Abstract
Portal fibroblasts are an important yet often overlooked non-parenchymal cell population in the liver. They are distinct from hepatic stellate cells, yet like stellate cells differentiate in the setting of chronic injury to fibrogenic myofibroblasts, playing an important role in collagen production in the fibrotic liver. Portal fibroblasts are located adjacent to bile duct epithelia and thus play a particularly significant role in biliary fibrosis. New data suggest that they may also have key functions independent of fibrogenesis. This review will address the definition and characteristics of PF as well as their signaling pathways, interactions with the biliary epithelium, and contributions to liver pathobiology. We conclude that portal fibroblasts are an important and multifunctional non-parenchymal cell population in need of further study.
Keywords: Biliary fibrosis, biliary duct epithelia, cholangiocytes, MCP-1, TGF-β, IL-6, P2Y, elastin, precursor cell niche, myofibroblast
Fibrosis and cirrhosis have been referred to as the “final common pathway” of chronic liver injury. Although anatomists and pathologists have always stressed the differences between biliary and non-biliary etiologies of fibrosis, the landmark isolation of hepatic stellate cells (HSC) and demonstration of their in vitro “activation” resulted in 20 years of fibrosis research focused on understanding HSC behavior in culture and applying these findings to animal models of disease. Recent work, however, has led to a renewed appreciation for the cellular complexity of fibrosis. We discuss here the fibroblasts and myofibroblasts of the portal tract, emphasizing new data demonstrating that these cells have important roles in liver fibrosis and other pathology, and highlighting promising areas for future research.
History, Nomenclature, and Markers
Portal fibroblasts (PF) were reported as distinct cells as early as 1961, when Carruthers and colleagues used light and electron microscopy to study the rat portal tract after bile duct ligation (BDL) (1, 2). These investigators observed fibroblast proliferation around newly formed bile ductules and reported that fibroblasts of the diseased portal tract had long processes and were often surrounded by fibrils, including elastic fibers (1). In 1963, Popper and colleagues described “mesenchymal cells not related to sinusoids” and later noted that fibroblast-like cells and matrix deposits were present in the region immediately surrounding proliferating bile ducts in biliary cirrhosis (3, 4). These early observations were coincident with the recognition by Gabbiani and colleagues that fibroblast-derived α-smooth muscle actin (α-SMA)-expressing myofibroblasts were the major matrix producing cells in wound healing (5), setting the stage for the study of PF as potential mediators of fibrosis. The study of PF as candidate myofibroblast precursors stalled, however, after methods to isolate HSC were first published, and Friedman reported that HSC in culture underwent “activation” to fibrogenic myofibroblasts (6, 7). The observation that HSC (and not hepatocytes) were matrix-producing cells (8, 9) led to a proliferation of research on HSC, and the majority of publications in the liver fibrosis literature over the last two decades have incorporated the assumption that all α-SMA positive myofibroblasts are activated HSC.
The recent resurgence of interest in PF has resulted in part from data showing that liver myofibroblasts are heterogeneous and not always derived from HSC (10-13). It has been appreciated for many years that biliary cirrhosis is distinct from non-biliary cirrhosis, occurring more rapidly and with the pathological signature of dysregulated bile ductular proliferation. As it became clear that the bile duct epithelia (BDE) are the primary site of injury in chronic cholangiopathies such as primary biliary cirrhosis and that fibrosis originates in the peri-ductular region in these diseases (14), the portal localization of PF (as opposed to the more distant, perisinusoidal location of HSC) made them attractive candidates as mediators of biliary fibrosis. Indeed, a model whereby PF were “first responders” in biliary fibrosis, later to be supplanted by HSC, was proposed in 2002 by Kinnman and Housset (15).
