Session of ECS was daily treated for 7days from P1 to P7, from P7 to P13 or from P14 to P20. Rats were sacrificed 6 h after the last ECS treatment. Concentration of total BDNF protein was determined by ELISA (A) and mature BDNF protein (~14 kDa) was measured by Western blot (B). Data are shown as mean ± SEM (n=6). * p<0.05 compared to sham-treated (control) group at the same age. # p<0.05 compared to sham-treated group at P7.
Changes in NGF protein following chronic ECS in postnatal development
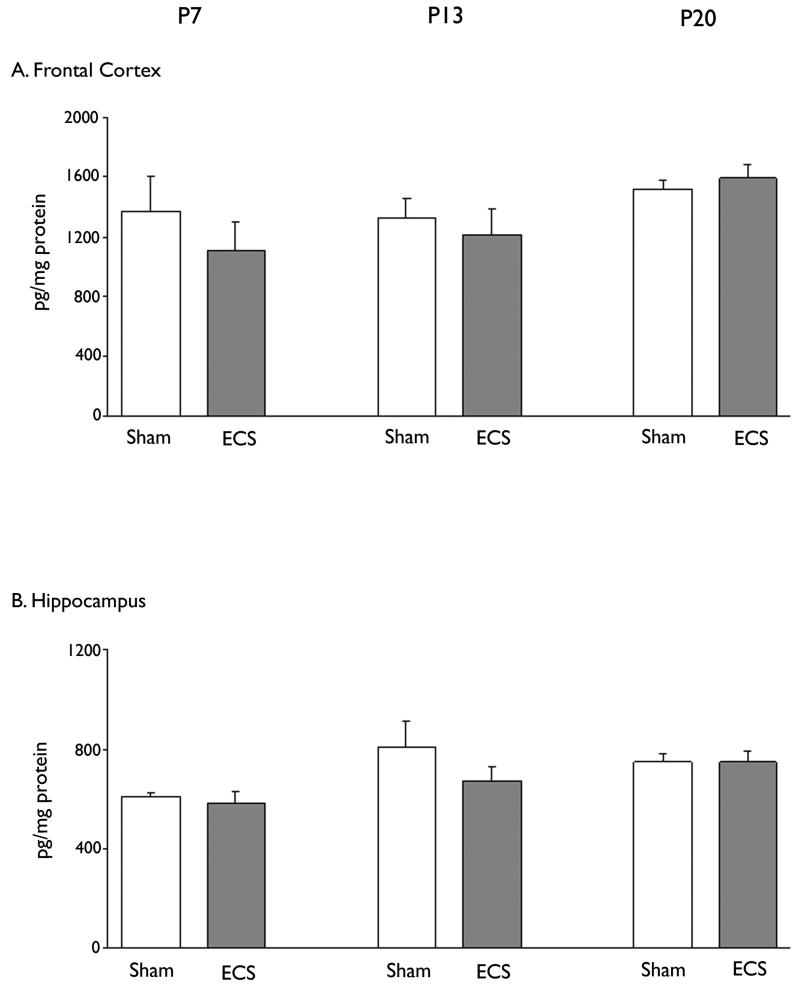
Repeated ECS seizures did not cause a significant increase in NGF protein either in the hippocampus or frontal cortex in any of the age groups examined (Fig. 4). This contrasted with the effects of ECS in the adult brain (Conti et al., 2009).
Figure 4. Effect of brief repeated ECS on NGF expression in frontal cortex and hippocampus during postnatal development.
1 Session of ECS consisting of 3 consecutive ECS with 30 minute interval was daily treated for 7days from P1 to P7, from P7 to P13 or from P14 to P20. Rats were sacrificed 6 h after the last ECS treatment. Concentration of NGF protein in frontal cortex (A) and hippocampus (B) was determined by ELISA. Data are shown as mean ± SEM (for each group of animals; n=6).
