Abstract
Wild berries are integral dietary components for Alaska Native tribes and a rich source of polyphenolic metabolites that can ameliorate metabolic disorders such as obesity and diabetes. In this study, five species of wild Alaskan berries (Vaccinium ovalifolium, V. uliginosum, Rubus chamaemorus, R. spectabilis, and Empetrum nigrum) were screened for bioactivity through a community-participatory research method involving three geographically-distinct tribal communities. Compositional analysis by HPLC and LC-MS2 revealed substantial site-specific variation in anthocyanins (0.01-4.39 mg/g-FW) and proanthocyanidins (0.74-6.25 mg/g-FW), and identified A-type proanthocyanidin polymers. R. spectabilis increased expression levels of preadipocyte-factor-1 (182%), and proanthocyanidin-enriched fractions from other species reduced lipid accumulation in 3T3-L1 adipocytes. Selected extracts reduced serum glucose levels in C57bl/6j mice by up to 45%. Local observations provided robust insights into effects of climatic fluctuations on berry abundance and quality, and preliminary site-specific compositional and bioactivity differences were noted, suggesting the need to monitor this Alaska Native resource as climate shifts impact the region.
Keywords: Anthocyanins, proanthocyanidins, Vaccinium ovalifolium, Vaccinium uliginosum, Rubus spectabilis, Rubus chamaemorus, Empetrum nigrum, Traditional Ecological Knowledge, pref-1, adipocytes, diabetes, obesity, metabolic syndrome
Introduction
Type 2 diabetes mellitus (T2DM) incidence rates in the United States have increased nearly 200% over the past two decades, rising from 5.6 million cases in 1980 to 15.8 million in 2005 (1). This has coincided with a surge in overweight and obese individuals; over 2/3 of the population of the United States is classified as overweight, with 32% of the population diagnosed as obese (2). American Indian/Alaska Native (AI/AN) populations suffer disproportionately high rates of T2DM and obesity and are twice as likely to have T2DM as non-Hispanic whites (1). This ethnic group has the highest obesity rates in all age classes (2, 3), which is in part attributed to a shift from a traditional to a more Western lifestyle, including higher calorie and fat diets (4) and lowered physical activity (5, 6). Alaska Natives are particularly at risk, experiencing increased glucose intolerance, T2DM, and obesity across all age groups (7-9).
Northern America sustains a wide range of berries that are integral parts of the traditional ecological knowledge (TEK) of indigenous Arctic tribes. Salmonberries (Rubus spectabilis and R. chamaemorus), in the same genus as raspberry, have been used for such diverse health remedies as wound healing and gynecological aids (10), and both species are important to tribal populations as a foodstuff (10, 11). The Tanaina, a tribal group near Anchorage, Alaska, have used the leaves and stems of Empetrum nigrum (alternatively known as crowberry, blackberry, or mossberry in various regions of the Arctic) to treat diarrhea, and the plant is noted for countering kidney trouble (12). Highbush and bog blueberries (Vaccinium ovalifolium and V. uliginosum) are also integral dietary resources, and are used both topically and orally as medicines (13). In addition to eating the raw berries, the Inupiat tribe - an Inuit tribe indigenous to Alaska's Northwest arctic - also ferment them to prepare a vinegar for cooking, and make berry-based relishes (14). Northern and Aleutian Alaska Natives also use the berries as part of agutuk, a traditional treat made with fish or seal oil (15).
Although scant phytochemical analyses have been documented for circumpolar berries such as those found in Alaska, significant circumstantial evidence suggests that they may be protective against diabetes-related health complications. Related berry species have demonstrated substantial bioactivity countering a wide variety of human diseases and conditions. Berries contain complex phytochemical mixtures including anthocyanins and proanthocyanidins, powerful antioxidants capable of neutralizing free radicals responsible for an array of cardiovascular diseases including atherosclerosis and ischemic stroke (16, 17), DNA damage (18), and neurodegeneration (19). Bilberry (Vaccinium myrtillus) extracts have been shown to improve night vision and decrease myopia symptoms (20). Berry species, including blueberry, strawberry, raspberry, and cranberry, inhibit multiple stages of carcinogenesis and stimulate apoptosis of carcinogenic cells (21, 22). The A-type proanthocyanidins found in blueberries and cranberries have antiadhesion and antiproliferation properties that are effective in preventing bacterial infections, especially in the urinary tract (23-25).
In addition to the wide breadth of diverse health-related benefits noted above, ingestion of the significant amounts of flavonoids contained in many berries may ameliorate complications associated with T2DM (26). Diet is increasingly recognized as an important avenue to preventing the onset of T2DM (27). Members of the genus Vaccinium and Rubus have demonstrated hypoglycemic and antidiabetic activity (28, 29), and feature in traditional anti-diabetic medicine; the Cree First Nation in Quebec, Canada used several species of fruit, including Vaccinium sp., to treat symptoms of diabetes (30). Blueberry fruits and leaves were prepared as decoctions for teas used to treat diabetes in European medicine (31). Strawberry, blueberry, and raspberry extracts decrease the activity of glucosidase enzymes such as α-amylase (32), showing potential for lowering postprandial hyperglycemic episodes. Berries also stimulate glucose uptake in various cell models, including C2C12 myotubes and 3T3-L1 adipocytes (28, 33).
In addition, the rich proanthocyanidin and anthocyanin complement in many berries is purported to offset the tendency towards obesity. Glycosylated anthocyanins actively regulate genetic markers associated with obesity (34). In several studies by Tsuda et al., anthocyanins prevented the onset of obesity in rats maintained on a high-fat diet, affecting adipocyte gene-expression level and adipocytokine secretion (26, 35). Proanthocyanidins have been shown to regulate adipocyte function and obesity. Procyanidin-rich fractions of apples inhibited pancreatic lipase activity in in vitro models (36, 37). Proanthocyanidins are also effective in lowering plasma triglyceride levels and cholesterol accumulation in a variety of in vivo studies (37-39).
Environmental stresses significantly modulate the phytochemical profiles of plants. Biotic and abiotic stresses present in the wild are endured by plants through defensive chemical adaptations, fostering a potentially more complex phytochemical composition in the wild fruit than in cultivated varieties (40). Wild berry fruits typically accumulate more concentrated phenolic compounds (including tannins) than their cultivated relatives (41, 42), as the latter are more buffered from environmental insults due to commercial agriculture inputs, and have modified composition due to selection and breeding. A comparative study between blackberries from the Pacific Northwest and similar genotypes from Mexico demonstrated that the cooler, wetter climate of the Pacific Northwest fostered berries with higher antioxidant bioactivity, which was correlated with their polyphenol content (43). Controlled experiments mimicking extreme cold or drought conditions revealed that some species of berry can alter their biosynthesis of photosynthetic and adaptive secondary biochemicals in response to stress (44, 45).
The Alaskan environment is one of the most stressful climates in North America. The Alaskan landscape is characterized by a short growing season, long photoperiods during the summer (approaching 24 hours above the Arctic circle), extreme shifts in temperature (as low as −50 °C in the winter and up to 27 °C in summer months), and the presence of a permafrost soil structure (46). These combinations of climate and geochemistry extremes significantly stress indigenous plants, which prompts adaptive biochemical responses.
Alaska's unique location has also made it especially environmentally vulnerable as worldwide evidence for global climate change has become incontrovertible. The Arctic has experienced a rapid increase in temperature over the last three decades (47), with a sustained temperature increase averaging 0.47 °C per decade over the last 80 years (48). In addition to shifting the weather patterns of the region (49), the warmer temperatures translate into a visual increase in shrub-like plant distribution along the tundra (50). The growing season has lengthened by 2.6 days per decade and the leaf onset date has shifted 1.1 days earlier per decade, yielding as much as 20% higher primary productivity for some species of vascular plants (48). Controlled experiments have demonstrated a marked change in vegetative composition in the ecosystem; increased densities of vascular plants, especially shrubs, and diminishing populations of other plants such as lichen and moss (51). By some measures, berry species may actually be favored by climatic shifts, as wider distribution ranges and longer growing seasons may be enabled. On the other hand, climate shifts could allow previously unadapted plant species to encroach on the habitat of berries, competing for scarce resources and disadvantaging the berry populations. In addition, climate changes may attenuate the characteristic environmental stresses that trigger adaptive production of biologically-active phytochemicals in berries. In this case, plant proliferation and survival may be enhanced, but secondary product accumulation could recede. Quantitative projections of climate change-induced impacts on berries would mandate extended term data analysis, however, traditional knowledge and local observations provide robust insights into climatic factors affecting berry abundance and quality.
The objectives of this study were to characterize the phytochemical composition, especially the anthocyanin and proanthocyanidin constituents, of wild Alaskan berries from differing climatic regimes, and to evaluate their potential efficacy against symptoms of T2DM and obesity via in vitro bioassays (adipogenesis and lipid accumulation in 3T3-L1 cells) (52) and in an in vivo hyperglycemic rodent model, and to integrate the bioscience discoveries with TEK, local climate change observations, and community health concerns. The present study uses a combination of participatory field research and laboratory investigations to identify and biochemically and functionally characterize wild Alaskan berries chosen from 3 distinct geographic sites.
Materials and Methods
Field Work
Research Sites
Three village community sites were selected to represent significant biogeographic and climatic variation in Alaska: Akutan Island (AK) (54.1373 °N, 165.7728 °W), Seldovia (SD) (59.4112 °N, 151.6866 °W), and Point Hope (PH) (68.1737 °N, 166.9940 °W). The population in Seldovia is approximately 30% Alaska Native (AN), whereas Point Hope and Akutan villages feature 90% and 95% AN members, respectively. Annual average precipitation ranges around 28″, 34″, and 10″ for Akutan, Seldovia, and Point Hope, respectively. Akutan has the narrowest yearly range of temperatures (−5.5 to 12.8 °C), whereas Point Hope demonstrates the greatest variation (−45 to 25.6 °C) (53). All three villages are located in coastal regions, and berry collection sites range from 3-80 m in elevation. Akutan and Point Hope are characterized by mesic graminoid herbaceous and dwarf shrub communities, while Seldovia consists of spruce-dominated coniferous forests (54).