PF are heterogeneous and have been given a variety of different names, some cumbersome, complicating research into their behavior. Similarly, PF have been identified (and differentiated from HSC) on the basis of expression of multiple markers, but these have not been consistently examined by different researchers. Names applied to fibroblasts found in the portal region have included “peribiliary fibrogenic cells distinct from hepatic stellate cells” as well as “periductular fibroblasts” and “portal/periportal mesenchymal cells” (16-18). Myofibroblasts have similarly been given different names when associated with the portal tract. Cassiman et al. identified three myofibroblast populations in cirrhotic rat and human livers: myofibroblasts clearly derived from HSC, portal/septal myofibroblasts postulated to be derived from PF, and interface myofibroblasts, with an intermediate phenotype and unclear origin (10, 19). For clarity, we refer here to all fibroblasts in the portal region (whether periductal or not) as PF and to all non-HSC derived myofibroblasts in the portal region as portal myofibroblasts, acknowledging that the cells in each category are heterogeneous. Portal myofibroblasts in particular may originate from different precursor cell populations, potentially including vascular smooth muscle cells from the walls of the hepatic artery and portal vein.
Isolated PF in culture, which undergo myofibroblastic differentiation, are a useful new tool for studying mechanisms of biliary fibrosis. Unfortunately, isolation techniques, nomenclature, and identification of these presumably heterogeneous cells vary. PF clearly distinct from HSC by marker analysis have been isolated by outgrowth from dissected bile duct segments and express α-SMA and type I collagen after undergoing growth in culture (17, 20). We have isolated PF from rat liver by sequential protease perfusion, bile duct dissection, and size selection and have observed that they undergo progressive myofibroblastic differentiation over 10-14 days (21, 22). In no case, however, is it understood how well isolated PF and portal myofibroblasts reflect the corresponding cell populations in vivo.
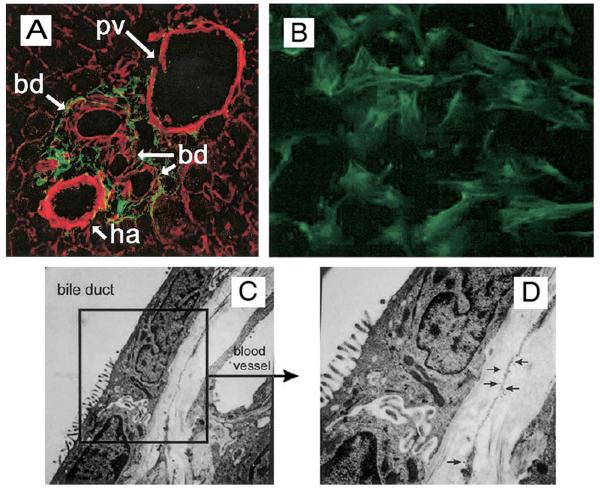
A variety of markers have been used to identify PF, although the findings of different groups have not always coincided. Markers considered specific for PF include fibulin-2, IL-6, elastin, and the ecto-ATPase nucleoside triphosphate diphosphohydrolase-2 (NTPD2; Fig. 1). Expression of P100, α2-macroglobulin, and neuronal proteins (including neuronal cell adhesion marker and synaptophysin) and the absence of lipid droplets have also been used to differentiate PF from HSC (for review, see (10, 18, 23)). As research on PF increases, the application of a uniform set of markers by different investigators would undoubtedly clarify many published results.
Figure 1. Expression of NTPD2 by PF.
A. Confocal immunofluorescent expression of NTPD2 in normal liver. Sections were obtained from normal rat liver, and a photomicrograph highlight of a portal area is shown. NTPD2 expression is demonstrated by indirect immunofluorescence (green), and filamentous actin is shown by staining with rhodamine phalloidin (red). NTPD2 is expressed in the stromal cells surrounding intrahepatic bile ducts, identified by apical and lateral actin staining. B. Immunofluorescent expression of NTPD2 in isolated rat PF on glass coverslips. NTPD2 is seen at the plasma membrane and intracellular structures. C, D. Immuno-electron microscopic expression of NTPD2 in normal liver. Sections were obtained from normal rat liver, and an area of the PF-bile duct interface is shown. Immuno-gold labeling is seen on PF and is plentiful on membranous extensions from the PF soma that are adjacent to the bile duct basolateral membrane, best seen in the inset (D). Adapted from (53).