Discussion
We used ECS to study the effect of repeated brief seizures on the levels of trophic factors in the developing brain. Repeated ECS exposure over 7 day periods had no significant impact on the health or growth of the rat pups, as indicated by measures of brain and body weight. This indicates that our seizure model is distinct from that of supramaximal ECS which negatively impacts both brain and body weight of immature rats (Wasterlain and Plum, 1973; Vernadakis et al., 1967). As the rat pups matured, the behavioral seizure manifestations became more organized, with the seizures resembling the adult phenotype by the third postnatal week. However, enhanced seizure severity is not likely to be responsible for the induction of neurotrophic factors at different developmental stages, since seizure intensity started to increase in the second postnatal week without significant upregulation of neurotrophic factors. As reported previously, acute brief non-injurious seizure activity lasting 5–10 sec is generally sufficient to induce significant increase of neurotrophic factors in adults (Conti et al., 2009; Gwinn et al., 2002).
The gradual increase in baseline expression of FGF-2 in the rhinal cortex over the first 3 postnatal weeks is consistent with the developmental profile documented in a previous report (Kuzis et al., 1995). Most of the postnatal increase derived from changes in the high molecular weight isoform. Similarly, baseline BDNF expression increased with age, but the most pronounced change was somewhat earlier than of FGF-2.
BDNF levels appeared to reach a peak and plateau by P13, as previously described (Das et al., 2001; Kim et al., 2007). During the third postnatal week, repeated ECS significantly upregulated the hippocampal levels of BDNF and all three isoforms of FGF-2 in the rhinal cortex. This resembles what has been observed previously in the adult brain following repeated ECS (Gwinn et al., 2002; Angelucci et al., 2002; Altar et al., 2003).
During the first postnatal week, no ECS-induced changes in FGF-2 or BDNF proteins were detected, but during the second postnatal week, repeated ECS evoked a small but significant increase in hippocampal BDNF. By the third postnatal week, ECS treatment caused a net increase in BDNF that was more than five times that observed at P13, and a significant increase in FGF-2 protein in rhinal cortex was seen. It is interesting to note that in the case of both FGF-2 and BDNF, the age of onset of responsiveness to seizure-induced upregulation corresponds to the period when baseline levels have reached peak values. This raises the possibility that during the postnatal maturational increase in BDNF and FGF-2 protein levels, their synthesis is largely regulated by activity-independent mechanisms, and that once a critical level of protein is achieved, activity-dependent regulation plays a greater role.
The most likely explanation for the relative lack of induction of FGF-2 and BDNF in response to seizures during the first two postnatal weeks is either the lack of activity-dependent regulation of these trophic factors or a highly constrained range for activity-dependent regulation, with seizure activity exceeding the upper limit of this window. It is also possible that the seizures in the early neonatal period propagate through networks other than those engaged by the seizures in the older pups. The different seizure patterns observed across different developmental stages would support this possibility. However, given the high susceptibility of the hippocampus to all types of seizures, it is unlikely that this area would not be engaged by the seizure activity in the younger animal (Stafstrom at al., 1992).
Baseline levels of NGF in the hippocampus and frontal cortex remained relatively constant over the developmental period examined in our study. This is in agreement with the observations of Das et al (2001) who found little change in NGF protein in hippocampus between P7 and P21. Repeated ECS seizures did not alter NGF expression in the hippocampus and frontal cortex in the rat pups during any of the postnatal ages examined. The absence of an effect in the immature frontal cortex contrasts with the ECS-induced increase, albeit modest, in NGF protein in adult frontal cortex (Conti et al., 2009; Angelucci et al., 2002).
The model of seizures that we employed allows for 24 h intervals between seizure sessions. This treatment regimen is sufficient to cause maximal induction of FGF-2 and BDNF in adults and, as we have seen here, in 3 week-old pups. To address the question of whether more frequent seizures may trigger an upregulation in less mature animals, we conducted pilot studies (unpublished data) in which ECS sessions were given at 6 h intervals between P6 and P8 (i.e., 10 sessions of 3 ECS/session over 54 h). Under these conditions, we saw a 40% increase in hippocampal BDNF, but because this was associated with a significant decrease in the growth rate of the pups, the findings are inconclusive. Nevertheless, this increase was still several fold lower as compared with the induction following seizures during the third postnatal week.