Community Participatory Research
This study involved NA/AN people in the biodiscovery process, in a community-participatory framework that investigated and validated traditional ecological knowledge, and conducted field surveys of bioactive constituents of local medicinal plants. The research team engaged local community members to implement a set of field bioassays known as “Screens-to-Nature” (STN), an innovation developed through the Global Institute for BioExploration (www.gibex.org). The STN are field-deployable bioassays that use visual chemical indicators to reveal preliminary data on the bioactivity of plant extracts. For this study, the antioxidant potential and α-amylase inhibition assays (two potential mechanisms of antidiabetic plants (55)) were chosen to investigate the bioactivity of local wild berries, along with protease activity and inhibition. Community members were trained by the research team in the process of data collection and analysis of bioassay readings. Assays were carried out according to defined protocols described in GIBEX field manuals, provided to each community.
Based on the preliminary STN analyses, as well as recommendations from community elders, five berry species were collected for in-depth analyses: Vaccinium ovalifolium, V. uliginosum, Empetrum nigrum, Rubus spectabilis, and R. chamaemorus. At least 500 g of each berry fruit at each site was harvested to ensure sufficient sample for analysis. Due to climatic and other site-specific considerations, only E. nigrum was available and collected at all three test sites. V. ovalifolium and R. spectabilis were harvested in both Seldovia and Akutan, V. uliginosum from both Akutan and Point Hope, and R. chamaemorus only from Point Hope. Upon collection, berry fruit samples were frozen and transported on dry ice in insulated shipping containers to the University of Illinois, where samples were immediately placed in a −20 °C freezer until analysis.
Interviews with community members and surveys were conducted by local students as well as a research team investigator, to gather information regarding local berry use, community and environmental well-being, and observations of climatic influence. The interviews were conducted over a two-year period for a total of 11 interviews in Akutan, 24 in Point Hope, and 30 in Seldovia. Interviews were recorded, transcribed, and analyzed thematically. Formal interviews were supplemented by numerous conversations with community members over the course of the project.
Phytochemical Analysis
Reagents
Except where noted, all chemicals were purchased from Fisher Scientific (Pittsburgh, PA) and were of reagent grade. All solvents for High Performance Liquid Chromatography (HPLC) were HPLC grade.
Extraction
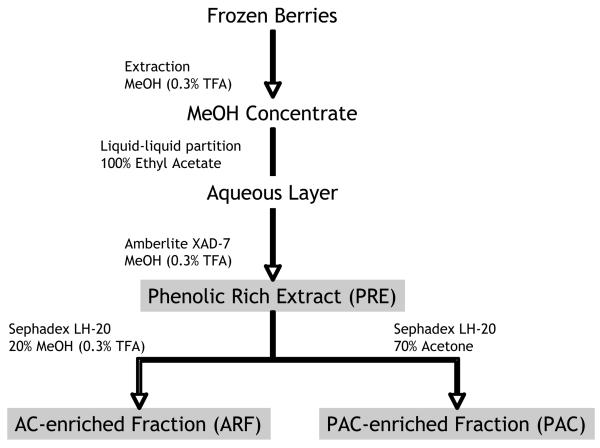
Procedure for frozen berry extraction was modified from Grace et al. (2009) (56). In brief, berries were homogenized with acidified methanol (99.7% methanol (MeOH): 0.3% trifluoroacetic acid (TFA) in a 2:1 v/w ratio). Homogenate was filtered and alcohol removed via rotary evaporation at temperatures ≥40 °C. Resulting aqueous suspension was partitioned with ethyl acetate (3 × 500 mL) to remove lipophilic constituents. The aqueous layer was retained, loaded on 1 L Amberlite XAD-7 resin (Sigma-Aldrich, St. Louis, MO) and polyphenols eluted with 2 L acidified methanol until all the colored residue was extracted from the column. Methanol was removed via reduced pressure, frozen, and lyophilized to yield the phenolic-rich extract (PRE). Fractions were prepared by placing 1.0 g PRE on a Sephadex LH-20 column (25 × 3 cm) (GE Life Sciences, Buckinghamshire, UK). Anthocyanins were obtained from an isocratic elution of acidified aqueous methanol (80% H2O: 20% methanol: 0.3% TFA), after which proanthocyanidins were eluted with 70% aqueous acetone. All fractions were individually concentrated and lyophilized to give the anthocyanin-enriched (ARF) or proanthocyanidin-enriched (PAC) fractions (Figure 1).
Figure 1.
Flow chart for frozen berry extraction. Abbreviations: PRE = phenolic-rich extract (obtained after Amberlite column); ARF = anthocyanin-rich fraction (obtained after 20% methanol elution from Sephadex column); PAC = proanthocyanidin-rich fraction (obtained after 70% acetone elution from Sephadex column). Adapted from Grace et al. (2009)
Phenolic Characterization
Anthocyanin separation was conducted on an 1100 HPLC (Agilent Technologies, Santa Clara, CA) using a reverse-phase Supelcosil-LC-18 column (250 mm × 4.6 mm × 5 μm) (Supelco, Bellefonte, PA). Samples were dissolved at a concentration of 5 mg/mL in 100% methanol and filtered through 0.45 μm nylon filters (Fisher Scientific, Pittsburg, PA). The mobile phase consisted of 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was held constant at 1 ml/min with a step-wise gradient of 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively. The same instrumentation set-up was used to separate proanthocyanidins. Mobile phases consisted of 94.9% H2O: 5% acetonitrile: 0.1% formic acid (A) and 94.9% acetonitrile: 5% H2O: 0.1% formic acid (B). The flow rate was held at 1 mL/min with a step gradient of 0%, 5%, 30%, 60%, 90%, 0%, and 0% of solvent B at 0, 3, 40, 45, 50, 55, and 60 min, respectively. Agilent's Chemstation software was used for both protocol control and data processing.
Proanthocyanidin LC-MS2 analyses were conducted on a Waters Alliance 2795 (Waters Corporation, Beverly, MA) with a reverse-phase C-18 column (150mm × 2.1mm i.d., particle size 5 μm, 90 Å) (Western Analytical, Murrieta, CA). The analyses were carried out at a constant flow rate of 200 μl/min using mobile phases consisting of 94.9% H2O: 5% acetonitrile: 0.1% formic acid (A), and 94.9% acetonitrile: 5% H2O: 0.1% formic acid (B), with a step-wise gradient of 5%, 30%, 60%, 90%, 90%, 5% and 5% of solvent B at 0, 40, 45, 50, 55, 60, and 70 min, respectively. All samples were dissolved in 100% MeOH at a concentration of 1 mg/mL and filtered through 0.45 μm nylon before injection at a volume of 10 μL. The column was connected to a Q-TOF Ultima mass spectrometer (Waters Corporation). An ESI source working in the positive ion mode was used for all MS analyses. The mass spectrometer was tuned for maximum intensity of the parent ions of interest. Acquisition of LC-PDA-MS data was performed under Mass Lynx 4.0 (Waters Corporation) and data processing was achieved using the XCalibur Qual Browser v. 1.4 software (Thermo Electron Corporation, Waltham, MA).
In vitro Assays
Cell Culture and Treatments
3T3-L1 fibroblasts from Swiss albino mice (ATCC CCL-92.1) (American Type Culture Collection, Rockville, MD) were seeded at a concentration of 6 × 104 cells/well in 6-well plates and 5 × 103 cells/well in 96-well plates. All cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 100 units/mL penicillin-streptomycin, 10 mM sodium pyruvate, and 10% calf serum (CS) (DMEM/CS medium) (Sigma-Aldrich). Adipocytes were continuously incubated at 37 °C in a 5% CO2 atmosphere during culturing and treatment.
Mature and immature adipocytes were treated with the phenolic-rich extract (PRE), as well as the two post-Sephadex fractions (anthocyanin-rich (ARF) and proanthocyanidin-rich (PAC)). Samples were dissolved in dd-H2O and applied to cells at a final concentration of 200 μg/mL.
Cytotoxicity Assay
All extracts and fractions were assayed for any increase in cell mortality before pref-1 or lipid quantification assays. CellTiter 96AQueous One Solution was used to determine the amount of viable cells (Promega, Madison, WI). Briefly, 20 μL of CellTiter 96AQueous One Solution was charged to each well containing 100 μL of DMEM without serum, and the plates were incubated at 37 °C and 5% CO2 atmosphere for two hours. The absorbance was measured on a 96-well plate reader (Biotek Instruments, Winooksi, VT) at 515 nm and compared against a vehicle-treated control. No samples significantly decreased cell viability (defined as cell counts >80% of control).
Preadipocyte pref-1 Analysis
Preadipocyte cells were seeded at a concentration of 1 × 105 cells/well in 6-well plates and cultured with DMEM/CS medium. After 24 hours cells were treated with berry extracts enumerated above. Cells were washed with media and Dulbecco's Phosphate Buffer Saline (DPBS) and then lysed with 200 μL Laemmeli buffer (Bio-Rad Laboratories, Hercules, CA). Proteins separated via electrophoresis on 4-20% Tris-HCl ready gels (Biorad Laboratories) with Precision Plus Dual Color Protein Standard (Bio-Rad) as a control between 10 and 250 kDa. Gels were run on a PowerPac 300 (Bio-Rad) at 200 V for 30 min.
The separated proteins were transferred to polyvinylidene difluoride (PVDF) (Millipore, Billerica, MA) membranes and blocked with 5% non-fat dry milk (NFDM) (Nestle S.A., Vevey, Switzerland) in 0.1% Tris-buffered saline Tween 20 (TBST) for 1 h at 4 °C. After blocking, membranes were incubated overnight with 10 mL anti-pref-1 goat polyclonal IgG antibody (1:2000 in TBST with 1% NFDM) (Applied Biosystems, Foster City, CA) at 4 °C. The membranes were washed with TBST, 5 × 5 min, and then incubated with 10 mL ECL anti-goat IgG horseradish peroxidase conjugates (1:1000 in TBST with 1% NFDM) (Applied Biosystems) for 2 h at room temperature. The membranes were washed a final time in 1% NFDM in TBST, 5 × 5 min. The membranes were imaged using chemiluminescence dye (GE Life Sciences, Piscataway, NJ) on Kodak image station 440 CF (Eastman Kodak, Rochester, NY).