Development and Embryonic Origins
The embryologic origins of PF are not known, and definitive lineage tracing has not been performed. The portal mesenchyme in the developing human liver includes α-SMA-positive cells at the ductal plate stage. During the ductal plate remodeling stage, these α-SMA-positive cells disappear, and cells expressing vimentin, believed to be PF, begin to appear (24); it appears likely that both PF and vascular smooth muscle cells are derived from the early α-SMA-positive mesenchymal cells. Immunostaining data suggest that portal myofibroblasts are important mediators of biliary development (potentially through production of extracellular matrix components such as laminin and collagen IV) and that they also contribute to hepatic arterial development (25).
A recent study showed that p75 neurotrophin receptor (p75NTR)-expressing mesenchymal cells in the mouse fetal liver include precursors for both HSC and PF. p75NTR-positive cells were initially localized to the periphery of the liver bud but then divided into distinct parenchymal and portal populations, presumably reflecting HSC and PF. Since the portal population of p75NTR-positive cells expressed the Notch ligand Jagged1, these cells may regulate the commitment of hepatoblasts to a biliary lineage (26). Lineage tracing analyses using mouse embryos expressing a LacZ reporter gene under the control of the mesodermal marker MesP1 demonstrated a mesodermal origin for HSC and perivascular mesenchymal cells (desmin+, p75NTR+, α-SMA+) as well as a population of submesothelial cells (27). The perivascular mesenchymal cells described may be PF precursors. Interestingly, this would suggest that HSC and PF originate from a common precursor in the early embryo.
Cell Biology of PF and Portal Myofibroblasts
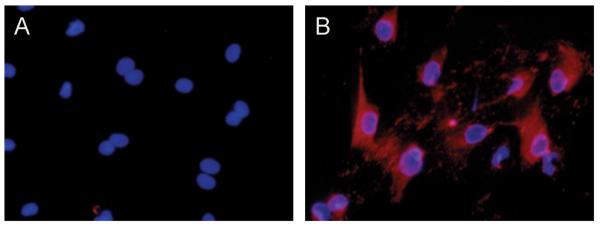
PF in the normal liver are similar to other fibroblasts, and elastin-expressing PF in culture can be stained with the marker TE-7, considered to be definitive for fibroblasts (Fig. 2) (28). PF, like most fibroblasts, are characterized by two key features: prominent endoplasmic reticulum, especially rough endoplasmic reticulum, and elongated and thin cytoplasmic processes (1). Their Golgi complexes are relatively small (29). PF have dendrite-like cell extensions that extend to within sub-micron distances of the basolateral membranes of BDE; these extensions have been reported to increase in number in response to injury (30).
Figure 2. PF, unlike HSC, express the fibroblast marker TE-7.
Myofibroblastic HSC (A) and PF (B), isolated as described from normal rat liver and differentiated in culture (22), were stained with the fibroblast marker TE-7 (red) and with the nuclear stain DAPI (blue). Note that this marker clearly distinguishes the two cell types, and identifies PF in culture as fibroblasts.
PF undergo myofibroblastic differentiation in the chronically injured liver and when cultured on plastic or glass. Portal myofibroblasts, like typical myofibroblasts, express large numbers of α-SMA-containing microfilament bundles arrayed in parallel to the long axis of the cell. In the livers of alcohol-fed but not normal baboons, portal myofibroblasts express pinocytic vesicles (31). Rough endoplasmic reticulum and Golgi complexes are more prominent in myofibroblastic PF than in normal PF (29). Relative to HSC-derived myofibroblasts, portal myofibroblasts demonstrate more variability in size (18). There are controversial reports that portal myofibroblasts proliferate and can be passaged in culture, whereas myofibroblastic HSC undergo DNA replication but do not divide, and rapidly undergo apoptosis after passaging (19, 32). Whether portal myofibroblasts, like HSC myofibroblasts, can revert to a non-myofibroblastic state, at least in culture, is not known, and whether such reversion occurs to any significant degree in vivo (for example during fibrosis regression) for either cell type is similarly unknown (33, 34).
PF as Mediators of Fibrosis
There is now convincing evidence that PF and portal myofibroblasts play an important role in liver scar formation, especially in biliary fibrosis. As early as 1991, PF in the post-BDL rat liver were reported to express fibronectin and fibrillar collagens (35), raising the possibility, later supported by data from other groups, that PF not only deposit matrix but do so before undergoing myofibroblastic differentiation (29, 35). Desmouliere and colleagues in particular noted that significant increases in portal matrix deposition preceded the appearance of myofibroblasts in the rat BDL model (35).
