Abstract
The prevalence of dementia is increasing with expansion of the older adult population. In the absence of effective therapy, preventive approaches are essential to address this public health problem. Blueberries contain polyphenolic compounds, most prominently anthocyanins, which have antioxidant and anti-inflammatory effects. In addition, anthocyanins have been associated with increased neuronal signaling in brain centers mediating memory function as well as improved glucose disposal, benefits that would be expected to mitigate neurodegeneration. We investigated the effects of daily consumption of wild blueberry juice in a sample of nine older adults with early memory changes. At 12 weeks, we observed improved paired associate learning (p = 0.009) and word list recall (p = 0.04). In addition, there were trends suggesting reduced depressive symptoms (p = 0.08) and lower glucose levels (p = 0.10). We also compared the memory performances of the blueberry subjects with a demographically-matched sample who consumed a berry placebo beverage in a companion trial of identical design and observed comparable results for paired associate learning. The findings of this preliminary study suggest that moderate-term blueberry supplementation can confer neurocognitive benefit and establish a basis for more comprehensive human trials to study preventive potential and neuronal mechanisms.
Keywords: blueberries, memory, metabolism, Mild Cognitive Impairment, prevention, neurodegeneration
Alzheimer’s disease (AD) accounts for 60% to 80% of cases of dementia (1). The prevalence of AD threatens to reach epidemic proportions in the coming decades, with projections of 16 million cases in the US by 2050 (1). There are several age-related health conditions that increase vulnerability to AD, most prominently cardiovascular risks (2). However, metabolic disturbance appears to be a fundamental factor driving both cardiovascular disorders and neurodegeneration (3). The presence of insulin resistance and diabetes increase risk for AD substantially, and the risk attributable solely to hyperinsulinemia was determined to be as high as 39% in one longitudinal study (4).
A number of concepts have been introduced to classify older adults at different stages of cognitive decline. Mild Cognitive Impairment (MCI) identifies individuals with increased risk for dementia and represents the first clinical appearance of neurodegeneration for a substantial subset of individuals who will progress to AD (5). There is no remedy for dementia, and it is not clear when or if effective therapy will be developed. However, it has been proposed that interventions initiated in individuals with pre-dementia conditions such as MCI might forestall progression of cognitive decline, and MCI may represent the final point at which intervention can be effective (6).
Dietary approaches hold promise as effective and safe preventive interventions. Dietary factors represent the most potent determinants of metabolic health and have been shown to mitigate specific mechanisms of neurodegeneration (7). Polyphenol consumption is important in this regard, and epidemiological studies indicate that consumption of fruits and vegetables is associated with lower risk of neurodegenerative disorders and better cognitive performance in the elderly (8). Furthermore, there is pre-clinical evidence that blueberry supplementation enhances memory and motor performance in aged animals (9–11). These effects have been attributed in large part to anthocyanins, which enter the brain and other organs (11–13). Enhanced signaling and neuroprotection have been observed in association with blueberry supplementation (14–15). Following blueberry feeding, anthocyanins have been identified in specific cerebral sites, including hippocampus and neocortex, regions essential for cognitive function (12), and anthocyanin distribution in the hippocampus has been related to increased neuronal signaling in that structure (11). Further, there are indications that anthocyanins have insulin-like and glitazone-like properties that contribute to improved metabolic function (16–17) and lipid lowering effects (18).
This body of pre-clinical findings involving several actions pertinent to neurodegeneration suggests that blueberry consumption may be beneficial with respect to memory function in older adults at risk for dementia. As an initial assessment of this hypothesis, we performed a moderate-term trial involving daily supplementation with wild blueberry juice and assessed changes in neurocognitive function.
Materials and Methods
Participants
The study protocol was approved by the University of Cincinnati Medical Institutional Review Board, and each enrolled participant signed the informed consent document. Older adult men and women were recruited from the Cincinnati region with print advertising in the form of flyers posted at senior centers and advertisements placed in the Cincinnati Enquirer, the major daily newspaper. The recruitment ads solicited participation of older adults with mild, acquired memory decline for a dietary supplement study. We enrolled nine participants (5 men, 4 women) who had experienced age-related memory decline such as forgetfulness and prospective memory lapses. The mean (± SD) age of the sample was 76.2 (± 5.2) years, and the mean (± SD) educational level was 15.6 (± 1.5) years. Subject recruitment for this study occurred as an extension of another, controlled berry juice trial evaluating effects of Concord grape juice against a placebo beverage in the same population (19). The design of that study with respect to the duration of the intervention, daily dosage, and the administration of pre- and post-intervention assessments was identical to the blueberry juice trial, and it afforded the opportunity to utilize data from its placebo group as a control. The placebo comparison sample was recruited and screened in the same manner and consisted of seven subjects with early memory decline. There was no statistical difference in age (80.2 ± 6.3 years) or educational level (13.4 ± 3.1 years) between this group and the blueberry sample. The placebo beverage was produced by Welch Foods, Inc., Concord, MA, USA. It contained no juice or natural polyphenol and was matched to Concord grape juice with respect to overall carbohydrate composition and caloric load (3.0 kJ/mL).