Lipid Quantification by Oil Red O Assay
Immature adipocytes were seeded in 6-well plates at 6 × 104 cell/well. Two days after reaching confluence preadipocytes were induced with DMEM containing 100 units/mL penicillin-streptomycin, 10 mM sodium pyruvate, and 10% fetal bovine serum (FBS) (DMEM/FBS medium) containing 172 nM insulin, 0.5 M isobutylmethylxanthine (IBMX), and 1.0 M dexamethasone (DXM) (Sigma-Aldrich) for 2 days. Cells were then differentiated using DMEM/FBS medium containing 1.72 μM insulin for 2 days. Medium was changed (DMEM/FBS without additional insulin) every two days for 6 days total until treatment. Mature adipocytes were treated with extracts for 48 hours before assaying using a procedure adapted from Martinez-Villaluenga et al. (2008) (57). In summary, mature adipocytes were washed with DPBS and fixed for one hour with 10% formaldehyde (v/v in DPBS) (Sigma-Aldrich). Cells were washed in 60% isopropanol and air-dried. The Oil Red O solution was prepared by dissolving 0.1 g Oil Red powder (Sigma-Aldrich) in 20 mL 100% isopropanol and diluted to a final volume of 50 mL with dd-H2O. Each well was stained with 2 mL for 60 min, after which the cells were washed with water four times and allowed to air dry. The Oil Red O dye was eluted from the lipid droplets by adding 2 mL 100% isopropanol for 10 min. The resulting eluant was analyzed on a SpectraMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA) at 510 nm. Percent accumulation was calculated by the following equation:
In vivo Assay
The bioassay for evaluation of hypoglycemic response (antidiabetic properties) due to extract administration to mice is described by Grace et al. (55). In summary, five-week-old male C57bl/6J mice (10-20 g) were purchased from Jackson Labs (Bar Harbor, ME) and acclimatized for 1 week in a cage with a regular ad libitum diet and water, a 12:12 h light-dark cycle, and constant 24±1 °C temperature. At 6 weeks of age, mice were randomly distributed into experimental groups fed either a low fat diet (LFD) or very high fat diet (VHFD). The LFD and VHFD (Research Diets Inc., New Brunswick, NJ) were similar in nutritional content, differing only in carbohydrates (70 kcal% vs. 20 kcal%) and fat content from lard (10 kcal% vs. 60 kcal%). Mice were maintained on assigned diet for 14 weeks, weighed weekly and blood was collected every other week for glucose analysis. Animals had food restricted for 4 h and then were gavaged with plant extracts (500 mg/kg), control vehicle (water), or metformin (positive control at 300 mg/kg). Blood glucose readings were taken at 0, 3, and 6 h after gavage (animals continued food restriction during this time), after which food was replaced until the next treatment. All protocols were approved by Rutgers University Institutional Care and Use Committee and followed state and federal laws.
Statistical Analysis
Data are presented as mean of triplicate runs ±SEM
Statistical significance of in vitro and in vivo assays was determined using the student's Ttest procedure of Statistical Analysis System (SAS) (version 9.2, SAS Institute Inc., Raleigh, NC). Linear correlation for lipid accumulation was achieved using Microsoft Excel (Microsoft Corporation, Redmond, WA). Mean separation of in vivo results were achieved through the LSD procedure of SAS (SAS Institute) with α=0.01.
Results
Community Participatory Research
The three STN bioassays provided a strong preliminary indication of bioactivity relevant to diabetes and obesity, and simultaneously served to engage local community members in the objectives of the research initiative. As an illustration of the outcomes, a representative data summary for the three berries assayed in Akutan is shown in Table 1. All berries demonstrated antioxidant capacity. E. nigrum and V. ovalifolium were effective α-amylase inhibitors, whereas R. spectabilis was not. All three berry species inhibited protease digestion of gel substrates, and correspondingly had undetectable levels of protease activity. Both ripe and unripe berries were assayed in each community, which illustrated to the community members that the secondary phytochemicals accumulated in ripe fruit were responsible for the observed bioactive properties. Berries from each of the other two sites (Seldovia and Point Hope) also demonstrated similar bioactivies and trends.
Table 1.
Screens-to-Nature (STN) Assay Results Demonstrating Antioxidant, Protease Inhibitor, and Amylase/Amylase Inhibitory Activity from Three Sampled Akutan Berry Species
| Berry | Ripe/ Unripe1 |
Protease Assay |
Protease Inhibitor Assay |
Amylase Assay |
Amylase Inhibitor Assay |
Antioxidant Assay |
|---|---|---|---|---|---|---|
| from Akutan, Alaska | ||||||
| Rubus spectabilis | R | 0 | 3 | 0 | 1 | 2 |
| Rubus spectabilis | U | 0 | 0 | 0 | 1 | 1 |
| Vaccinium ovalifolium | R | 0 | 3 | 0 | 2 | 3 |
| Vaccinium ovalifolium | U | 0 | 0 | 0 | 1 | 1 |
| Empetrum nigrum | R | 0 | 3 | 0 | 2 | 2 |
| Empetrum nigrum | U | 0 | 0 | 0 | 1 | 2 |
Responses are evaluated on a 0-3 scale, with 3 reflecting the highest activity.
Interviews and surveys with residents in all communities conducted in tandem with the field screening training sessions indicated that berries are considered a key local food resource and are valued for their nutritional contribution to local diets as well as for the traditional cultural practice of berry harvesting. In all communities, participants voiced concern about younger people moving away from local subsistence foods and consequent negative health implications. Local residents were particularly concerned about the rapid rise in diabetes and other health problems, including cancer.
Local tribal community members in each of the three test sites were keenly aware that berries are easily compromised or enhanced by fluctuations in climate. Local observations confirmed the importance of winter conditions, particularly abundant precipitation in the form of snow as crucial to developing a high yield, high quality berry harvest, with warmer, drier winters resulting in fewer berries with a decrease in taste. Moderate summer warmth, sunshine and adequate precipitation were also seen as essential to berry abundance and timing. Residents widely agreed that local climates were changing, albeit with mixed perspectives in Seldovia on the validity of anthropogenic sources of climate change. Point Hope participants reported substantial climate fluctuation with warming trends affecting sea ice, tundra conditions, wildlife migration patterns, and presence of insects. Akutan participants reported climate fluctuations, with greater uncertainty regarding weather trajectories and impacts from year to year. Seldovia participants noted local experience with climatic cycling, but rarely acknowledged a linear warming trend. In each community, climate change or fluctuation from year to year was highlighted as posing one of the greatest risks to local berry resources.
Phenolic Composition
Anthocyanins
The total anthocyanin content of the berries was calculated as cyanidin-3-glucoside equivalents from the total sum of peak areas as determined by HPLC. Berries showed substantial differences in anthocyanin content, ranging between 0.01 and 4.39 mg/g fresh weight of fruit (mg/g FW) (Table 2) with Point Hope's E. nigrum containing the highest levels of anthocyanins. Identification and peak assignment of the anthocyanins were based upon retention time comparison with previously reported values (56,58), MS spectral data, and comparison to standards. The HPLC chromatograms of each berry, measured at 520 nm, revealed a complex mixture of anthocyanins (Table 3). The distribution of anthocyanin aglycones varied between locations, but some species-level trends emerged.
Table 2.
Total Anthocyanin and Proanthocyanin Content of Wild Alaskan Berries in the Phenolic-rich Extract (PRE) and Enriched Fractions (ARF and PAC)
| Berry1 | Yield | Anthocyanin Content2 | Proanthocyanidin Content3 |
||
|---|---|---|---|---|---|
| PRE | ARF | PRE | PAC | ||
| g PRE extract (500 g fruit) |
mg equiv / g fruit |
mg equiv | mg equiv / g fruit |
mg equiv | |
| EN-AK | 4.249 | 2.762 | 238.02 | 0.746 | 21.248 |
| EN-PH | 9.768 | 4.386 | 302.96 | 3.741 | 62.005 |
| EN-SD | 4.460 | 2.648 | 154.03 | 2.861 | 42.607 |
| RC-PH | 1.960 | 0.010 | 0.09 | 1.256 | 23.367 |
| RS-AK | 2.183 | 0.391 | 29.73 | 0.486 | 16.708 |
| RS-SD | 1.323 | 0.086 | 32.09 | 0.737 | 21.372 |
| VO-AK | 6.350 | 3.337 | 154.62 | 2.420 | 38.494 |
| VO-SD | 3.644 | 2.364 | 102.73 | 1.732 | 25.363 |
| VU-AK | 5.388 | 3.100 | 130.52 | 2.969 | 56.352 |
| VU-PH | 7.308 | 2.063 | 76.57 | 6.252 | 80.41 |
Abbreviations: AK=Akutan, SD=Seldovia, PH=Point Hope, VO=V. ovalifolium, VU=V. uliginosum, EN=E. nigrum, RS=R. spectabilis, RC=R. chamaemorus.
measured by HPLC at 520 nm
measured by HPLC at 280 nm
Table 3.