The relative absence of upregulation of trophic factors by recurrent seizures in early postnatal development suggests that seizure activity in this period may not promote neuroplasticity and synaptic remodeling to the extent that it does in the adult brain. This may explain the fact that long-term outcomes following seizures in the immature brain are different from those in adults. For example, an increase in BDNF and TrkB activation is thought to contribute to epileptogenesis (Binder et al., 1999), so the relative lack of seizure-induced BDNF induction may explain the decreased epileptogenicity (i.e., induction of spontaneous seizures) following exposure to status epilepticus during the first two postnatal weeks (Danzer et al., 2004). Spontaneous seizure activity developed after status epilepticus only in pups older than P18 (Danzer et al., 2004; Priel et al., 1996; Stafstrom et al., 1992). In addition, repeated pentylenetetrazol-induced recurrent seizures during the second postnatal week did not induce later spontaneous seizures (Huang et al., 2002).
Our data has implications for the potential therapeutic impact of ECT in children (Stein et al., 2006). If induction of neurotrophic factors is an important component of the therapeutic action of ECT, the relative lack of this component in early childhood may predict reduced efficacy of this treatment approach in this population. On the other hand, we have found a substantial ECS-induction of at least two neurotrophic factors in pre-adolescent animals, suggesting that by late childhood, these mechanisms are operative. However, it should be noted that the lack of increase in NGF protein in frontal cortex and hippocampus and the lack of increase in FGF-2 in frontal cortex are features that distinguish the effect of ECS on the immature brain from that in adults. The extent to which these differences may affect the therapeutic efficacy of ECT are unknown, but they underscore the need for further characterization of the molecular and behavioral consequences of ECS in immature animals.
In conclusion, while the upregulation of neurotrophic factors may mediate many of the short and long-term effects of seizures in the mature brain, whether therapeutic (as in the case of ECT for affective disorders) or deleterious (as in the case of epileptogenesis), a similar extent of seizure-related upregulation does not occur in the immature brain, corresponding to the period of early childhood. This may have implications for the application of ECT to children, as well as for understanding the neurodevelopmental impact of childhood seizures.
Acknowledgments
This study was supported by a Predoctoral Fellowship from the Epilepsy Foundation, and a research grant from the Partnership for Pediatric Epilepsy Research, administered through the Epilepsy Foundation, and NIH grants NS 20576, NS 041231, MH 02040, MH079991, U10 HD047890. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has any conflict of interest to disclose.
Reference List
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol. Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA. Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J. ECT. 2002;18:138–143. doi: 10.1097/00124509-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Soderstrom S, Kokaia Z, Kokaia M, Ernfors P, Persson H, Ebendal T, Lindvall O. Widespread increase of nerve growth factor protein in the rat forebrain after kindling-induced seizures. Brain Res. 1992;587:338–342. doi: 10.1016/0006-8993(92)91016-8. [DOI] [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J. Neurosci. 1999;19:4616–4626. doi: 10.1523/JNEUROSCI.19-11-04616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987;465:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Conti G, Gale K, Kondratyev A. Immunohistochemical evaluation of the protein expression of nerver growth factor and its TrkA receptor in rat limbic regions following electroshock seizures. Neuroscience Res. 2009;65:201–209. doi: 10.1016/j.neures.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J. Neurosci. 1993;13:3818–3826. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, He X, McNamara JO. Ontogeny of seizure-induced increases in BDNF immunoreactivity and TrkB receptor activation in rat hippocampus. Hippocampus. 2004;14:345–355. doi: 10.1002/hipo.10190. [DOI] [PubMed] [Google Scholar]
- Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S., Jr. Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103:739–761. doi: 10.1016/s0306-4522(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic MM, Tocco G, Willoughby DA, Najm I, Pasinetti G, Thompson RF, Baudry M, Lapchak PA, Hefti F. BDNF mRNA expression in the developing rat brain following kainic acid-induced seizure activity. Neuron. 1992;8:1127–1138. doi: 10.1016/0896-6273(92)90133-x. [DOI] [PubMed] [Google Scholar]
- Elfving B, Bonefeld BE, Rosenberg R, Wegener G. Differential expression of synaptic vesicle proteins after repeated electroconvulsive seizures in rat frontal cortex and hippocampus. Synapse. 2008;62:662–670. doi: 10.1002/syn.20538. [DOI] [PubMed] [Google Scholar]
- Follesa P, Gale K, Mocchetti I. Regional and temporal pattern of expression of nerve growth factor and basic fibroblast growth factor mRNA in rat brain following electroconvulsive shock. Exp. Neurol. 1994;127:37–44. doi: 10.1006/exnr.1994.1077. [DOI] [PubMed] [Google Scholar]
- Gwinn RP, Kondratyev A, Gale K. Time-dependent increase in basic fibroblast growth factor protein in limbic regions following electroshock seizures. Neuroscience. 2002;114:403–409. doi: 10.1016/s0306-4522(02)00265-8. [DOI] [PubMed] [Google Scholar]
- Huang LT, Yang SN, Liou CW, Hung PL, Lai MC, Wang CL, Wang TJ. Pentylenetetrazol-induced recurrent seizures in rat pups: time course on spatial learning and long-term effects. Epilepsia. 2002;43:567–573. doi: 10.1046/j.1528-1157.2002.29101.x. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog. Neurobiol. 2001;63:125–149. doi: 10.1016/s0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Kim JK, Jeon SM, Lee KM, Park ES, Cho HJ. Expression of brain-derived neurotrophic factor in the rat forebrain and upper brain stem during postnatal development: an immunohistochemical study. Neuroscience. 2007;146:1128–1136. doi: 10.1016/j.neuroscience.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Sankar R, Shin DH, Wasterlain CG, Gall CM. Induction of brain derived neurotrophic factor mRNA by seizures in neonatal and juvenile rat brain. Brain Res. Mol. Brain Res. 1997;44:219–228. doi: 10.1016/s0169-328x(96)00224-0. [DOI] [PubMed] [Google Scholar]
- Kuzis K, Reed S, Cherry NJ, Woodward WR, Eckenstein FP. Developmental time course of acidic and basic fibroblast growth factors’ expression in distinct cellular populations of the rat central nervous system. J. Comp Neurol. 1995;358:142–153. doi: 10.1002/cne.903580109. [DOI] [PubMed] [Google Scholar]
- Masco D, Sahibzada N, Switzer R, Gale K. Electroshock seizures protect against apoptotic hippocampal cell death induced by adrenalectomy. Neuroscience. 1999;91:1315–1319. doi: 10.1016/s0306-4522(98)00636-8. [DOI] [PubMed] [Google Scholar]
- Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Russell PS, Tharyan P, Arun Kumar K, Cherian A. Electro convulsive therapy in a pre-pubertal child with severe depression. J. Postgrad. Med. 2002;48:290–291. [PubMed] [Google Scholar]
- Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res. Dev. Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- Stein D, Weizman A, Bloch Y. Electroconvulsive therapy and transcranial magnetic stimulation: can they be considered valid modalities in the treatment of pediatric mood disorders? Child Adolesc. Psychiatr. Clin. N. Am. 2006;15:1035–1056. doi: 10.1016/j.chc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Taylor SM. Electroconvulsive therapy, brain-derived neurotrophic factor, and possible neurorestorative benefit of the clinical application of electroconvulsive therapy. J. ECT. 2008;24:160–165. doi: 10.1097/YCT.0b013e3181571ad0. [DOI] [PubMed] [Google Scholar]
- Vernadakis A, Valcana T, Curry JJ, Maletta GJ, Hudson D, Timiras PS. Alterations in growth of brain and other organs after electroshock in rats. Exp. Neurol. 1967;17:505–516. doi: 10.1016/0014-4886(67)90135-5. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Plum F. Vulnerability of developing rat brain to electroconvulsive seizures. Arch. Neurol. 1973;29:38–45. doi: 10.1001/archneur.1973.00490250056006. [DOI] [PubMed] [Google Scholar]
- Zaw FK. ECT and the youth: catatonia in context. Int. Rev. Neurobiol. 2006;72:207–231. doi: 10.1016/S0074-7742(05)72013-4. [DOI] [PubMed] [Google Scholar]
- Zetterstrom TS, Pei Q, Grahame-Smith DG. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res. Mol. Brain Res. 1998;57:106–110. doi: 10.1016/s0169-328x(98)00077-1. [DOI] [PubMed] [Google Scholar]