Procedure
Prospective participants were assessed with structured interview instruments to determine eligibility for study inclusion. The Academic and Medical History Questionnaire (20) was used to obtain demographic information and information regarding academic attainment, current and past medical conditions, and medication and substance use. Those with diabetes, substance abuse disorder, or diagnosed psychiatric or neurological condition were excluded as well as those using medications that might affect outcome measures such as benzodiazepines. Level of memory impairment was determined with the Clinical Dementia Rating (CDR), which elicits information from the participant and an informant (typically, spouse or adult child) concerning the nature and extent of cognitive decline as manifested in everyday activities at home and in the community (21). The domains memory, orientation, problem solving, community affairs, home activities, and personal care were evaluated, and the ratings for each domain contributed to a global CDR classification with the memory domain weighted most heavily. CDR classifications include no impairment, mild decline, and mild, moderate, and severe dementia. We enrolled individuals with mild decline corresponding to Mild Cognitive Impairment and excluded those with CDR classifications indicating no impairment and those with mild, moderate, and severe dementia. In addition to the global CDR classification, the sum of boxes score also was derived. This score represented the arithmetic sum of the category ratings across the six domains of functioning and served as a means of quantifying the overall level of functional decline (22).
Wild blueberry juice was commercially prepared from ripe, frozen wild (lowbush) blueberries (Vaccinium angustifolium Aiton) by Van Dyk’s Health Juice Products Ltd (Caledonia, Nova Scotia, Canada) and was provided for this research by the Wild Blueberry Association of North America, Old Town, ME, USA. Berries were thawed, pressed, filtered, pasteurized, and then bottled in one liter amber glass bottles. One kg of blueberry fruit produced approximately 735 mL of single-strength juice.
Analyses were performed on samples of the juice used in this study. The most abundant dissolved components in the wild blueberry juice were glucose, fructose, and malic and citric acid (23). Colorimetric measurement of total juice phenolics indicated a concentration of 2.38 g gallic acid equivalents/L (24). The major phenolics in the juice were the hydroxycinnamic acid ester, chlorogenic acid at approximately 734 mg/L, and flavonoid anthocyanins at 877 mg cyanidin 3-glucoside equivalents/L juice based on HPLC analysis as described elsewhere (25). Losses in anthocyanins and other phenolics in blueberry juice can occur during storage. During the approximately three month storage of the juice samples, losses in the total phenolics and anthocyanins were determined to be 23% and 20%, respectively, for juice that was stored under refrigeration in amber bottles.
Daily consumption was maintained between 6 mL/kg and 9 mL/kg by using a dosing schedule determined by body weight. Individuals weighing 54 to 64 kg were prescribed 444 mL/day, those weighing between 65 and 76 kg consumed 532 mL/day, and those weighing between 77 and 91 kg consumed 621 mL/day. Table 1 contains data on phenolic and anthocyanin intake determined from samples of the blueberry juice used in this study. This dosage range corresponded to the volume used in human trials with Concord grape juice (19,26). The study participants were blind to the supplement they received and were told that the study product might be grape juice, blueberry juice, or a berry placebo beverage. The juice was stored in a cold room at 4 °C prior to distribution. The subjects were instructed to refrigerate the juice at home and to take prescribed daily quantities in equal, divided dosages with the morning, midday, and evening meals. We provided containers with individual dosages marked for each subject to minimize risk of mis-measuring. The period of the intervention was 12 weeks. Despite the fact that this represented a much smaller percentage of total lifespan for humans than for rodents, we expected that the biological response would occur in substantially the same timeframe in view of the correspondence between previous human and animal studies. Blueberry supplementation for similar timeframes in experiments with aged animals have demonstrated cognitive performance improvements (12,14). In addition, human trials of this duration with berry juice have shown beneficial changes in inflammatory markers and antioxidant capacity (26).