Identification of Anthocyanins in Anthocyanin-Rich Fraction (ARF) of Wild Alaskan Berries
| Peak | RT (min) |
MS/MS (m/z) |
Anthocyanin | Peak | RT (min) |
MS/MS (m/z) |
Anthocyanin |
|---|---|---|---|---|---|---|---|
|
V. ovalifolium – Akutan (VO-AK)
| |||||||
| 1 | 19.7 | 465/303 | Delphinidin-3-galactoside | 7 | 27.1 | 463/301 | Peonidin-3-galactoside |
| 2 | 21.6 | 465/303 | Delphinidin-3-glucoside | 8 | 27.8 | 479/317 | Petunidin-3-glucoside |
| 3 | 23.0 | 435/303 | Delphinidin-3-arabinoside | 9 | 28.8 | 449/317 | Petunidin-3-arabinoside |
| 4 | 24.2 | 419/287 | Cyanidin-3-galactoside | 10 | 30.3 | 493/331 | Malvidin-3-galactoside |
| 5 | 25.0 | 449/287 | Cyanidin-3-glucoside | 11 | 31.5 | 493/331 | Malvidin-3-glucoside |
| 6 | 26.2 | 479/317 | Petunidin-3-galactoside | 12 | 33.4 | 463/331 | Malvidin-3-arabinoside |
|
V. ovalifolium – Seldovia (VO-SD)
| |||||||
| 1 | 19.6 | 465/303 | Delphinidin-3-galactoside | 7 | 26.9 | 463/301 | Peonidin-3-galactoside |
| 2 | 21.4 | 465/303 | Delphinidin-3-glucoside | 8 | 27.6 | 479/317 | Petunidin-3-glucoside |
| 3 | 22.8 | 435/303 | Delphinidin-3-arabinoside | 9 | 28.7 | 449/317 | Petunidin-3-arabinoside |
| 4 | 23.9 | 419/287 | Cyanidin-3-galactoside | 10 | 30.2 | 493/331 | Malvidin-3-galactoside |
| 5 | 24.7 | 449/287 | Cyanidin-3-glucoside | 11 | 31.5 | 493/331 | Malvidin-3-glucoside |
| 6 | 26.1 | 479/317 | Petunidin-3-galactoside | 12 | 33.3 | 463/331 | Malvidin-3-arabinoside |
|
V. uliginosum – Akutan (VU-AK)
| |||||||
| 1 | 19.8 | 465/303 | Delphinidin-3-galactoside | 7 | 27.4 | 479/317 | Petunidin-3-glucoside |
| 2 | 21.5 | 465/303 | Delphinidin-3-glucoside | 8 | 28.6 | 449/317 | Petunidin-3-arabinoside |
| 3 | 22.7 | 435/303 | Delphinidin-3-arabinoside | 9 | 29.9 | 493/331 | Malvidin-3-galactoside |
| 4 | 24.5 | 449/287 | Cyanidin-3-galactoside | 10 | 30.1 | 493/331 | Malvidin-3-glucoside |
| 5 | 25.2 | 449/287 | Cyanidin-3-glucoside | 11 | 31.8 | 443/301 | Peonidin-3-arabinoside |
| 6 | 26.4 | 463/301 | Peonidin-3-galactoside | 12 | 32.8 | 463/331 | Malvidin-3-arainoside |
|
V. uliginosum – Point Hope (VU-PH)
| |||||||
| 1 | 20.2 | 465/303 | Delphinidin-3-galactoside | 7 | 28.1 | 479/317 | Petunidin-3-glucoside |
| 2 | 22.1 | 465/303 | Delphinidin-3-glucoside | 8 | 29.4 | 449/317 | Petunidin-3-arabinoside |
| 3 | 23.5 | 435/303 | Delphinidin-3-arabinoside | 9 | 30.6 | 493/331 | Malvidin-3-galactoside |
| 4 | 24.6 | 449/287 | Cyanidin-3-galactoside | 10 | 31.9 | 493/331 | Malvidin-3-glucoside |
| 5 | 25.4 | 449/287 | Cyanidin-3-glucoside | 11 | 32.8 | 443/301 | Peonidin-3-arabinoside |
| 6 | 26.7 | 463/301 | Peonidin-3-galactoside | 12 | 33.9 | 463/331 | Malvidin-3-arainoside |
|
E. nigrum – Akutan (EN-AK)
| |||||||
| 1 | 19.3 | 465/303 | Delphinidin-3-galactoside | 6 | 26.9 | 419/287 | Cyanidin-3-arabinoside |
| 2 | 22.8 | 449/287 | Cyanidin-3-galactoside | 7 | 28.7 | 463/301 | Peonidin-3-galactoside |
| 3 | 24.1 | 435/303 | Delphinidin-3-arabinoside | 8 | 30.2 | 493/331 | Malvidin-3-galactoside |
| 4 | 25.2 | 449/287 | Cyanidin-3-glucoside | 9 | 31.9 | 493/331 | Malvidin-3-glucoside |
| 5 | 25.9 | 479/317 | Petunidin-3-galactoside | 10 | 33.3 | 463/331 | Malvidin-3-arabinoside |
|
E. nigrum – Point Hope (EN-PH)
| |||||||
| 1 | 19.7 | 465/303 | Delphinidin-3-galactoside | 6 | 27.2 | 419/287 | Cyanidin-3-arabinoside |
| 2 | 22.9 | 449/287 | Cyanidin-3-galactoside | 7 | 28.7 | 463/301 | Peonidin-3-galactoside |
| 3 | 24.3 | 435/303 | Delphinidin-3-arabinoside | 8 | 30.4 | 493/331 | Malvidin-3-galactoside |
| 4 | 25.3 | 449/287 | Cyanidin-3-glucoside | 9 | 31.9 | 493/331 | Malvidin-3-glucoside |
| 5 | 26.1 | 479/317 | Petunidin-3-galactoside | 10 | 33.3 | 463/331 | Malvidin-3-arabinoside |
|
E. nigrum - Seldovia (EN-SD)
| |||||||
| 1 | 19.4 | 465/303 | Delphinidin-3-galactoside | 6 | 26.8 | 419/287 | Cyanidin-3-arabinoside |
| 1′ | 20.2 | 465/303 | Delphinidin-3-galactoside | 7 | 27.9 | 449/317 | Petunidin-3-glucoside |
| 2 | 22.2 | 435/303 | Delphinidin-3-arabinoside | 8 | 29.6 | 463/301 | Peonidin-3-galactoside |
| 3 | 23.1 | 449/287 | Cyanidin-3-galactoside | 9 | 31.0 | 493/331 | Malvidin-3-galactoside |
| 3′ | 23.7 | 419/287 | Cyanidin-3-galactoside | 10 | 32.3 | 493/331 | Malvidin-3-glucoside |
| 4 | 24.9 | 449/287 | Cyanidin-3-glucoside | 11 | 32.9 | 433/301 | Peonidin-3-arabinoside |
| 5 | 25.9 | 479/317 | Petunidin-3-galactoside | 12 | 34.1 | 463/331 | Malvidin-3-arabinoside |
|
R. chamaemorus – Point Hope (RC-PH)
| |||||||
| 1 | 24.8 | 449/287 | Cyanidin-3-glucoside | 3 | 28.0 | 449/317 | Petunidin-3-glucoside |
| 2 | 26.7 | 419/287 | Cyanidin-3-arabinoside | 4 | 30.3 | 493/331 | Malvidin-3-galactoside |
|
R. spectabilis – Akutan (RS-AK)
| |||||||
| 1 | 24.9 | 449/287 | Cyanidin-3-glucoside | 3 | 33.8 | 463/331 | Malvidin-3-arabinoside |
| 2 | 27.5 | 419/287 | Cyanidin-3-arabinoside | ||||
|
R. spectabilis – Seldovia (RS-SD)
| |||||||
| 1 | 26.5 | 449/287 | Cyanidin-3-glucoside | 2 | 28.8 | 419/287 | Cyanidin-3-arabinoside |
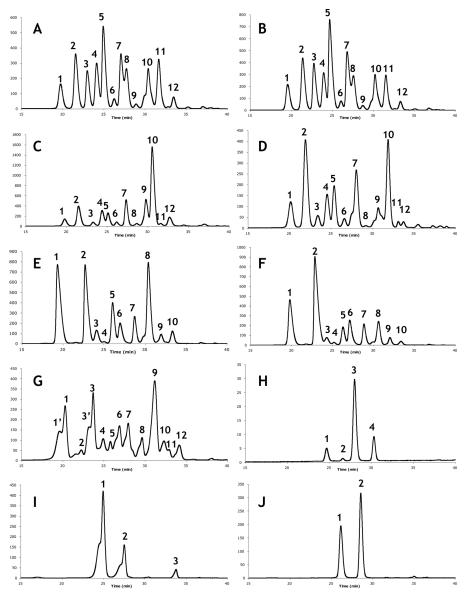
HPLC analysis of the V. ovalifolium samples from Akutan (VO-AK) and from Seldovia (VO-SD) revealed markedly different levels of anthocyanins (Table 2). VO-AK contained 3.34 mg AC/g FW, whereas VO-SD contained 2.36 mg AC/g FW. The anthocyanin content of Seldovia's berries were comparable to the mean value of 2.65 mg AC/g FW which has been reported for cultivated V. ovalifolium genotypes around the Pacific Northwest (59, 60), whereas the Akutan V. uliginosum exceeded the 3.11 mg/g FW reported for the same species of wild blueberries from Washington (59). Both VO-AK and VO-SD contained 12 distinct anthocyanin structures (Table 3). The major species present (Figs. 2A,B) were delphinidin glycosides (galactoside (peak 1), glucoside (peak 2) and arabinoside (peak 3)), cyanidin glycosides (galactoside (peak 4) and glucoside (peak 5)), and malvidin glycosides (galactoside (peak 10), glucoside (peak 11), and arabinoside (peak 12)). Also present were smaller amounts of petunidin-3-galactoside (peak 6), petunidin-3-glucoside (peak 8), and peonidin-3-galactoside (peak 7), consistent with anthocyanin profiles from previous V. ovalifolium analyses (61).
Figure 2.