Several seminal papers established a role for portal myofibroblasts in fibrosis. Tuchweber et al. reported in 1996 that both PF and BDE in the rat liver proliferated dramatically in the first 48 hours after BDL, and that many α-SMA positive myofibroblasts, negative for the HSC marker desmin, appeared adjacent to the proliferating ductules (30). This supported a model in which portal myofibroblasts play an important role in early portal fibrosis. Other groups, using both BDL and chronic carbon tetrachloride toxicity models, demonstrated that myofibroblasts accumulating in different regions of the liver expressed different markers; correlation with in vitro data suggested that myofibroblasts derived from both PF and HSC caused fibrosis (11, 36).
Beaussier et al. recently used two different models of cholestatic liver injury to conclude that HSC do not undergo myofibroblastic differentiation in biliary fibrosis. In rat livers subjected to either BDL or arterial ischemia, HSC upregulated desmin expression, but did not contribute to the large population of α-SMA-expressing myofibroblasts in the portal region associated with the fibrotic scar (16). Although Beaussier et al. did not stain their sections with markers specific for PF, the portal myofibroblasts they observed were likely derived from PF. Future fibrosis research will need to incorporate standardized marker analyses in order to delineate the relative contributions of HSC and PF. Nonetheless, it is clear that the cellular basis of fibrosis is variable, depending on the nature of the injury, and that PF and portal myofibroblasts make significant contributions independent of HSC.
Signaling and Cell-Cell Interactions
Factors regulating the behavior of PF have primarily been studied in cells in culture. Transforming growth factor-β (TGF-β), widely appreciated as one of the most important mediators of liver fibrosis, is required for PF differentiation. PF require a relatively stiff surrounding environment as well as TGF-β in order to express α-SMA and become fibrogenic in culture (22). This is in contrast to HSC in culture, which express α-SMA independent of TGF-β (22, 37, 38), but is consistent with the model of Gabbiani and colleagues that TGF-β as well as mechanical tension are required for differentiation of myofibroblasts in all tissues (39). Interestingly, PF-derived myofibroblasts secrete large amounts of the TGF-β isoform TGF-β2 and express high levels of the TGF-β receptor betaglycan, which is required for high affinity signaling by TGF-β2 (40). Combined with evidence that damaged BDE in human tissue express TGF-β2 and release inflammatory mediators (discussed below), this suggests a model whereby BDE damage leads to initial PF myofibroblastic differentiation, followed by autocrine perpetuation of the process (41).
The impact of other growth factors on the function of PF is less clear. Tumor necrosis factor-α, although upregulated in patients with advanced primary biliary cirrhosis (42), has not been shown to regulate PF activity, and our unpublished data suggest that it has no effect on PF myofibroblastic differentiation or type I collagen production. Similarly, the role of connective tissue growth factor (CCN2) in PF biology has not yet been studied, although it often enhances or mediates the effects of TGF-β and is upregulated in human biliary fibrosis and in animal models of chronic liver disease (43, 44). Conflicting results have been reported for platelet-derived growth factor (PDGF). One group demonstrated that PDGF upregulated α-SMA expression in PF in culture; in rats subjected to BDL, injections of the protein-tyrosine kinase inhibitor ST1571 resulted in decreased α-SMA expression without altering bile ductular proliferation (17). Another group, however, observed that PDGF enhanced PF proliferation in culture but decreased α-SMA expression (22). PDGF is of particular interest given that it is expressed by cultured bile duct segments from BDL-treated rats, suggesting a possible mechanism for fibrosis after BDL (45). Interestingly, PDGF induces production of sonic hedgehog by myofibroblastic HSC, which enhances HSC growth in an autocrine fashion (46). Although the role of hedgehog has not been studied in PF, the hedgehog pathway is activated in rat livers after BDL, raising the possibility that PF also produce or respond to hedgehog ligands (47).
