Abstract
Background
Eosinophilic inflammation is closely related to angiogenesis in asthmatic airway remodeling. In ovalbumin-sensitized mice, bone marrow-derived pro-angiogenic endothelial progenitor cells (EPCs) are rapidly recruited into the lungs after ovalbumin aerosol challenge, and promptly followed by mobilization and recruitment of eosinophils.
Objective
We hypothesized that bone marrow-derived EPCs initiate the recruitment of eosinophils through expression of eosinophil chemoattractant eotaxin-1.
Methods
EPCs were isolated from ovalbumin murine model of allergic airway inflammation and from asthma patients. Endothelial and smooth muscle cells were isolated from mice. Eotaxin-1 expression was analyzed by immunofluorescence, real-time PCR or by ELISA. In vivo recruitment of eosinophils by EPCs was analyzed in mice.
Results
Circulating EPCs of asthmatic individuals had higher levels of eotaxin-1 as compared to controls. In the murine model, ovalbumin allergen exposure augmented eotaxin-1 mRNA and protein levels in EPCs. The EPCs from ovalbumin-sensitized and challenged mice released high levels of eotaxin-1 upon contact with lung endothelial cells from sensitized and challenged mice, but not from control animals, and not upon contact with cardiac or hepatic endothelial cells from sensitized and challenged mice. Intranasal administration of the eotaxin-rich media overlying cultures of EPCs caused recruitment into lungs, confirming functional chemoattractant activity.
Conclusions
Bone marrow-derived EPCs are early responders to environmental allergen exposures, and initiate a parallel switch to a pro-angiogenic and pro-eosinophilic environment in the asthmatic lungs.
Keywords: eosinophils, allergy, airway inflammation, angiogenesis, bone marrow, eotaxin
Introduction
Increasing blood vessel numbers and density in the airway wall is a defining characteristic of asthma1-5. Studies suggest that the increased vascularity is related to pathology of inflammation in asthma6, 7. Over-expression of vascular endothelial growth factor (VEGF) in airways of mice leads to neovascularization, and to the development of inflammation and airway remodeling that strikingly mimics an asthma-like condition6. Although angiogenesis is strongly implicated in the mechanisms driving the allergic inflammatory lung process, how new vessel formation in allergic animals leads to infiltration of eosinophils remains unknown.
Bone marrow-derived endothelial progenitors (EPCs) in postnatal neovascularization are a relatively new concept in vascular biology8-14. Whether these cells differentiate into true endothelial cells is controversial, however animal tumor and hind leg ischemia models provide conclusive support that EPCs are essential for angiogenesis via paracrine mechanisms 8-14,15-18. These pro-angiogenic cells originate from hematopoietic stem cells and are subtyped by specific biologic assays and cell surface markers, such as the Colony Forming Unit – Endothelial Cell (CFU-EC) assay, Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2), Sca-1, C-kit and lectin positivity and low density lipoprotein uptake (Dil-AcLDL 8, 15, 16, 19.
Using the ovalbumin (OVA) mouse model of allergic airway inflammation, we recently reported that bone marrow-derived pro-angiogenic EPCs are initiators of an early angiogenic switch in asthmatic lungs7. EPCs progressively home to the lungs within hours after an initial allergen challenge. Blood vessel formation and influx of eosinophils follow the EPCs in a temporal pattern sequentially7. Eosinophils are considered the typical effector cells in asthma20-23. These inflammatory cells release abundant factors that mediate airway epithelial damage, airway remodeling and bronchoconstriction20-23. Eotaxins, a family of C-C chemokines including eotaxin-1, -2 and -3 in humans24-26 and eotaxin-1, -2 in mice,27, 28 are the primary eosinophil specific29 chemoattractants as shown by recent elegant studies in mice genetically deficient for eotaxin-1/2 22. Eotaxin-1 is found in lung structural cells including endothelial cells (EC), epithelial cells, airway and vascular smooth muscle cells (SMC), while eotaxin-2 is secreted by macrophages and T-cells30. Eotaxin-1 induces mobilization of eosinophils and their progenitors from the bone marrow into the blood circulation31, 32 and their adhesion to the endothelium33-35, while eotaxin-2 is mainly expressed by airway lumen macrophages and primarily directs the recruitment of eosinophils from the vasculature into the airway28. Studies with eotaxin-1 blocking antibodies36, 37 and strain-specific eotaxin-1 knockout mice38, 39 show reduction of eosinophil numbers in the lungs, but eotaxin-1 instillation into the airways of naïve mice fails to induce accumulation of eosinophils in the airways40, 32, 41, 42. Based on the rapid mobilization and recruitment of EPCs to the lung following allergen challenge in sensitized mice, and the temporal relationship of EPCs to angiogenesis and eosinophilic inflammation, we hypothesized that EPC may contribute to the genesis of lung eosinophilia via the expression and secretion of eotaxin-1, which is triggered upon contact of the EPCs with vascular cells in the allergen-sensitized and challenged lung.