HPLC chromatographs of phenolic-rich extracts (PRE) of 10 wild Alaskan berry samples. Chromatograms: (A)-V. ovalifolium - Akutan (VO-AK), (B)-V. ovalifolium - Seldovia (VO-SD), (C)-V. uliginosum - Akutan (VU-AK), (D)-V. uliginosum - Point Hope (VU-PH), (E)-E. nigrum - Akutan (EN-AK), (F)-E. nigrum - Point Hope (EN-PH), (G)-E. nigrum - Seldovia (EN-SD), (H)-R. chamaemorus - Point Hope (RC-PH), (I)-R. spectabilis - Akutan (RS-AK), (J)-R. spectabilis - Seldovia (RS-SD).
Samples of the other wild blueberry species, V. uliginosum, from Akutan (VU-AK) and Point Hope (VU-PH) contained anthocyanin levels of 3.10 and 2.06 mg/g FW, respectively. This was consistent with V. uliginosum berries found in northern Finland, which also demonstrated considerable variability in the content of anthocyanins present, ranging from 2.61-4.32 mg/g FW (62). However, both samples were significantly higher than the 1.24 mg/g FW found in V. uliginosum samples from Wyoming (61). HPLC and LC/MS analysis, compared with previous studies (63, 64) led to the identification of 12 structures (Table 3) consisting of five aglycones - delphinidin, cyanidin, malvidin, peonidin, and petunidin - congruous with the previous results (62). The major anthocyanins present in VU-AK (Figs. 2C) were delphinidin-3-glucoside (peak 2), cyanidin-3-galactoside (peak 4), cyanidin-3-glucoside (peak 5), petunidin-3-glucoside (peak 7), malvidin-3-galactoside (peak 9), and malvidin-3-glucoside (peak 10). Results from VU-PH (Figure 2D) were similar, but substantial amounts of delphinidin-3-galactoside (peak 1) were also present.
E. nigrum, the only species available and collected at all three sites of this study, provided a 3-way comparison of phytochemical components. The total content of anthocyanins was similar for berries collected at Akutan (2.76 mg/g FW, EN-AK) and Seldovia (2.65 mg/g FW, EN-SD), but berries from Point Hope (EN-PH) revealed markedly higher anthocyanin concentrations (4.39 mg/g FW, the highest level of any berry tested in the study). While all three samples were higher than the reported average of 1.25 mg/g (65), EN-PH demonstrated higher anthocyanin content than E. nigrum found in northern Finland (4.08 mg/g FW) (62). EN-AK and EN-PH showed similar chromatographs (Figs. 2E,F), with 10 individual anthocyanin species. EN-AK (Figure 2E) had two major species present - delphinidin-3-galactoside (peak 1) and cyanidin-3-galactoside (peak 2) - while EN-PH (Figure 2F) possessed three major species - delphinidin-3-galactoside (peak 1), cyanidin-3-galactoside (peak 2), and malvidin-3-galactoside (peak 8). These results agree with other E. nigrum studies (65-67). The chromatograph of EN-SD (Figure 2G) contained a third delphinidin (delphinidin-3-arabinoside, peak 2), an additional petunidin residue (petunidin-3-glucoside, peak 8), and peonidin-3-arabinoside, which eluted immediately after malvidin-3-glucoside (65). Peaks 1 (delphinidin-3-galactoside) and 3 (cyanidin-3-galactoside) had shoulders that could not be resolved (marked as peaks 1′ and 3′, respectively).
Cloudberry (R. chamaemorus), available only at the Point Hope site (RC-PH), had the lowest concentration of anthocyanins of all berries tested, 0.01 mg/g FW. HPLC analysis revealed four structures (Figure 2H), identified as cyanidin-3-glucoside (peak 1), cyanidin-3-arabinoside (peak 2), petunidin-3-glucoside (peak 3), and malvidin-3-galactoside (peak 4) (Table 3). There has been a single report of the anthocyanins in R. chamaemorus, a qualitative study by Jennings and Carmichael (1979) suggesting there were cyanidin structures present (68). Our research corroborates the previous report in 2 of the 4 identified structures, which are reported here for the first time in R. chamaemorus.
R. spectabilis samples from Akutan (RS-AK) and Seldovia (RS-SD) were also found to have low concentrations of anthocyanins, 0.39 and 0.09 mg/g FW, respectively. Akutan and Seldovia's R. spectabilis both contained two cyanidin glycosides (Figs. 2I,J) (cyanidin-3-glucoside (peak 1) and cyanidin-3-arabinoside (peak 2)), with RS-AK containing an additional species, malvidin-3-arabinoside (peak 3). While R. spectabilis has been reported to contain anthocyanins (68), no quantitative data has been previously reported, and these specific anthocyanin structures have also not been previously reported for R. spectabilis.
Proanthocyanidins
HPLC chromatograms gave an estimate of the total proanthocyanidin (PAC) content, calculated as (epi)-catechin equivalents from the total peak area measured at 280 nm (Table 2). Concentrations ranged greatly, between 0.49 and 6.25 mg/g FW, with Point Hope's V. uliginosum containing the greatest concentration overall.
The most common flavan-3-ol subunits of PACs are (epi)afzelechin, (epi)catechin, and (epi)gallocatechin (‘epi’ refers to the epimer of the flavanoid). B-type PACs contain a single linkage, either C4 → C8′ or C4 → C6′. A-types possess an additional linkage between monomers, an ether bridge from C2 → O → C7′ (69). A-type proanthocyanidins are rarer than B-types in plants consumed as food (69, 70), and can vary in degree of polymerization (DP), with multiple DP possible within a single fruit (25).
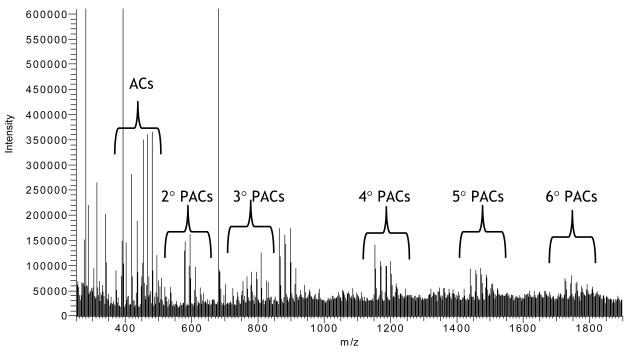
LC-MS analysis revealed a series of PAC oligomers and polymers ranging between dimers and hexamers (Figure 3). Preliminary indication of A-type PACs arose from MS spectra through the visualization of the [M+H]+ ions, which are 2 Da smaller than the corresponding B-type PAC. All berry species in this study were found to possess A-type PACs. For further structural information and characterization of A-type PAC dimers through tetramers, tandem ESI-MS2 was used to fragment the parent ions. Fragments were identified through neutral mass losses resulting from quinone methide (QM) cleavage, retro-Diels-Alder (RDA) fission, heterocyclic ring fission (HRF), benzofuran-forming (BFF) fission, and water loss (Table 4). Based upon the identity of these structural fragments and the daughter ions resulting from the fission events, a unique PAC assignment can be made, which includes the A-linkage placements in the molecule (71).
Figure 3.
Representative LC-MS spectrum from phenolic-rich extract (PRE) of V. uliginosum - Akutan (VU-AK) showing ACs and PAC dimers through hexamers.
Table 4.
Neutral Mass Losses in MS-MS Fission Events (adapted from Li and Deinzer, 2008)
| Monomer | Retro-Diels Alder RDA Da |
Heterocyclic Ring Fission HRF Da |
Benzofuran- forming Fission BFF Da |
|---|---|---|---|
| (epi)afzelechin | −136 | −126 | −106 |
| (epi)catechin | −152 | −126 | −122 |
| (epi)gallocatechin | −168 | −126 | −156 |
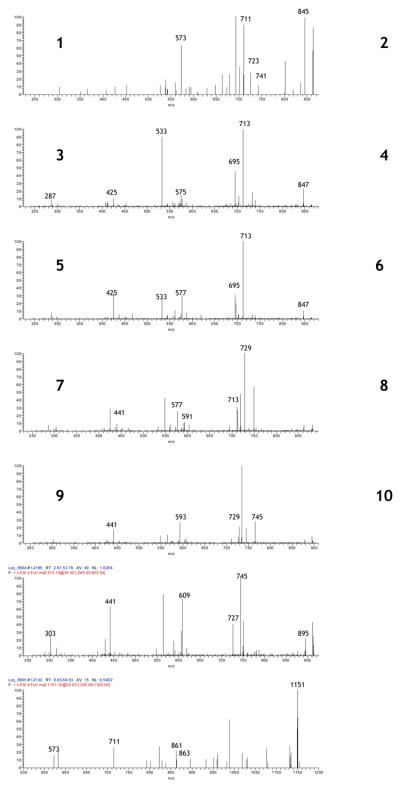
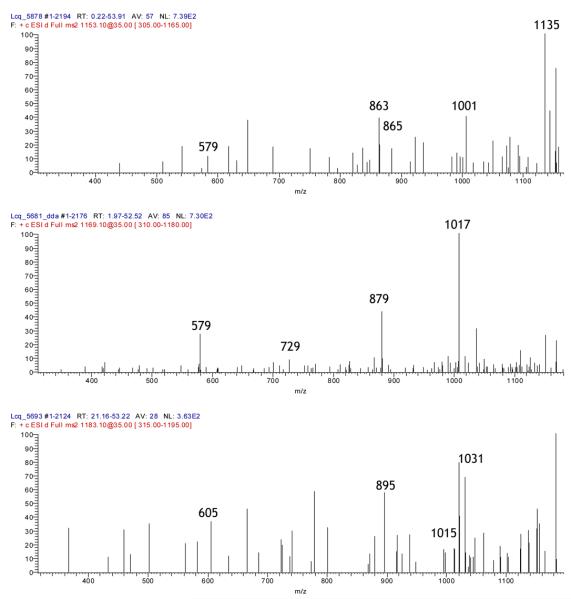
Compounds 1-3 represent A-type trimers (Figure 4). The degree of A-linkage can be determined from the difference in parent ion mass compared to a B-type trimer; 1 (m/z 863) is 4 Da less than the corresponding B-type trimer and thus has 2 A-linkages, while 2 and 3 (m/z 865) are only 2 Da smaller and thus have only one A-link. The MS2 spectrum of 1 yields a fragment peak at m/z 711, representing an RDA fission event of the upper monomer and identifying it as (epi)catechin. The peak at m/z 573 (863 Da - 290 Da) results from the loss of a terminal (epi)catechin unit via QM loss. Difference in masses reveals the central unit to be (epi)catechin as well, giving an identification for compound 1 as (epi)catechin-A-(epi)catechin-A-(epi)catechin (with “-A-“ representing an A-link between monomers). 2 and 3 are very similar in their degradation patterns. Both contain peaks at m/z 713, representing an RDA fission of the upper unit and identifying it as (epi)catechin. 2 undergoes QM fission of the terminal unit to yield a peak at m/z 575, which is indicative of an A-type dimer (865 Da - 290 Da), whereas the QM fission of 3 yields a peak at m/z 577, a B-type dimer (865 Da - 288 Da). Thus, the A-linkage in each trimer is reversed; compound 2 is assigned the structure (epi)catechin-A-(epi)catechin-(epi)catechin while compound 3 is designated as (epi)catechin-(epi)catechin-A-(epi)catechin.