Table 1.
Daily Blueberry Juice Intake by Weight, Total Phenolics, and Anthocyanins.
| Body weight, kg | Blueberry juice intake, mL/d | Phenolics, g gallic acid eq. | Anthocyanins, g cyanidin 3-glucoside eq. |
|---|---|---|---|
| 54–64 | 444 | 1.056 | 0.428 |
| 65–76 | 532 | 1.266 | 0.512 |
| 77–91 | 621 | 1.478 | 0.598 |
The subjects were given bottled juice at the baseline visit and at an interim visit during week 6 of the intervention. Adherence to the consumption protocol and side effects were assessed with weekly telephone contacts and by direct interview during the interim and final visits. The subjects were instructed to avoid berry fruits and juices and berry extracts for the duration of the trial and were provided a list of foods and supplements to avoid. This list included fruits and beverages such as blueberries, blackberries, cherries, grapes, grape juice, pomegranates, strawberries, and wine among others.
Assessments were performed at pre-treatment baseline and during the final week of the intervention. The primary outcomes were measures of memory function, including the Verbal Paired Associate Learning Test (V-PAL; 27) and the California Verbal Learning Test (CVLT; 28). Paired associate tasks have identified those with progressive neurodegeneration and demonstrated sensitivity to early and more advanced Alzheimer’s disease (29), and the V-PAL has been shown to be sensitive to developmental performance changes between young, middle-aged, and elderly women (30). This task calls for the subject to learn new associations between common one- and two-syllable semantically unrelated words (e.g., help-years). The V-PAL performance score represents the cumulative number of correct responses summed across four learning and testing trials. The CVLT is a widely-used list learning and recall task that has demonstrated sensitivity to age-related memory changes, MCI, and dementia (31). It involves a 16-item list of common words that can be grouped into semantic categories. However, there is no demand to encode new associations. The free recall score was used to assess word list retention. Both of these verbal memory tests were included because they induce somewhat different cognitive demands. Whereas the V-PAL requires the formation of novel associations, the CVLT involves acquisition and retention of a list of individual words. Both rely on hippocampal processing, although the paired associate task may be more resource intensive and potentially more sensitive to cognitive aging effects (32).
Alternate forms of each memory task were used at the baseline and final visits so that the specific test item content was not repeated. The use of alternate forms substantially mitigates practice effects associated with test-retest designs, in particular with respect to memory tests (33). However, alternate forms may not eliminate performance gain at re-test related to procedural practice effects; that is, familiarity with the test procedure (34).
Mood was assessed with the Geriatric Depression Scale (GDS; 35), a 30-item inventory designed to evaluate symptoms of depression in older adults. We also measured weight and waist circumference, and obtained fasting blood samples for determinations of serum glucose and insulin values by the biochemistry laboratory of the General Clinical Research Center (GCRC) at the University of Cincinnati.
The primary statistical analyses included dependent sample t-tests to determine change from baseline to final visit in memory performance, mood, body anthropometrics, and metabolic parameters. We set α probability of type 1 error at 0.05 to report statistically significant effects. Given the preliminary nature of this trial, we reported trends at α ≤ 0.10. We also computed Cohen’s d effect size statistics (36) for these analyses, which are characterized as small (0.2), medium (0.5), and large (0.8).
Because of concern regarding performance gain with repeated memory testing, we also performed analyses of covariance (ANCOVA) to control for potential procedural practice effects by comparing memory performances of the blueberry sample with that of the grape placebo sample from the companion study. These analyses isolated the effect of the intervention using outcome scores from the final visit as the dependent measure and the corresponding score from the baseline visit as covariate measure (37). Cohen’s f (36) represents the effect size statistic for these analyses, which are small (0.1), medium (0.25), and large (0.40).
Results
Table 2 contains information concerning the sample characteristics. The mean age of our sample and the mean Clinical Dementia Rating sum boxes score are consistent with other studies of Mild Cognitive Impairment (22). The level of depressive symptoms as measured by the Geriatric Depression Scale was within the non-depressed, normal range (35). The average waist circumference was high (98 cm), with the mean (SD) waist circumference for the men at 102.5 (5.0) cm and at 92.5 (15.1) cm for the women. These waist circumference values were, respectively, near and just above gender-specific cutoffs established as markers of insulin resistance and metabolic pathology (38). While baseline fasting glucose was within normal limits for our GCRC laboratory standards (65 to 115 mg/dl), mean fasting insulin was in the hyperinsulinemic range (≥ 15 μU/mL). As would be expected, waist circumference was significantly correlated with fasting insulin, r = .72, p = 0.04.