Methods
Human Subjects
Non-smoking healthy persons and asthma patients were recruited for this study. Exclusion criteria for both groups included age younger than 18 years or older than 65 years, pregnancy, human immunodeficiency virus infection, and history of respiratory infection in the previous 6 weeks, prolonged exposure to second hand smoke at home or work, exposure to dusty environments or known pulmonary disease-producing agents. Additional exclusion criteria for healthy controls included history or symptoms of lung disease, or allergies. None of the volunteers with asthma had a recent asthma exacerbation, hospitalization, or change in medications for 6 weeks before the study. Asthma was defined based on the National Asthma Education Prevention Program Guidelines, including episodic respiratory symptoms, reversible airway obstruction by documentation of variability of forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC) by 12% and 200ml either spontaneously or after two puffs of inhaled Albuterol, and/or a positive methacholine challenge 43. For methacholine challenge testing, increasing concentrations of methacholine were delivered until FEV1 fell at least 20% when compared to a control level. The measure used to compare the sensitivity of one individual to another was PC20, the first provocative concentration that caused a 20% fall in FEV1. Studies on human subjects were approved by the Institutional Review Board of the Cleveland Clinic and written informed consent was obtained from all participating individuals.
Animals
Immunocompetent 6-8 week old female BALB/c mice from the Jackson Laboratory (Bar Harbor, ME) were used for OVA allergen sensitization and challenge44, 45. All mice were maintained in specific pathogen-free conditions using micro-isolator cages and were used in accordance with applicable regulations after institutional approval.
Ovalbumin sensitization
Typical induction of allergic airways disease, was performed as described earlier7, 44, 45. In short, BALB/c mice were immunized by intraperitoneal injection with ovalbumin (OVA) (Sigma Chemicals) (10 μg, adsorbed in Al(OH)3). Two weeks later, a series of daily inhalations (40 min per day) were started, with mice placed as a group in a chamber kept saturated with nebulized OVA solution (1% w/v in sterile PBS). Twenty-four hours after the first challenge or at indicated time points (day 3 or day 6) animals were anesthetized by intraperitoneal injection with pentobarbital. Blood was drawn in EDTA syringes by cardiac puncture and lungs were dissected. Lungs were gently squeezed through 40mm pore size nylon mesh in PBS. In some experiments whole lung digestion was performed to isolate lung vascular cells. Hind legs and vertebrae were also dissected and bone marrow cells were isolated in sterile PBS. Mononuclear cells were obtained from blood, lung and bone marrow by centrifugation on Lympholyte (Cedarlane, Ontario, Canada).
Isolation of mouse EPCs
EPCs with > 95% purity were isolated as initially described by others 46-49. Briefly, bone marrow mononuclear cells were seeded on fibronection (1mg/cm2 fibronectin, Sigma-Aldrich, Milwaukee, WI) coated 24-well plates at a concentration of 4 × 106 cells/0.5ml in 20% FBS and 1% penicillin/ streptomycin (complete EBM-2) supplemented with 20ng/ml VEGF (Invitrogen, Carlsbad, CA) added. Lung mononuclear cells were seeded at a density of 0.5 × 106 cells/0.5ml. Adherent cells were trypsinized between days 7 and 12 and characterized by analyzing for 1,1′ – dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil)-acetylated LDL (Dil-AcLDL) (Molecular probes, OR) uptake (15mg/ml) and by staining with Ulex lectin-FITC (Sigma MO) (2.5mg/ml) as described 7, 46-49. Phase-contrast and fluorescent images were captured by using a 40× objective on a Leica DM IRB inverted fluorescent microscope equipped with a Retiga SRC CCD camera and Q-Imaging software. EPC were also characterized by flow cytometric staining using anti-mouse CD45-FITC (eBioscience), CD11b-FITC (eBioscience), Sca-1-APC (eBioscience), C-kit (eBioscience), CD31-FITC (BD Bioscience, San Jose, CA) and VEGFR2-PE (BD Bioscience San Jose, CA). Isotype matched irrelevant antibodies were used as controls. Cytospins were made with a cell concentration of 40,000 cells in 200ul PBS-5% BSA using a Cytospin3 centrifuge (1200 RPM, 4 min) (Thermo Scientific Waltham. MA).
Isolation of human EPCs
Human EPCs with > 95% purity were obtained from the peripheral blood circulation using the Colony Forming Unit – Endothelial Cell (CFU-EC) assay 7, 50. Cells were trypsinized and cytospins were frozen for batch analysis.