Figure 4.
LC-MS2 spectra of A-type proanthocyanidin trimers and tetramers from proanthocyanidin-rich fraction (PAC) of wild Alaskan berries. 1 - m/z 863, 2 - m/z 865, 3 - m/z 865, 4 - m/z 881, 5 - m/z 897, 6 - m/z 913, 7 - m/z 1151, 8 - m/z 1153, 9 - m/z 1169, 10 - m/z 1183.
Compounds 4-6 (Figure 4) are oligomeric or polymeric trimer A-type proanthocyanidins containing (epi)gallocatechin. All three weigh 2 Da less than the corresponding B-type trimer, thus all three contain a single A-type linkage. An RDA fission event of the upper unit of compound 4 (m/z 881) yields the peak m/z 729 and identifies the upper unit as (epi)catechin. The fragment ion at m/z 577 represents an B-type dimer (881 Da - 304 Da) formed through the QM loss of (epi)gallocatechin. The corresponding peak at m/z 591 (881 Da - 290 Da) identifies the A-type dimer ion after a QM fission of (epi)catechin. Thus, the final assignment of compound 4 is (epi)catechin-(epi)catechin-A-(epi)gallocatechin. Structure 5 (m/z 897) undergoes two RDA fission events; the terminal unit yields the peak at m/z 745 and identifies the terminal unit as (epi)catechin, while the upper unit yields the peak at m/z 729, identifying the upper unit as (epi)gallocatechin. The peak at m/z 593 is an A-type unit (897 Da - 304 Da) formed from the QM fission of an (epi)gallocatechin unit. Thus, the final characterization of compound 5 is (epi)gallocatechin-(epi)gallocatechin-A-(epi)catechin. Compound 6 fragments via an RDA fission to form the daughter ion m/z 745, yielding an (epi)gallocatechin terminal unit. The peak at m/z 609 (913 Da - 304 Da) is an A-type dimer formed from a QM fission of the terminal unit. The upper unit of this dimer, also (epi)gallocatechin, undergoes another RDA fission, yielding the peak at m/z 441, and thus the final assignment of compound 6 is (epi)gallocatechin-A-(epi)gallocatechin-(epi)gallocatechin.
Compounds 7 and 8 are A-type tetramer proanthocyanidins comprised of all (epi)catechin units. Structure 7 (m/z 1151) is 4 Da less than the corresponding B-type tetramer, indicating the presence of 2 A-linkages. The peak at m/z 863 (1151 Da - 288 Da) is an A-type trimer containing 2 A-links formed from the QM fission of the upper unit ((epi)catechin), while m/z 861 (1151 Da - 290 Da) is an A-type trimer containing a single A-link formed from the QM fission of the terminal unit, identified as (epi)catechin. The trimer at m/z 863 immediately undergoes a second QM fission, yielding the peak m/z 573, which represents an A-type dimer (863 Da - 290 Da). The central unit must be (epi)catechin by mass difference, and the final assignment of structure 7 is (epi)catechin-(epi)catechin-A-(epi)catechin-A-(epi)catechin. Compound 8 is only 2 Da smaller than a B-type tetramer proanthocyanidin, and thus has a single A-link. The fragment ion at m/z 863 (1153 Da - 290 Da) represents a QM fission of the terminal unit, identifying it as (epi)catechin. The upper unit undergoes QM fission (1153 Da - 288 Da), yielding an A-type trimer at m/z 865. The trimer further fragments to give peak m/z 579 (1153 Da - 574 Da), a B-type dimer, indicating the A-link lies between the 2nd and 3rd units. Final assignment of compound 8 is given as (epi)catechin-(epi)catechin-A-(epi)catechin-(epi)catechin.
Compounds 9 and 10 are oligomeric A-type tetramer proanthocyanidins consisting of both (epi)catechin and (epi)gallocatechin monomers. Compound 9 is 2 Da smaller than the corresponding B-type tetramer, having a single A-type link. The upper unit undergoes QM fission, yielding the A-type trimer m/z 879 (1169 Da - 290 Da), identifying it as (epi)catechin. This trimer then fragments again, giving a B-type (epi)catechin-(epi)catechin dimer, m/z 579 (1169 Da - 590 Da), demonstrating the 2nd as (epi)gallocatechin by mass difference and yielding the final structure of compound 9 as (epi)catechin-(epi)gallocatechin-A-(epi)catechin-(epi)catechin. Structure 10 is 4 Da less than it's corresponding B-type proanthocyanidin, and thus contains 2 A-links. The parent ion undergoes two RDA fission events, yielding peaks at m/z 1031 and 1015 and identifying the upper and terminal units as (epi)catechin and (epi)gallocatechin, respectively. The fragment ion with m/z 895 (1183 Da - 288 Da) arises from the QM fission of the upper (epi)catechin, yielding a trimer with 2 A-links. This trimer undergoes a second QM fission at m/z 605 (1183 Da - 578 Da), identifying the second unit as an A-type (epi)catechin, and leaving an A-type (epi)gallocatechin dimer. The final structure of compound 10 is given as (epi)catechin-(epi)catechin-A-(epi)gallocatechin-A-(epi)gallocatechin.
Table 5 shows the results of the tandem ESI-MS2 analysis. Every berry studied contained multiple proanthocyanidin structures, most possessing A-type proanthocyanidins of varying DP. All berries save Seldovia's R. spectabilis contained A-type dimers, and Point Hope's R. chamaemorus was lacking in trimer A-type proanthocyanidins. There was considerable variation between samples from different locations, and several A-type proanthocyanidin trimers (compounds 1,2, and 5) were found to be species-specific.
Table 5.
A-type Proanthocyanidins Polymers in Proanthocyanidin-rich (PAC) Fraction of Alaskan Berries
| Proanthocyanidin1 | m/z | VO- AK |
VO- SD |
EN- AK |
EN- PH |
EN- SD |
VU- AK |
VU- PH |
RS- AK |
RS- SD |
RC- PH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimers | |||||||||||
| (e)C-A-(e)C | 577,425,287 | • | • | • | • | • | • | • | |||
| (e)C-A-(e)Gc | 593,303,287 | • | • | • | • | • | • | • | |||
| (e)Gc-A-(e)Gc | 609,303,287 | • | • | • | • | ||||||
| Trimers | |||||||||||
| (e)C-A-(e)C-A-(e)C | 863,573 | • | • | ||||||||
| (e)C-(e)C-A-(e)C | 865,577 | • | • | • | • | • | • | • | |||
| (e)C-A-(e)C-(e)C | 865, 575 | • | • | ||||||||
| (e)C-(e)C-A-(e)Gc | 881,729,577 | • | • | • | • | • | |||||
| (e)Gc-(e)Gc-A-(e)C | 897,593,441 | • | • | • | |||||||
| (e)Gc-A-(e)Gc-(e)Gc | 913,609 | • | |||||||||
| Tetramers | |||||||||||
| (e)C-(e)C-A-(e)C-A-(e)C | 1151,863,573 | • | |||||||||
| (e)C-(e)C-A-(e)C-(e)C | 1153,865,575 | • | • | ||||||||
| (e)C-(e)Gc-A-(e)C-(e)C | 1169,881, 729,577 |
• | • | ||||||||
| (e)C-(e)C-A-(e)Gc-A- (e)Gc |
1183,1021, 895,605 |
• | |||||||||
Abbreviations: (e)C=(epi)catechin, (e)Gc=(epi)gallocatechin, -A- =A-linkage, AK=Akutan, SD=Seldovia, PH=Point Hope, VO=V. ovalifolium, VU=V. uliginosum, EN=E. nigrum, RS=R. spectabilis, RC=R. chamaemorus.
HPLC analysis of V. ovalifolium revealed proanthocyanidin concentrations of 1.73 mg/g FW (VO-SD) and 2.42 mg/g FW (VO-AK) (Table 2). Both samples of V. ovalifolium contained A-type dimers (epi)catechin-A-(epi)gallocatechin and (epi)gallocatechin-A-(epi)gallocatechin, and VO-SD contained the additional dimer (epi)catechin-A-(epi)catechin. Compound 2 ((epi)catechin-A-(epi)catechin-(epi)catechin) was found exclusively in VO-AK and VO-SD, and VO-SD was found to possess compound 4 as well. The two V. ovalifolium samples contained A-type tetramers, albeit different structures; VO-AK was the only sample to incorporate compound 7, whereas VO-SD contained compound 8.
The other Vaccinium species, V. uliginosum, contained some of the highest levels of proanthocyanidins. VU-AK was found to contain 2.97 mg/g FW of proanthocyanidins, while VU-PH contained 6.25 mg/g FW; the highest level measured from all samples (Table 2). The two V. uliginosum samples also differed in their A-type dimer composition. VU-AK contained all three A-type dimers, but VU-PH only possessed (epi)catechin-A-(epi)catechin. Both VU-AK and VU-PH contained compound 3, and VU-PH also contained compound 4. The presence of compound 3 in VU-PH was previously reported in a study by Määttä-Riihinen et al. (2005) (72). Only Point Hope's V. uliginosum sample contained an A-type tetramer, compound 9.