Table 2.
Subject Sample Characteristics
| N | 9 |
|---|---|
| Age, years | 76.2 (5.2) |
| Education, years | 15.6 (1.5) |
| CDRa sum boxes score | .88 (.48) |
| GDSb score | 5.8 (6.2) |
| Weight, kg | 80.3 (12.0) |
| Waist, cm | 98.0 (11.2) |
| Fasting glucose, mg/dl | 94.6 (10.1) |
| Fasting insulin, μU/mL | 15.9 (14.1) |
Note. CDR = Clinical Dementia Rating.
GDS = Geriatric Depression Scale.
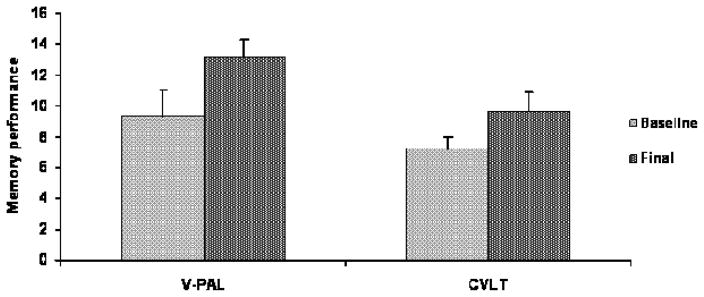
Figure 1 shows changes in memory performance from pre-intervention baseline to the final assessment. The V-PAL cumulative learning score was significantly improved at 12 weeks (13.2 v 9.3), t(8) = 3.42, p = 0.009, effect size d = 1.78. With α = .05, power = 0.95. In addition, word list recall performance on the CVLT improved significantly (9.6 v 7.2), t(8) = 2.34, p = 0.04, d = 1.18. Power for this effect was 0.65 at α = 0.05.
Figure 1.
Memory performances for the blueberry juice sample at the 12-week final visit relative to pre-intervention baseline as measured by the Verbal Paired Associate Learning Test (V-PAL) and the California Verbal Learning Test (CVLT) free recall task. Data show significantly improved performances for both the V-PAL, p = 0.009, and CVLT recall, p = 0.04.
Weight (80.3 v 80.3 kg), t(8) = .05, p = 0.95, and waist circumference (98.0 v 98.9 cm), t(8) = 1.10, p = 0.30, did not change as a result of the intervention. There was a nonsignificant trend indicating reduced depressive symptoms at 12 weeks relative to baseline (3.5 v 5.8), t(8) = 1.96, p = 0.08. There also was a trend toward lower fasting glucose for the blueberry group at 12 weeks compared with baseline (91.2 v 94.6 mg/dl), t(8) = 1.85, p = 0.10. The mean fasting insulin level was reduced to within the normal range at 12 weeks (12.1 v 15.9 μU/mL), although this reduction from baseline level was not statistically significant, t(8) = 1.21, p = 0.26.
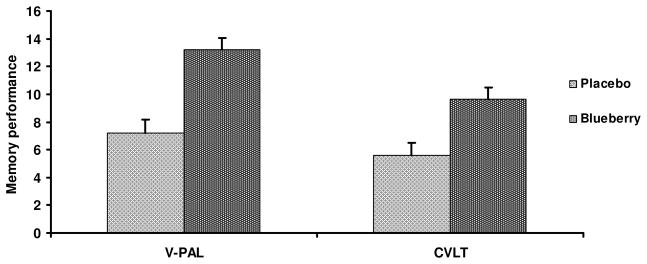
Ancillary ANCOVA tests compared changes in memory performance for the blueberry sample with that of the placebo beverage group from the companion study (19). Figure 2 shows that the performance of the blueberry juice group exceeded that of the placebo beverage group, with a significant effect for V-PAL performance (p = 0.03, Cohen’s effect size, f = 0.48), although the effect for list recall was not maintained (p = 0.12, Cohen’s f = 0.32).
Figure 2.