Eotaxin immunofluorescence staining
Cytospins of EPC were completely air dried after spinning and stored at -20°C till batch staining. Frozen cytospins were brought to room temperature and then fixed in 95% ethanol. A one hour blocking step was used with 2% normal mouse serum or 2% FBS for human cells after which slides were incubated overnight at 4°C in a moist chamber with rabbit anti-mouse or goat anti-human antibodies against eotaxin-1 (Santa Cruz Biotechnology, Santa Cruz, CA), as appropriate. Specifity of both antibodies were verified by preabsorption with pure mouse or human eotaxin-1. For mouse cytospins biotin donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA) and streptavidin alexa Fluor 568 (Invitrogen) were added as secondary and tertiary reagents, respectively, and incubated for an hour at room temperature each. For human cells donkey anti-goat alexa fluor 594 (Invitrogen) was used as second step. Between each incubation slides were washed three times with PBS. After the final wash, slides were mounted with DAPI (Vectashield Laboratories, Burlingame, CA) and sealed. Pictures were taken using identical settings within each batch, at a final magnification of 400× with a Leica DMR fluorescence microscope equipped with a Retiga EX digital camera and by using QImaging software. Background substraction was performed by applying the black point function in an area with no cells using the Levels Tool in Adobe Photoshop 8.0 and was verified using slides stained with secondary (and tertiary, for mouse samples) reagent(s) only. In Image Pro, grayscale images were used to count EPC and to calculate the sum intensity. We used the count/size tool to manually select and set the threshold for positive labeling for analysis of all images. Average intensity of Eotaxin-1 expression per cell was calculated by determining total intensity for eotaxin-1 and dividing by the total number of cells per image. All selected objects were manually verified for inclusion.
Real-Time Quantitative PCR (qRT-PCR)
Total RNA was extracted from bone marrow-derived EPCs using a standardized TRIzol method of phenol extraction (Invitrogen, Carlsbad, CA). A260/A280 ratio was between 1.9 and 2.2 for all samples. Transcripts were analyzed by qRT-PCR in triplicate. Transcript-specific primers were generated based on mouse sequences from GenBank and were designed using Primer Express software (Applied Biosystems, Inc. ABI, Foster City, CA). NCBI BLAST was used to ensure specificity of each primer pairs for each transcript. Primer sequences are eotaxin-1 (CCL11) forward:ATTGTGTTGTTTGTTTGCTTGC and reverse:GTCAGCCTGGTCTACACAGTGA. Briefly, reverse transcription was carried out using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) to generate first strand cDNA. qRT-PCR was performed with SYBR green PCR Master mix (Applied Biosystems, Foster City, CA) and was carried out with an ABI 7300 sequence detection instrument (Applied Biosystems, Foster City, CA). Mouse glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal control and relative transcript abundance was normalized to the amount of GAPDH for the qRT-PCR data. Mean fold changes were calculated by averaging the triplicate measurements for each gene. The relative fold difference calculation uses the 2-ΔΔCT method51.
Isolation and culture of mouse lung EC and SMC
Lungs were perfused with 10ml sterile warm PBS via the heart to remove all blood cells. Lungs were then dissected, minced, and completely dissociated in digestion buffer (0.1% Collagenase A (Roche Applied Science, Indianapolis, IN), 0.04% DNA-se II (Sigma-Aldrich), 0.5mM CaCl2 (Sigma-Aldrich) in 1ml of Dispase II (Roche Applied Science) for 1.5 hours at 37°C. The cell suspension was filtered through a 40μm cell strainer (BD Biosciences) and number of cells was counted. Dead cells were removed by using MACS Dead Cell Removal Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufactures instructions and remaining viable cells were then labeled with biotin anti-mouse CD31 (eBioscience, San Diego, CA). MACS anti-biotin microbeads (Miltenyi Biotec) was used as secondary reagent and CD31+ cells were isolated using MS columns (Miltenyi Biotec). CD31- fraction was collected for SMC cultures while the positive fraction was run through a second column to increase the purity of the CD31+ EC. EC were seeded on rat tail collagen I coated plates (50μg/ml (BD Biosciences) in MCDB-131 complete medium (VEC Technologies, Rensselaer, NY). Purity was assessed by staining of the cells with anti-biotin-PE (Miltenyi Biotec) for flow cytometric analysis.
Eotaxin-1 ELISA
Single cultures of lung, heart or liver EC and SMC or co-cultures with bone marrow-derived EPCs were performed. A quantity of 0.5 ×106 EC or SMC were incubated on fibronectin coated 24-wells and after 12-hours 100 ×103 bone marrow-derived EPCs were seeded on top of the vascular cells in a final volume of 0.5ml MCDB-131 complete medium (VEC Technologies, Rensselaer, NY). Media overlaying the cells were conditioned for 5 days and eotaxin-1 concentration was quantified using mouse CCL11/Eotaxin DuoSet (R&D, Minneapolis, MN).
Instillation of EPC conditioned medium
Mice were mildly anesthetized by isoflurane inhalation and 50μl conditioned medium was instillated into the airways. Where indicated neutralizing eotaxin-1 or isotype matched control antibodies (75μg/ml) were added to the conditioned medium 30 min prior to instillation. After overnight incubation mice were euthanized by intravenous injection of overdose Pentobarbital, ETDA blood was drawn by cardiac puncture, lungs were dissected for whole organ digestion as described above. Eosinophils in whole blood were analyzed using Advia 120 Hematology Analyzer (Bayer, Pittsburgh, PA). To analyze recruitment of eosinophils into the lungs, red blood cells were lysed and samples were stained with anti-mouse CD45-FITC (eBioscience, San Diego, CA) and anti-mouse CCR3-PE (R&D) antibodies for flow cytometric analysis52. Isotype irrelevant antibodies were used as controls. Samples were analyzed on a FACScan (BD Biosciences) flow cytometer. Fifty thousand events were acquired and analyzed using CellQuest 3.3 soft- software (BD Biosciences).