Strong evidence suggests that signals between BDE and PF are instrumental in the progression of biliary fibrosis and cirrhosis. Several investigators have shown that there is a direct correlation between the intensity of the ductular reaction and the severity of fibrosis in human liver disease of a variety of etiologies, including hepatitis C and non-alcoholic fatty liver disease, as well as in animal models (16, 48-50). Cytokines and chemokines, in particular interleukin-6 (IL-6) and monocyte chemotactic protein-1 (MCP-1; CCL2), are emerging as important mediators of cell-cell communication between BDE and PF. In humans, IL-6 is expressed specifically by BDE in biliary as opposed to other forms of fibrosis (51); correspondingly, PF express the IL-6 co-receptor gp130 (52). There appears to be a paracrine loop operative in which IL-6 potently downregulates NTPD2 on PF (without altering PF myofibroblastic differentiation); NTPD2 in turn regulates G protein-coupled P2Y receptors for extracellular ATP and other nucleotides, which mediate BDE proliferation (53). Additionally, non-HSC rat liver myofibroblasts express IL-6, providing a mechanism for autocrine regulation as well (54). The result is that loss of NTPD2, which is observed in biliary cirrhosis in rats and humans, leads to BDE hyper-proliferation (55, 56). To complete the loop, IL-6 is released by BDE in response to extracellular ATP, providing a feed-forward mechanism for the continued production of IL-6 and the perpetuation of bile ductular proliferation (52).
MCP-1 was first identified as a regulator of monocyte/macrophage chemotaxis (57), and may be important as a regulator of PF myofibroblastic differentiation and fibrogenesis. BDE express MCP-1 mRNA and are the liver cells expressing MCP-1 mRNA most strongly in chronic hepatitis (58). There is now direct evidence that BDE signal to PF via MCP-1 release. MCP-1 upregulates PF proliferation, myofibroblastic differentiation, and procollagen-1 mRNA expression and downregulates NTPD2 expression. Furthermore, conditioned medium from BDE isolated from BDL-treated rats induced PF myofibroblastic differentiation, which was inhibited by an MCP-1 blocking antibody (59). The identity of the MCP-1 receptor expressed by PF is not known. These cells fail to express the MCP-1 cognate receptor CCR2, although CCR2 null mice are protected from BDL-induced cirrhosis (59, 60).
Additional Roles for PF
HSC, initially studied almost exclusively as matrix-producing cells, are now known to have many complex functions (61). Although our understanding of PF-mediated fibrogenesis is still in its infancy, two additional points warrant mention. PF appear to be the major elastin-expressing cells (aside from vascular smooth muscle cells) in the liver, and some investigators have shown that elastin deposition increases as PF proliferate and fibrosis progresses (35, 36). Although not all groups agree that elastin is specific for PF (as opposed to HSC) (62), the data raise interesting points beyond the use of elastin as a marker for PF. Elastic fibers, which are formed from fibrillin microfibrils, with or without a core of elastin, are important determinants of the mechanical properties of tissues, providing resilience, in contrast to the fibrillar collagens (typical of the liver scar) which provide rigidity. Fibrillin is expressed by both HSC and PF (36, 63); thus, the elastic fibers around HSC and PF are structurally different. Whether this is important to cell behavior, to the mechanical properties of livers with biliary versus non-biliary fibrosis and cirrhosis, or to matrix remodeling and fibrosis regression is an important question for future research.
PF may also participate in progenitor cell expansion and differentiation in the liver. In a dietary model of progenitor cell activation, myofibroblast activation and extracellular matrix deposition preceded progenitor cell expansion, and progenitor cells were surrounded by myofibroblasts and embedded in matrix proteins (64). New data on the identity of Thy-1 positive cells in the regenerating liver (previously believed to be oval cells) suggests that a subpopulation may actually be myofibroblasts closely apposed to oval cells, although they appear to be elastin negative (65, 66). Interestingly, in studies of the transcription factor FoxL1, bipotential progenitor cells were encircled by elastin-positive, α-SMA-negative cells, which may be PF (67). Thus, there is now suggestive evidence that PF and portal myofibroblasts play an important role in the liver progenitor cell niche.