Post-intervention final visit mean values comparing memory performance for subjects (n = 7) who consumed placebo beverage and subjects who consumed wild blueberry juice (n=9) for 12 weeks. The ANCOVA analyses indicated significantly improved paired associate learning (V-PAL) performance, F(1,13) = 5.58, p = 0.03, although improved recall on the CVLT for the blueberry juice subjects did not achieve statistical significance, F(1,13) = 2.27, p = 0.12.
Discussion
This study indicated that wild blueberry juice supplementation for 12 weeks improved memory function in older adults with early memory decline. To our knowledge, this is the first human trial assessing the potential benefit of blueberry supplementation on neurocognitive function in older adults with increased risk for dementia. Although the sample size was relatively small, effect sizes were moderate to large for both the primary and secondary analyses. The magnitude of the effect for the paired associate task exceeded that for the list learning task and was maintained in the ANCOVA analysis. While both paired associate and list learning tasks rely on hippocampal mediation, the former engages integrated parahippocampal processing to encode associations between unrelated terms (32). Accordingly, the greater magnitude of effect for the V-PAL may reflect the greater resource demand and greater sensitivity of the paired associate task in these subjects with mild decline and, correspondingly, greater performance enhancement in response to blueberry treatment.
We also observed trends suggesting diminished depressive symptoms and reduced fasting glucose levels, measures which would not be expected to be susceptible to practice effects. While the mean depression symptom score at the pre-intervention assessment was below clinically significant levels (35), the reduction is notable and provides further corroboration of neurocognitive benefit associated with the blueberry intervention. The trend toward lower glucose levels along with correction of fasting insulin to the normal range is interesting and suggests one possible mechanism of effect. While much of the preclinical research has focused on antioxidant, anti-inflammatory, and neuronal signaling properties, there also are recent data supporting the notion that anthocyanins can enhance glucose disposal through a number of mechanisms (16,17). This factor also may contribute to improved neurocognitive function. Improved glucose disposal and correction of hyperinsulinemia would be expected to be associated with reduction of inflammation and greater clearance of central beta-amyloid as well as enhanced signaling in memory centers (39). It will be of interest to pursue investigations of blueberry supplementation on metabolic and other putative mechanisms of neurodegeneration to determine whether changes in cognitive function can be associated with metabolic enhancement and downstream mechanisms such as inflammation and neuroplasticity.
One of the primary limitations of this study was the small sample size. Although the significant effects and substantial effect sizes are encouraging, there is a clear need for larger trials. The absence of a fully matched control product also was a limitation. While data from the grape juice placebo sample did provide adequate control on a number of dimensions, including identical study design and duration of the intervention period, use of a product designed to simulate characteristics of berry juice, daily dosage, and control for potential practice effects, it was not matched to the blueberry juice for glycemic load. This raises the possibility that the relatively greater glycemic content of the placebo beverage might have influenced cognitive performance. While this concern is mitigated by the fact that change in metabolic function was not observed in the companion study (27), use of a fully matched placebo product in future studies will be essential.
These preliminary memory findings are encouraging and suggest that consistent supplementation with blueberries may offer an approach to forestall or mitigate neurodegeneration. Interpretation of our findings should be tempered because of the relatively small sample size and the absence of a blueberry-specific control, although comparison with the analogous placebo beverage data provides some assurance that the observed changes in memory performance were not attributable to practice effects. Replication of the findings in a larger, controlled trial will be important to corroborate and amplify these data. On balance, this initial study establishes a basis for further human research of blueberry supplementation as a preventive intervention with respect to cognitive aging.