Statistical analysis
Data were analyzed by using JMP 5.1 software program. ANOVA or Student's T-test were used for comparisons of parametric data, and Wilcoxon test was used for comparison of nonparametric data, as appropriate. p-values <0.05 were considered as significant. Mean ± SEM value for each group is shown.
Results
Expression of eotaxin-1 by bone marrow-derived EPCs
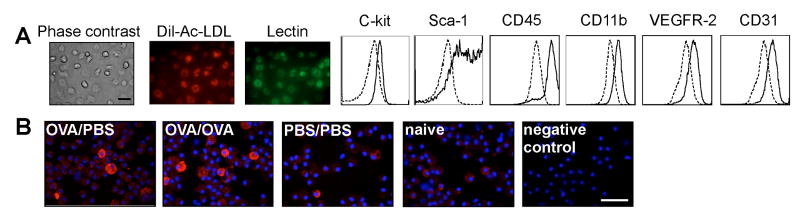
EPCs were obtained from mouse bone marrow and characterized by immunofluoresence and flow cytometry. The cells were positive for Dil-Ac-LDL uptake and lectin and expressed progenitor cell markers Sca-1 and C-kit and myeloid markers such as CD45 and CD11b were positive for EC markers VEGFR2, CD31 (see Figure 1A) as previously described7, 48, 53-55. Cytospins of EPCs isolated from different experimental conditions were stained for eotaxin-1. The majority of EPCs obtained from OVA sensitized/sham challenged (OVA/PBS) and OVA sensitized/OVA challenged (OVA/OVA) mice were positive for eotaxin-1 with a heterogeneous expression pattern (see Figure 1B). Some eotaxin-1 positive EPCs were found in naïve mice and in sham sensitized/sham challenged (PBS/PBS) or in sham sensitized/OVA challenged (PBS/OVA) mice. The intracellular eotaxin-1 content was higher in EPCs from OVA/PBS and OVA/OVA group compared to PBS/PBS or PBS/OVA (all p<0.04) [eotaxin-1 content (103)/cell: OVA/PBS 96 ± 14; OVA/OVA 122 ± 5; PBS/PBS 65 ± 5; PBS/OVA 69 ± 1, n=5 each, ANOVA p<0.002]. Quantitative real time PCR indicated that the gene expression of eotaxin-1 in EPCs was increased at mRNA level in OVA/PBS and OVA/OVA mice [eotaxin-1 mRNA EPC: [OVA/PBS: 1.25 ± 0.20; OVA/OVA 1.33 ± 0.27; PBS/PBS 1.02 ± 0.26; PBS/OVA 1.02 ± 0.38 ANOVA p<0.038, n=3 each]. Thus, OVA allergen exposure increases eotaxin-1 expression in bone marrow EPCs.
Figure 1.
Expression of eotaxin by bone marrow-Derived EPCs. A, EPCs were isolated from the bone marrow and characterized for the expression of endothelial, progenitor cell and myeloid markers. Dotted lines indicate autofluorescence and background staining. B, Eotaxin-1 expression by EPCs in different experimental conditions. Bar 200mm.
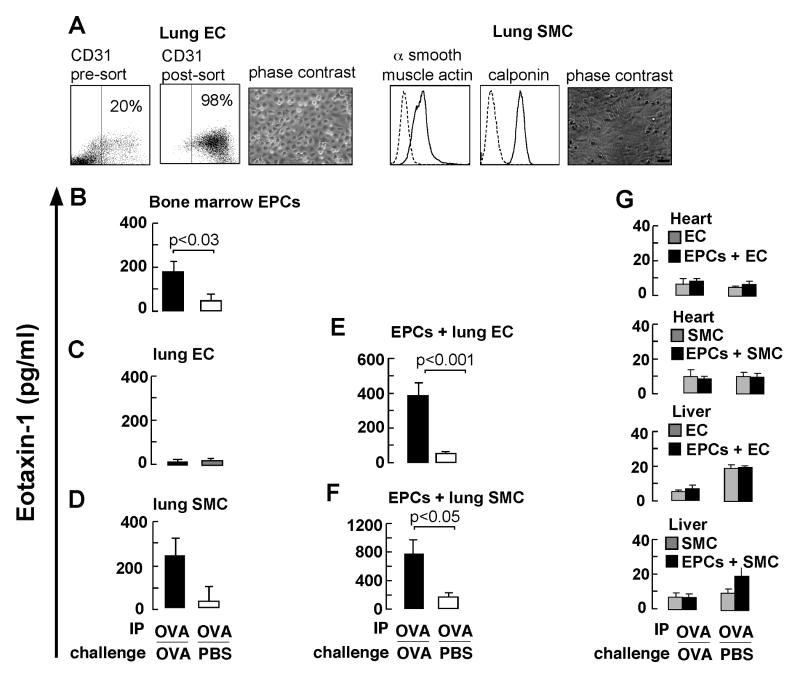
Secretion of eotaxin-1 by EPCs and in co-culture with lung EC and SMC
To determine whether EPCs secrete eotaxin-1 and whether release is affected by contact with vascular cells from the lungs, lung EC and SMC were harvested from mouse lungs under various conditions of OVA sensitization and challenge. Purity of cell cultures was > 95% (see Figure 2A). Co-cultures with EPC were performed to analyze the effect of lung EC and SMC on EPC eotaxin-1 secretion. EPC isolated from OVA/OVA or OVA/PBS mice released eotaxin-1 in media overlying cells (see Figure 2B). Lung EC had low levels of eotaxin-1 in overlying media (see Figure 2C), but this increased significantly in co-cultures of EPC and lung EC from the OVA/OVA group, but not in cells obtained from the control group (see Figure 2E). EC-derived eotaxin is stored in Weibel pallade bodies and is co-secreted with von Willebrand factor (vWF), also stored in these