The E. nigrum samples differed considerably in their proanthocyanidin content. The samples from Seldovia and Point Hope contained fairly large amounts of proanthocyanidins (2.86 mg/g FW and 3.74 mg/g FW, respectively), while EN-AK contained only 0.75 mg/g FW (Table 2). All three contained the same two A-type dimers, (epi)catechin-A-(epi)catechin and (epi)catechin-A-(epi)gallocatechin. They also possessed three of the same trimers: compounds 3, 4, and 5. Compound 5 ((epi)gallocatechin-(epi)gallocatechin-A-(epi)catechin) was unique to E. nigrum, not found in any other samples. Only EN-PH contained an A-type tetramer, compound 9.
R. spectabilis contained the lowest levels of proanthocyanidins of all berries sampled, possessing 0.49 and 0.74 mg/g FW for RS-AK and RS-SD, respectively. RS-AK contained a single A-type dimer, (epi)catechin-A-(epi)catechin, while RS-SD was found to contain no A-type proanthocyanidin dimers. RS-AK and RS-SD both contained an A-type trimer with two A-links, compound 1, as well as compound 3. The presence of 1 was unique to the R. spectabilis samples, not seen in any other berry. Both samples contained A-type tetramers, albeit different structures; RS-AK having compound 8 while RS-SD contained a double A-linked tetramer, 10.
Point Hope's R. chamaemorus contained modest amounts of proanthocyanidins, with HPLC analyses indicating 1.26 mg/g FW. Two A-type dimers were identified; (epi)catechin-A-(epi)gallocatechin and (epi)gallocatechin-A-(epi)gallocatechin. While B-type dimers have previously been described in this species (73), this marks the first time that A-type dimers have been identified in R. chamaemorus.
In vitro assays
Preadipocyte pref-1 Analysis
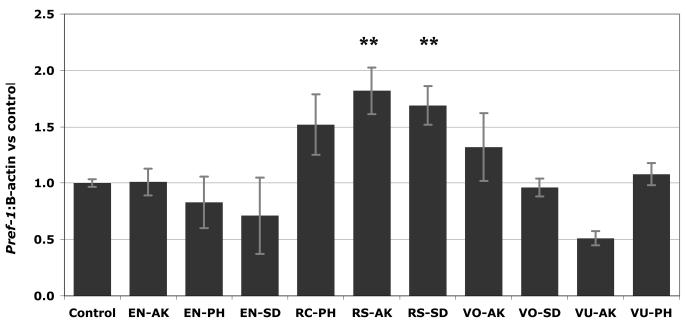
Pref-1 is an inhibitory protein which is responsible for preventing the maturation of adipocyte cells. The ability of an extract to increase or maintain high pref-1 levels is a marker of potential anti-obesity activity. The phenolic-rich extracts of Alaskan berries were assessed as to their capacity to increase the expression of pref-1. Total pref-1 expression was obtained via chemiluminescent Western blot imaging (Figure 5), and calculated as the ratio between pref-1 levels and β-actin levels, compared against control. Figure 6 shows the effect of PRE extracts on pref-1 expression levels in immature 3T3-L1 adipocytes. Of the ten Alaskan berry samples, most demonstrated no substantial increase in expression levels; 4 samples possessed moderate increases of pref-1, and of those only two were found to significantly increase the amount of pref-1 in immature adipocytes (Figure 6). RS-AK and RS-SD heightened expression levels 82% and 69%, respectively.
Figure 5.
Western blot analysis of several representative wild Alaskan phenolic-rich extracts (PRE). (a) - pref-1 expression levels, (b)- β-actin expression levels. Protein bands are denoted by arrows. Overall pref-1 expression is calculated as the ratio between the two for each sample, compared against control. Abbreviations: AK=Akutan, SD=Seldovia, VO=V. ovalifolium, EN=E. nigrum, RS=R. spectabilis.
Figure 6.
Effect of wild Alaska berry phenolic-rich extracts (PRE) on pref-1 expression levels of immature 3T3-L1 adipocytes. Run in triplicate, figure represents mean response ± SE. Asterisks denote significant activity, ** p≤0.05. Abbreviations: PRE = phenolic-rich extract, ARF = anthocyanin-rich fraction, PAC = proanthocyanidin-rich fraction, AK=Akutan, SD=Seldovia, PH=Point Hope, VO=V. ovalifolium, VU=V. uliginosum, EN=E. nigrum, RS=R. spectabilis, RC=R. chamaemorus.
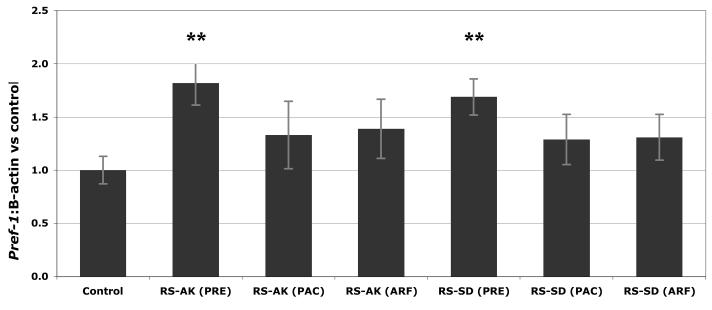
In addition to the PRE extracts, the anthocyanin-rich (ARF) and proanthocyanidin-rich (PAC) fractions of the two active samples (RS-AK and RS-SD) were evaluated in order to further investigate which group of berry phytochemicals affect the activity of the adipogenesis inhibitor pref-1 (Figure 7). The two enriched fractions did not significantly increase pref-1 expression compared to the control, while the PRE from each continued to demonstrate consistently high levels of pref-1 expression.
Figure 7.
Effects of RS-AK and RS-SD fractions on pref-1 expression levels in immature 3T3-L1 adipocytes. Run in triplicate, figure represents mean response ± SE. Asterisks denote significant activity, ** p≤0.05. Abbreviations: PRE = phenolic-rich extract, ARF = anthocyanin-rich fraction, PAC = proanthocyanidin-rich fraction, AK=Akutan, SD=Seldovia, RS=R. spectabilis.
Oil Red O Assay
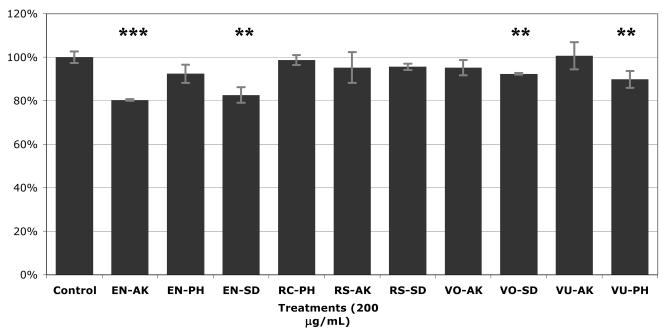
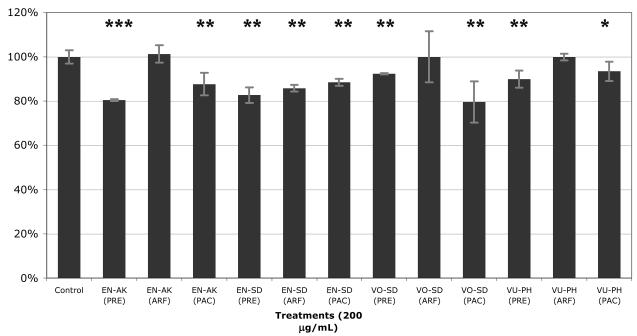
Phenolic-rich extract (PRE) of the ten wild Alaskan berry samples were assayed as to their ability to attenuate lipid accumulation in mature 3T3-L1 adipocytes (Figure 8). Accumulation levels ranged from 80.43% to 100.7% of the control. Four of the ten samples exhibited significant inhibition of aggregation of intracellular lipids; EN-AK (80.43% of control lipid levels), EN-SD (82.67%), VU-PH (89.85%), and VO-SD (92.33%).
Figure 8.
Effect of phenolic-rich extracts (PRE) of Alaskan berries on lipid accumulation in mature 3T3-L1 adipocytes. Results from Red Oil O Assay given as percent lipid accumulation versus control. Run in triplicate, figure represents mean response ± SE. Asterisks denote significant activity, ** p≤0.05, *** p≤0.01. Abbreviations: PRE = phenolic-rich extract, ARF = anthocyanin-rich fraction, PAC = proanthocyanidin-rich fraction, AK=Akutan, SD=Seldovia, PH=Point Hope, VO=V. ovalifolium, VU=V. uliginosum, EN=E. nigrum, RS=R. spectabilis, RC=R. chamaemorus.
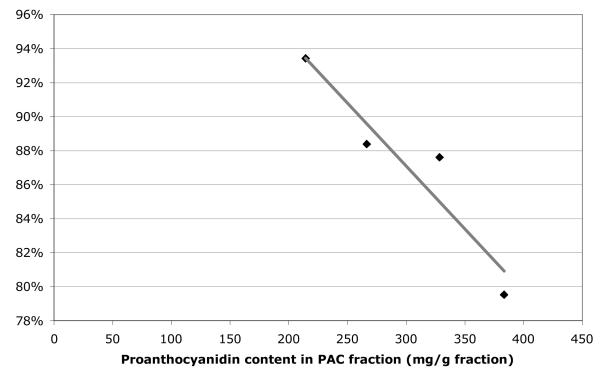
The enriched fractions (ARF and PAC) of the four active berry samples were also investigated for their effects on lipid accumulation (Figure 9). While most of the PRE extracts were active, only one of the ARF fractions (EN-SD) significantly reduced lipid levels in the cells, while all four PAC fractions significantly inhibited the aggregation of cellular lipids. As the PAC fractions possessed the highest activity, the inhibition levels of the active PAC fractions were compared on the basis of their respective proanthocyanidin content, revealing a linear response (Figure 10). The proanthocyanidin content (as mg/g fraction) was positively correlated with lipid accumulation inhibition, with a correlation coefficient (R2) of 0.896.