Acknowledgments
Material and funding support provided by the Wild Blueberry Association of North America and NIH grant # R21AG024484
Literature Cited
- 1.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: Common links. J Int Med. 2006;2(260):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Craft S. Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity related effect on memory, amyloid, and inflammation. Neurobio Aging. 2005;26S:S65–S69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Ming-Xiu T, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer’s disease. Neurol. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 6.Cotman CW. Homeostatic processes in brain aging: The role of apoptosis, inflammation, and oxidative stress in regulating healthy neural circuitry in the aging brain. In: Stern P, Carstensen L, editors. The aging mind: Opportunities in cognitive research. National Academy Press; Washington DC: 2000. pp. 114–143. [Google Scholar]
- 7.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: A diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JG, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 9.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits in blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova N, Bickford PC, Joseph JA. Short-term dietary supplementation of blueberry polyphenolics: Beneficial effects on aging brain performance and peripheral tissue function. Nutri Neurosci. 2000;3:383–397. [Google Scholar]
- 11.Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee MG, Smith MA, Joseph JA. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutri Neurosci . 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 12.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lameula-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutri Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 13.Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillore SA, Graf BA, O’Leary JM, Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 14.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer J. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Rad Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Galli RL, Bielinshi DF, Szprengiel A, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol Aging. 2006;27:344–350. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, Le PM, Prentki M, Bennett SA, Arnason JT, Haddad PS. Anti-diagetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomed. 2006;13:612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem. 2008;56:642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- 18.Kalt W, Foote K, Fillmore SA, Lyon M, Van Lunen TA, McRae KB. Effect of blueberry feeding on plasma lipids in pigs. Brit J Nutri. 2008;100:70–78. doi: 10.1017/S0007114507877658. [DOI] [PubMed] [Google Scholar]
- 19.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord Grape Juice Supplementation Improves Memory Function In Older Adults with Mild Cognitive Impairment. Brit J Nutri. doi: 10.1017/S0007114509992364. in press. [DOI] [PubMed] [Google Scholar]
- 20.Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in Obsessive- Compulsive Disorder. Brain Cogn. 2004;54:257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical Scale for the staging of dementia. Br J Psychiat. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 22.O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R. Staging dementia using clinical dementia rating scale sum of boxes scores. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalt W, McDonald JE. Chemical composition of lowbush blueberry cultivars. J Am Soc Hort Sci. 1996;121:142–146. [Google Scholar]
- 24.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer J Enol Vitic. 1965;6:144–158. [Google Scholar]
- 25.Kalt W, MacKinnon SL, McDonald JE, Vinqvist MR, Craft C, Howell AB. Phenolics of Vaccinium berries and other fruit crops. J Sci Food Agric. 2008;88:68–76. [Google Scholar]
- 26.O’Byrne DJ, Devaraj S, Grundy SM, Jialal I. Comparison of the antioxidant effects of Concord grape juice flavonoids and α-tocopherol on markers of oxidative stress in healthy adults. Am J Clin Nutri. 2002;76:1367–1374. doi: 10.1093/ajcn/76.6.1367. [DOI] [PubMed] [Google Scholar]
- 27.Krikorian R. Independence of verbal and spatial paired associate learning. Brain Cogn. 1996;32:219–223. [Google Scholar]
- 28.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- 29.Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- 30.Krikorian R. Cognitive changes in perimenopause. In: Liu J, Gass M, editors. Management of the perimenopause. McGraw-Hill; New York: 2006. pp. 57–74. [Google Scholar]
- 31.Greenaway MC, Lacritz LH, Binegar D, Weiner MF, Lipton A, Munro CC. Patterns of verbal memory performance in Mild Cognitive Impairment, Alzheimer disease, and normal aging. Cog Behav Neurol. 2006;19:79–84. doi: 10.1097/01.wnn.0000208290.57370.a3. [DOI] [PubMed] [Google Scholar]
- 32.Jackson O, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 33.Beglinger LJ, Crawford-Miller J, Kareken DA. Neuropsychological practice effects and change detection in people with schizophrenia. Schizophrenia Res. 2003;62:191–194. doi: 10.1016/s0920-9964(02)00425-5. [DOI] [PubMed] [Google Scholar]
- 34.McCaffrey RJ, Westervelt HJ. Issues associated with repeated neuropsychological assessments. Neuropsychol Rev. 1995;5:203–221. doi: 10.1007/BF02214762. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depresssion rating scale: A preliminary report. J Psychiat Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37.Sheeber LB, Sorensen ED, Howe SR. Data analytic techniques for treatment outcome studies with pretest/posttest measurements: An extensive primer. J Psychia Res. 1996;30:185–199. doi: 10.1016/0022-3956(96)00012-X. [DOI] [PubMed] [Google Scholar]
- 38.Wahrenberg H, Hertel K, Leijonhufvud B, Pearson L, Toft E. Use of waist circumference to predict insulin resistance: retrospective study. Brit Med J. 2005;330:1363–1364. doi: 10.1136/bmj.38429.473310.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson GS, Cholerton BA, Reger MA, Baker L, Plymate S, Asthana S, Fishel MA, Kulstad J, Jacob BA, Green PS, Cook DG, Kahn SE, Keeling M, Craft S. Preserved cognition in patients with early Alzheimer’s disease and amnestic Mild Cognitive Impairment during treatment with rosiglitazone. Am J Geria Psychia. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]