granules56. Increased levels of vWF in the co-cultures were not observed (not shown). This suggest EPCs as major source for eotaxin under these conditions. SMC cells from OVA/OVA animals released more eotaxin-1 as compared to SMC from the control group (see Figure 2D). The greatest levels of eotaxin-1 were detected in the co-cultures of EPCs and SMC obtained from the OVA/OVA group (see Figure 2F). In contrast to eotaxin, IL-4, IL-5 and IL-13, other important pro-eosinophilic cytokines in asthma30 were undetectable in media overlying EPCs or in co-cultures.
Figure 2.
Secretion of eotaxin-1 in co-cultures of EPCs and lung vascular cells. A, Isolation of EC and SMC from lungs. Dot plots of CD31+ cells before and after purification are shown. Histograms for α-SMC actin (solid line) and calponin (solid line) expression in SMC are shown. Dashed lines indicate staining with control antibodies. Phase contrast images of the EC and SMC are also shown. Eotaxin-1 secretion by B, EPCs, C, lung EC, C, lung SMC and E, co-cultures of EPCs with lung EC and F, co-cultures of EPCs with lung SMC, H, co-cultures of EPCs with heart and liver vascular cells. Data represent mean ± SE values of 6 mice in each group.
To analyze if the eotaxin release was lung specific, cells derived from heart and liver were evaluated in co-cultures with EPC. Eotaxin-1 release was low (<10pg/ml) in cultures of liver or heart EC and remained low in co-cultures with EPCs (see Figure 2G). Similar data were obtained in co-cultures of heart or liver SMC (see Figure 2G). Thus, EPCs from airway allergen challenged mice have increased eotaxin-1 secretion and this is further enhanced after contact with lung EC or SMC isolated from the allergen challenged lung, but not in the presence of heart or liver vascular cells.
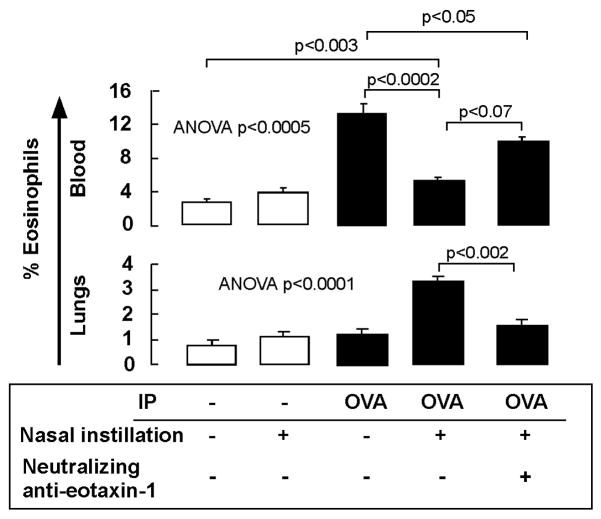
In vivo recruitment of eosinophils by EPC conditioned medium
To analyze the capacity of EPC conditioned media to recruit eosinophils into the lungs, mice were intranasally instilled with medium obtained from the EPC and lung EC co-cultures (that contained eotaxin-1 ∼ 400pg/ml). After 18 hours, mice were sacrificed and eosinophils in peripheral blood and lungs were quantified by staining for CCR322, 57, 58. Naïve mice had low levels of eosinophils in the blood circulation and lungs and these numbers did not increase after intranasal instillation of EPC conditioned medium (see Figure 3). Allergen sensitized mice had increased levels of circulating eosinophils, but normal levels in the lungs. However, after nasal instillation of the conditioned media OVA sensitized mice had three fold higher levels of eosinophils in the lungs, while the percentage of circulating eosinophils decreased (see Figure 3), indicating migration of eosinophils from the blood circulation into the lungs. Pre-incubation of the conditioned media with neutralizing anti-eotaxin-1 antibodies reduced lung recruitment of eosinophils by 40% (see Figure 3). Together these data indicate that eosinophil recruitment to lungs requires EPC secreted products but also alterations of resident vascular cells by allergen sensitization.