Summary
The published literature now clearly demonstrates that PF and portal myofibroblasts are mediators of biliary fibrosis. Our knowledge of PF, however, lags far behind our knowledge of HSC. We suggest several areas for future research. First, it is essential to study the heterogeneity of the portal mesenchymal cell population. Evaluation and standardization of markers should be a priority. This will provide the additional benefit of addressing how well PF in culture mimic the population in vivo. Second, there needs to be a better understanding of the differences between HSC and PF as regards their relative contribution to fibrosis and their molecular regulation. This should have significant implications for the development of antifibrotic therapies tailored to distinct disease etiologies. Finally, since PF may be as multifunctional as HSC, it is critical that hepatology researchers explore functions of PF beyond fibrosis.
Acknowledgments
Financial support: This work was supported by NIH R01 DK58123 and the Fred and Suzanne Biesecker Pediatric Liver Center (R.G.W.) and by NIH R01 DK070849 and the Yale Liver Center (DK34989) (J.A.D.).
The abbreviations used are
- HSC
hepatic stellate cells
- PF
portal fibroblasts
- BDL
bile duct ligation
- α-SMA
α-smooth muscle actin
- BDE
bile duct epithelia
- NTPD2
nucleoside triphosphate diphosphohydrolase-2
- p75NTR
p75 neurotrophin receptor
- TGF-β
transforming growth factor-β
- PDGF
platelet-derived growth factor
- IL-6
interleukin-6
- MCP-1
monocyte chemotactic protein-1
References
- 1.Carruthers JS, Kalifat SR, Steiner JW. The ductular cell reaction of rat liver in extrahepatic cholestasis. II. The proliferation of connective tissue. Exp Mol Pathol. 1962;1:377–396. doi: 10.1016/0014-4800(62)90032-1. [DOI] [PubMed] [Google Scholar]
- 2.Steiner JW, Carruthers JS. Studies on the fine structure of proliferated bile ductules. II. Changes of the ductule-connective tissue envelope relationship. Can Med Assoc J. 1961;85:1275–1287. [PMC free article] [PubMed] [Google Scholar]
- 3.Popper H, Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970;49:707–721. doi: 10.1016/s0002-9343(70)80135-8. [DOI] [PubMed] [Google Scholar]
- 4.Schaffner F, Barka T, Popper H. Hepatic Mesenchymal Cell Reaction in Liver Disease. Exp Mol Pathol. 1963;31:419–441. doi: 10.1016/0014-4800(63)90020-0. [DOI] [PubMed] [Google Scholar]
- 5.Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22:141–147. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Knook DL, Seffelaar AM, de Leeuw AM. Fat-storing cells of the rat liver. Their isolation and purification. Exp Cell Res. 1982;139:468–471. doi: 10.1016/0014-4827(82)90283-x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 8.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 11.Knittel T, Kobold D, Piscaglia F, Saile B, Neubauer K, Mehde M, Timpl R, et al. Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochem Cell Biol. 1999;112:387–401. doi: 10.1007/s004180050421. [DOI] [PubMed] [Google Scholar]
- 12.Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 13.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–1159. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 14.Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 15.Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:d496–503. doi: 10.2741/A790. [DOI] [PubMed] [Google Scholar]
- 16.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 17.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 18.Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84:153–159. doi: 10.1038/labinvest.3700030. [DOI] [PubMed] [Google Scholar]
- 19.Cassiman D, Roskams T. Beauty is in the eye of the beholder: emerging concepts and pitfalls in hepatic stellate cell research. J Hepatol. 2002;37:527–535. doi: 10.1016/s0168-8278(02)00263-5. [DOI] [PubMed] [Google Scholar]
- 20.Uchio K, Tuchweber B, Manabe N, Gabbiani G, Rosenbaum J, Desmouliere A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619–628. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- 21.Kruglov EA, Jain D, Dranoff JA. Isolation of primary rat liver fibroblasts. J Investig Med. 2002;50:179–184. doi: 10.2310/6650.2002.33431. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 23.Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmouliere A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Villeneuve J, Pelluard-Nehme F, Combe C, Carles D, Chaponnier C, Ripoche J, Balabaud C, et al. Immunohistochemical study of the phenotypic change of the mesenchymal cells during portal tract maturation in normal and fibrous (ductal plate malformation) fetal liver. Comp Hepatol. 2009;8:5. doi: 10.1186/1476-5926-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libbrecht L, Cassiman D, Desmet V, Roskams T. The correlation between portal myofibroblasts and development of intrahepatic bile ducts and arterial branches in human liver. Liver. 2002;22:252–258. doi: 10.1046/j.0106-9543.2002.01674.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Tanaka M, Watanabe N, Saito S, Nonaka H, Miyajima A. p75 Neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology. 2008;135:270–281. doi: 10.1053/j.gastro.2008.03.075. e273. [DOI] [PubMed] [Google Scholar]