Figure 3.
Recruitment of eosinophils into the lungs by EPCs conditioned medium. EPC conditioned supernatants (eotaxin-1 concentration ∼ 400pg/ml) were intranasally administered into mice that had undergone sham (-) or OVA intraperitoneal (IP) sensitization. Eosinophils in the peripheral blood circulation and recruitment into lungs was analyzed by flow cytometry. Pre-incubation of the conditioned medium with neutralizing eotaxin-1 antibody was performed to block eotaxin-1. Isotype matched control antibodies had no significant effects (not shown). Data represent mean ± SE values from 3 mice in each group.
Eotaxin-1 expressing EPCs are increased in lungs during allergen challenge
In the next set of experiments we analyzed the presence of eotaxin-1 positive EPCs in the lungs during allergen challenge. A time course experiment was performed during which mice were challenged daily with OVA allergen or PBS for 6 days. At days 1, 3 and 6 mononuclear cells were isolated from the lungs for EPC isolation. There was progressive homing of EPCs into the lungs as reported previously7(not shown). Immunofluorescence quantification of EPC-derived eotaxin-1 indicated that the OVA/OVA EPCs expressed high levels of eotaxin-1 temporally related to OVA challenge, and which was greater than control mice (see Figure 4A and B). Together the data indicate that increased numbers of eotaxin-1 expressing EPCs home to the lungs during allergen challenge.
Figure 4.
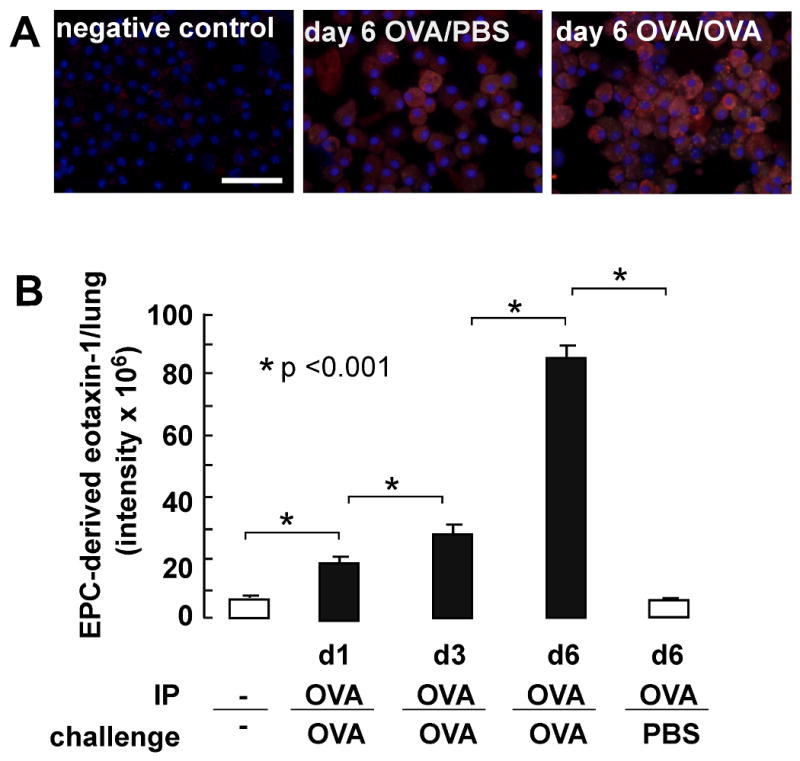
Eotaxin-1 positive EPCs in allergen exposed lungs. A, Mononuclear cells were isolated from lungs and evaluated for eotaxin-1 expression. Representative for 3 mice in each group are shown. B, Eotaxin-1 content per positive cells quantified using Image Pro software.
Expression of eotaxin-1 by human EPCs
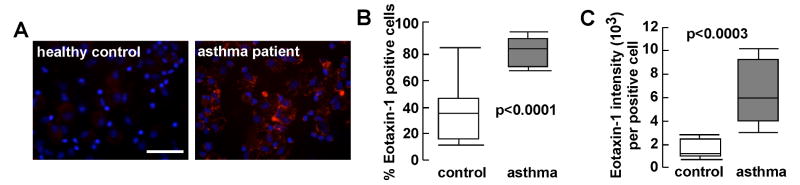
The data from the murine OVA model prompted us to analyze if there is increased eotaxin-1 expression by human asthmatic EPC. We recently reported increased numbers of circulating bone-marrow-derived EPC in patients with asthma 7. The study population consisted of 16 subjects, including 9 asthma patients (6 females, 36 ± 4 y, 5 African Americans, 4 Caucasians, FEV1% 75 ± 6, FEV1/FVC 0.69 ± 0.02) and 7 healthy controls (4 females, 34 ± 4 y, 4 African Americans, 2 Asians, 1 Caucasian, FEV1% 91 ± 5, FEV1/FVC 0.79 ± 0.03). Cytospins from healthy control and asthmatic individuals were immunostained for eotaxin-1 (see Figure 5A). Asthma-derived CFU-ECs had significantly higher numbers of cells positive for eotaxin [% eotaxin-1+ cells: control 37.6 ± 7.4; asthma 83.1 ± 3.5, p<0.0001 mean ± SE values] (see Figure 5B). Moreover, quantification of the eotaxin content among the positive cells indicated ∼ 4 fold higher levels in asthma-derived CFU-EC (see Figure 5C) [eotaxin-1 intensity (103)/cell): control 1.5 ± 0.29; asthma 6.2 ± 0.90, p<0.0003 mean ± SE values].
Figure 5.
Expression of eotaxin-1 by circulating human EPCs. A, Eotaxin-1 expression analyzed by immunofluorescence staining. Bar is 200mm. B, Percentage of eotaxin-1 positive cells EPCs. C, Eotaxin-1 content per positive cells was quantified using Image Pro software. Median values, upper and lower quartiles are shown.
Discussion
Neovascularization in asthmatic airways has been reported for decades, but its role in the genesis of the eosinophilic inflammatory disease remains unknown. Here, we show that pro-angiogenic bone marrow-derived EPCs in the OVA/OVA mouse model of allergic airway inflammation express high levels of eotaxin-1, an important eosinophil chemoattractant. In analogy to the mouse model, EPCs from asthmatic patients also exhibit high levels of eotaxin-1 expression. The EPCs isolated from allergen sensitized and challenged mice release eotaxin-1 upon contact with lung EC and SMC. Thus, the findings provide mechanistic insight into how the angiogenic process may be coupled to the generation of eosinophilia via eotaxin-1 expressing pro-angiogenic cells during their interaction with lung vascular cells.
Almost half a century ago Dunnill2 was the first to report that submucosal blood vessels in asthmatic airways are increased in both number and in size. Since then several groups, including ours, have confirmed these findings 1, 3-5, 7, 59. The number of blood vessels is known to be related to severity of asthma4, 5, 59, but a pathogenic link between angiogenesis and asthmatic allergic inflammation have been lacking. As in most angiogenic processes, pro-angiogenic EPCs are an intrinsic component of the neovascularization in asthma. In addition, they strongly correlate with recruitment of eosinophils7, main effector cells in this Th2-driven airway disease20-22. Eotaxin-1 and eotaxin-2 have critical and specific roles in the recruitment of eosinophils in asthma. Expressed by EC, SMC, epithelial cells28, 30, and here shown to be expressed by EPCs, eotaxin-1 is important in the genesis of eosinophilia by recruitment of eosinophils from the circulation28. Previous study shows that eotaxin-1 instillation into naïve C57Bl/6J mice fails to induce accumulation of eosinophils in the airways 40, while administration via endotracheal tube in BALB/c mice leads to recruitment of eosinophils60. In this study lung recruitment of eosinophils was not observed upon the nasal instillation of EPC-conditioned media containing eotaxin-1 in naïve BALB/c mice. These different findings may reflect differences in strain specific eosinophil mobilization and/or methods of eotaxin delivery. Thus, although eotaxin-1 is clearly important in the initial stages of lung eosinophilia32, 41, 42, allergen sensitization of the animal was required for the eotaxin-1 in the EPCs conditioned media to result in the influx of eosinophils into the lungs. Allergen sensitization also induced increased eotaxin expression in the EPCs. This suggests that allergic sensitization acts via angiogenic pathways inducing eosinophilic inflammation, specifically. Collectively, the data suggest a cellular mechanism in which ‘allergen-sensitized’ EPCs promote eosinophilic lung inflammation. Upon allergen sensitization, bone marrow EPCs increase eotaxin-1 expression. Within hours after airway allergen challenge, the eotaxin-rich EPCs are mobilized and blood borne7. The EPCs release eotaxin-1 upon contact with the lung allergen-sensitized EC and SMC, however the exact mechanisms remain to be elucidated. The highest level secretion occurs upon contact with SMC as the EPCs migrate into the vascular media, which creates a gradient of eotaxin that can draw the circulating mobilized eosinophils inward to the lung. Once within the lung tissues, the eosinophils would follow the eotaxin-1 and -2 gradients that are produced by other lung cells, including the airway smooth muscle and epithelial cells and alveolar macrophages. Thus, this study reveals a previously unrecognized and unsuspected role for the pro-angiogenic bone marrow progenitor as an early envoy for allergen-induced (asthmatic) eosinophilic lung inflammation, and helps to explain the coordinate relationship of angiogenesis and eosinophil influx during an allergen induced inflammatory response.
Acknowledgments
We thank L. Vargo, Dr. J. Drazba, Dr. A. J. Peterson in the Lerner Research Institute Digital Imaging Core, C. Shemo and S. O'Bryant in the Lerner Research Institute Flow Cytometry Core and S. Maximuk from the Department of Cell Biology for technical advise and excellent technical assistance. M. Baaklini and M. Cleggett-Mattox, for patient recruitment and Dr. S.A. Comhair for clinical sample database management. We also thank Dr. K. Litwak and his team from our Biological Resources Unit for excellent animal care.