- 27.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr., Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–358. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 30.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 31.Mak KM, Lieber CS. Portal fibroblasts and myofibroblasts in baboons after long-term alcohol consumption. Arch Pathol Lab Med. 1986;110:513–516. [PubMed] [Google Scholar]
- 32.Saile B, Matthes N, Neubauer K, Eisenbach C, El-Armouche H, Dudas J, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells differ in CD95-mediated apoptosis and response to TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2002;283:G435–444. doi: 10.1152/ajpgi.00441.2001. [DOI] [PubMed] [Google Scholar]
- 33.Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 34.Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–221. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 35.Desmouliere A, Darby I, Costa AM, Raccurt M, Tuchweber B, Sommer P, Gabbiani G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765–778. [PubMed] [Google Scholar]
- 36.Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, Desmouliere A. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest. 2004;84:203–212. doi: 10.1038/labinvest.3700023. [DOI] [PubMed] [Google Scholar]
- 37.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 38.Uemura M, Swenson ES, Gaca MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol Biol Cell. 2005;16:4214–4224. doi: 10.1091/mbc.E05-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 40.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 41.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 42.Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, et al. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol. 2002;17:196–202. doi: 10.1046/j.1440-1746.2002.02672.x. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Shady M, Friess H, Zimmermann A, di Mola FF, Guo XZ, Baer HU, Buchler MW. Connective tissue growth factor in human liver cirrhosis. Liver. 2000;20:296–304. doi: 10.1034/j.1600-0676.2000.020004296.x. [DOI] [PubMed] [Google Scholar]
- 44.Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 48.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 49.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78:89–100. [PubMed] [Google Scholar]
- 52.Yu J, Lavoie EG, Sheung N, Tremblay JJ, Sevigny J, Dranoff JA. IL-6 downregulates transcription of NTPDase2 via specific promoter elements. Am J Physiol Gastrointest Liver Physiol. 2008;294:G748–756. doi: 10.1152/ajpgi.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sevigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- 54.Ramadori G, Saile B. Mesenchymal cells in the liver--one cell type or two? Liver. 2002;22:283–294. doi: 10.1034/j.1600-0676.2002.01726.x. [DOI] [PubMed] [Google Scholar]
- 55.Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D, Knowles AF, et al. Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Investig Med. 2004;52:475–482. doi: 10.1136/jim-52-07-42. [DOI] [PubMed] [Google Scholar]
- 56.Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 57.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152:423–430. [PMC free article] [PubMed] [Google Scholar]
- 59.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 60.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanta J, Dooley S, Delvoux B, Breuer S, D'Amico T, Gressner AM. Tropoelastin expression is up-regulated during activation of hepatic stellate cells and in the livers of CCl(4)-cirrhotic rats. Liver. 2002;22:220–227. doi: 10.1046/j.0106-9543.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 63.Dubuisson L, Lepreux S, Bioulac-Sage P, Balabaud C, Costa AM, Rosenbaum J, Desmouliere A. Expression and cellular localization of fibrillin-1 in normal and pathological human liver. J Hepatol. 2001;34:514–522. doi: 10.1016/s0168-8278(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 64.Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–1635. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]
- 65.Dezso K, Jelnes P, Laszlo V, Baghy K, Bodor C, Paku S, Tygstrup N, et al. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. Am J Pathol. 2007;171:1529–1537. doi: 10.2353/ajpath.2007.070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dudas J, Mansuroglu T, Batusic D, Ramadori G. Thy-1 is expressed in myofibroblasts but not found in hepatic stellate cells following liver injury. Histochem Cell Biol. 2009;131:115–127. doi: 10.1007/s00418-008-0503-y. [DOI] [PubMed] [Google Scholar]
- 67.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]