Grant support: This study was supported by grants from the National Institutes of Health HL60917, HL69170, AI70649, HL081064, AI067816, HL04449 and UL1 RR024989 from the National Center for Research Resources
Abbreviations used
- CFU-EC
Colony Forming Unit – Endothelial Cell
- Dil-AcLDL
1,1′ –dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil)-Acetylated Low-Density Lipoprotein
- EC
endothelial cells
- EPCs
endothelial progenitor cells
- FEV1
Forced Expiratory Volume in 1 Second
- FVC
Forced Vital Capacity
- GAPDH
glyceraldehyde phosphate dehydrogenase
- OVA
Ovalbumin
- SMC
smooth muscle cells
- VEGF
Vascular Endothelial Growth Factor
- VEGFR2
Vascular Endothelial Growth Factor Receptor-2
- vWF
von Willebrand Factor
Footnotes
Key Message:
Bone marrow-derived pro-angiogenic progenitors home to the lungs during allergen challenge and release eotaxin-1 upon contact with lung vascular cells, which contributes to recruitment of eosinophils.
Capsule Summary:
Angiogenesis is a consistent process in asthmatic airway remodeling. Bone marrow-derived pro-angiogenic progenitors promote neovascularization and initiate eosinophilic inflammation through the expression of eotaxin, and thus participate in the genesis of asthma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148:1220–5. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 2.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–33. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 4.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–6. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrugt B, Wilson S, Bron A, Holgate ST, Djukanovic R, Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J. 2000;15:1014–21. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–94. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 8.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–63. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerbel RS, Benezra R, Lyden DC, Hattori K, Heissig B, Nolan DJ, et al. Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci U S A. 2008;105:E54. doi: 10.1073/pnas.0804876105. author reply E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, et al. Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest. 2008;118:2062–75. doi: 10.1172/JCI32832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116:3266–76. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–15. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 15.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 16.Pearson JD. Endothelial progenitor cells - hype or hope? J Thromb Haemost. 2009;7:255–62. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 17.Shantsila E, Watson T, Tse HF, Lip GY. New insights on endothelial progenitor cell subpopulations and their angiogenic properties. J Am Coll Cardiol. 2008;51:669–71. doi: 10.1016/j.jacc.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 18.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–8. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep. 2007;7:18–26. doi: 10.1007/s11882-007-0026-y. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 22.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–23. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humbles AA, Conroy DM, Marleau S, Rankin SM, Palframan RT, Proudfoot AE, et al. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo. J Exp Med. 1997;186:601–12. doi: 10.1084/jem.186.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 25.Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothenberg ME. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol. 1999;21:291–5. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 28.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–61. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–56. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 30.Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533:277–88. doi: 10.1016/j.ejphar.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 31.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 32.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–71. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenscher K, Metzner B, Schopf E, Norgauer J, Czech W. Recombinant human eotaxin induces oxygen radical production, Ca(2+)-mobilization, actin reorganization, and CD11b upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood. 1996;88:3195–9. [PubMed] [Google Scholar]
- 35.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–53. [PubMed] [Google Scholar]
- 37.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–90. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–23. [PubMed] [Google Scholar]
- 40.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 41.Brown JR, Kleimberg J, Marini M, Sun G, Bellini A, Mattoli S. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–46. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–9. [PubMed] [Google Scholar]
- 43.Asthma GftDatMo. Expert Panel Report II. Bethesda: National Institutes of Health; 1997. pp. 97–4051. [Google Scholar]
- 44.Aronica MA, McCarthy S, Swaidani S, Mitchell D, Goral M, Sheller JR, et al. Recall helper T cell response: T helper 1 cell-resistant allergic susceptibility without biasing uncommitted CD4 T cells. Am J Respir Crit Care Med. 2004;169:587–95. doi: 10.1164/rccm.200301-100OC. [DOI] [PubMed] [Google Scholar]
- 45.Aronica MA, Swaidani S, Zhang YH, Mitchell D, Mora AL, McCarthy S, et al. Susceptibility to allergic lung disease regulated by recall responses of dual-receptor memory T cells. J Allergy Clin Immunol. 2004;114:1441–8. doi: 10.1016/j.jaci.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 47.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 48.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossig L, Urbich C, Bruhl T, Dernbach E, Heeschen C, Chavakis E, et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med. 2005;201:1825–35. doi: 10.1084/jem.20042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 52.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–6. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 53.Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, et al. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells. 2008;26:1017–26. doi: 10.1634/stemcells.2007-0562. [DOI] [PubMed] [Google Scholar]
- 54.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 55.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 56.Oynebraten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–20. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- 57.Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–78. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 58.Grimaldi JC, Yu NX, Grunig G, Seymour BW, Cottrez F, Robinson DS, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–53. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]
- 59.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116:477–86. doi: 10.1016/j.jaci.2005.07.011. quiz 87. [DOI] [PubMed] [Google Scholar]
- 60.Ganzalo JA, Jia GQ, Aguirre V, Friend D, Coyle AJ, Jenkins NA, et al. Mouse Eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–14. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]