Figure 9.
Effect of enriched fractions from Alaskan berries on lipid accumulation in mature 3T3-L1 adipocytes. Results from Red Oil O Assay given as percent lipid accumulation versus control. Run in triplicate, figure represents mean response ± SE. Asterisks denote significant activity, * p≤0.1, ** p≤0.05, *** p≤0.01. Abbreviations: PRE = phenolic-rich extract, ARF = anthocyanin-rich fraction, PAC = proanthocyanidin-rich fraction, AK=Akutan, SD=Seldovia, PH=Point Hope, VO=V. ovalifolium, VU=V. uliginosum, EN=E. nigrum.
Figure 10.
Response of proanthocyanidin-rich fractions (PACs) on lipid accumulation in Red Oil O Assay correlated with proanthocyanidin content (in mg/g fraction). Linear correlation coefficient R2=0.896.
In vivo Assay
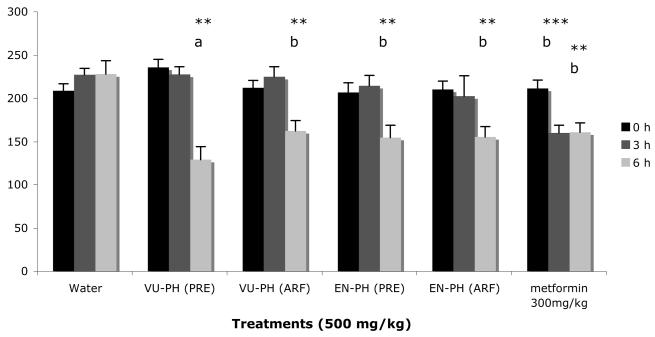
The PRE and ARF extracts of VU-PH and EN-PH were tested for potential hypoglycemic activity using an acute model of T2DM. Insulin resistance was induced after maintaining the mice on the VHF diet for 14 weeks. The berry extracts were administered to the mice orally following a 4 hr food restriction. Both the ARF and PRE preparations from VU or EN effectively lowered blood glucose levels in the hyperglycemic C57Bl/6J mice (Figure 11). The PRE extract exhibited greater hypoglycemic activity (45% decrease in blood glucose level) relative to the ARF extract from V. uliginosum (23% decrease in blood glucose level). The VU-PH PRE extract showed greater absolute hypoglycemic activity than the drug metformin (24% decrease in blood glucose level), which was included as a positive control. The animals treated with the water vehicle only showed a slight increase in blood glucose concentrations.
Figure11.
Glucose levels in dietary-induced obese C57BL/6 mice at 3 and 6 h post-treatment. The experiment was repeated with similar results, figure represents mean response ± SE. Abbreviations: VU-PH=Vaccinium uliginosum from Point Hope, EN-PH= Empetrum nigrum from Point Hope, PRE=phenolic-rich extract, ARF=anthocyanin-rich fraction. Asterisks denote significant activity, ** p≤0.01 vs initial, *** p≤0.001 vs initial; letters designate significantly different treatment means at α=0.01 (n=5).
Discussion
Wild Alaskan berry species continue to play a strong role in traditional tribal culture and diet, recognized by AN tribes as a contributing factor towards a healthy lifestyle. Not only are tribal members aware of the health contribution of the berries within the diet, but berry harvesting also provides a constructive means of exercise, which has been shown to help lower incidence rates of T2DM (74). The positive results from the STN assays conducted in the tribal communities gave first-hand scientific evidence as to the health properties of the berries, thereby providing a validation of the traditional perceptions of these fruits. They also generated additional interest in the berries as a part of tradition and source of nutrition, especially with the younger members participating in the berry screening; one high school student commented that “learning if our berries were healthy” was the most interesting part of the STN project.
Wild Alaskan berries contain a complex mixture of bioactive flavonoids, including anthocyanins and proanthocyanidins. The presence of such chemicals may play an important role in health promotion and metabolic disease prevention, including T2DM and obesity. The anthocyanin profile of the berries revealed a varied number of potentially beneficial anthocyanin compounds. All berries tested were found to possess A-type proanthocyanidin dimers, trimers, and/or tetramers. However, the phytochemical content and characterization was different for each berry, dependant on both species and biogeographic location. The berries from Point Hope yielded the highest concentrations of anthocyanins (EN-PH) and proanthocyanidins (VU-PH); concentrations larger than other circumpolar berries (62, 65). Point Hope is the site with the most extreme annual climate, and thus the severity of the environment could be a contributing factor to the overall composition of these particular fruits. However, additional years of collection are needed to analyze year-to-year variation in these adaptive chemicals and better correlate these values with climate metrics such as precipitation levels and type, temperature, and sunlight availability.
Adipocyte differentiation is controlled by multiple feedbacks (75-77), one of which is the negative control exerted by preadipocyte factor-1 (pref-1) (78). Levels of pref-1 remain high in immature adipocyte cells, decreasing to nearly zero as differentiation begins. Mice with decreased levels of pref-1 demonstrated increased adipose tissue formation (79). While numerous studies have demonstrated that various proteins and small organic compounds actively inhibit adipogenesis, only one study to date (on grape-seed procyanidins with varying degrees of polymerization) has investigated the effect of flavonoids on pref-1 expression levels (80). In the current study, the mixture of phytochemical compounds in the phenolic-rich extract of R. spectabilis increased pref-1 expression. Activity was lost when the extract was fractionated and component phytochemicals were administered separately. This result suggests that interactions between phytochemicals in the mixed PRE extract are required to potentiate biological activity. Both Rubus berries, which possessed the highest pref-1 activity, contained the lowest levels of anthocyanins and proanthocyanidins. This could indicate that minor compounds not quantified in this study are responsible for pref-1 activity. For example, Rubus fruits are known to have incredibly high levels of ellagic acid conjugates such as ellagitannins (81, 82).
Lipid accumulation is one mechanism of energy balance and regulation within adipocytes (75). Excess caloric intake can lead to a metabolic overload of adipocyte tissue, forcing adipocytes to enlarge to accommodate the larger influx of triglycerides. Prolonged enlargement and hypertrophy develops within the adipocytes, initiating a chronic inflammatory condition. This adipocyte dysfunction results in a disruption of the dynamic equilibrium of triglyceride metabolism within the cells, thereby causing obesity and insulin resistance (83). Multiple phenolic-rich extracts of Alaskan berries were able to decrease the lipid levels within mature adipocytes, but this activity was not conserved across all locations or species. Proanthocyanidin-enriched fractions were the preparations that most actively decreased lipid accumulation. The effect was reinforced by the linearity of the response between proanthocyanidin content of the enriched fractions and the lipid accumulation observed in the treated cells. Proanthocyanidins, especially procyanidins, from apples and grape seed have demonstrated similar activity against tissue lipid absorption in both cellular (84, 85) and organismal (38, 39) models.
In addition, both V. uliginosum and E. nigrum preparations from Point Hope (VU-PH and EN-PH) exhibited hypoglycemic activity in the acute in vivo T2DM model. These two samples were chosen as they represented the highest levels of the two most intensively investigated phenols: anthocyanins (EN-PH) and proanthocyanidins (VU-PH). Both samples possessed activity comparable to metformin, the anti-diabetic drug used as the positive control for the study. Both the PRE and ARF extracts of these hypoglycemic berries showed similar activities despite the dramatic differences in their phytochemical content. The robust hypoglycemic activity observed from treatments of Alaskan berry preparations was also observed when presented to animals with a vehicle of water. In previous work with comparable lowbush blueberry (V. angustifolium) extracts, hypoglycemic activity was pronounced only when provided in a formulation containing Labrasol®, an emulsifing agent that enhances absorption in the gastrointestinal tract (56).
The current study investigated the diverse phytochemical composition of wild Alaskan berries, with a focus on the identification of anthocyanin and proanthocyanidin compounds. The berry extracts demonstrated the ability to influence specific cellular targets related to metabolic disorders, with clear differences between the various fractions used in each of the assays. The two berries studied in the acute in vivo T2DM model exhibited significant hypoglycemic activity. This evidence exhibiting the hypoglycemic activity of wild Alaskan berries is particularly relevant in light of the high incidence of T2DM in Alaska Native populations. More detailed studies, including activity-based fractionation of the extracts, mechanistic studies in animals and investigations into the bioavailability and metabolism of the active compounds will further validate this biological activity.
Local observations support the hypothesis that climate fluctuations affect both berry quantity and quality, and thus the impacts of continued climate change on these berries and their bioactive health properties could be significant. Negative impacts to wild Alaskan berries could be possible if winter precipitation decreases or if summer temperatures substantially increase and affect the sun/precipitation balance. However, changing berry abundance and quality from year to year was considered a longstanding pattern. The Arctic's growing fluctuations in meteorological, hydrological, and biological processes (86) cast an uncertain pall questioning the continued availability and potency of the berry populations, and their implications for community health.
Acknowledgements
We are grateful to the Alaskan communities of Akutan, Point Hope, and Seldovia for their assistance in collecting and screening all the berries used in this study and in conducting and participating in interviews and surveys. We are thankful for the expertise and guidance of Drs. Gad Yousef and Mary Grace in HPLC and LC-MS2 protocols and data processing, and to Elisa Schreckinger and Dr. Cristina Martinez-Villaluenga for their technical help in the cell culture and in vitro assays. This research is supported by the Environmental Protection Agency's Science to Achieve Results (STAR) Program (EPA R833707). Some additional resources were provided through the National Center for Complementary and Alternative Medicine sponsored Purdue-University of Alabama-Birmingham Botanicals Center for Dietary Supplement Research (NIH, 2 P50 AT000477-06) and the NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome (NIH, 1-P50 AT002776-01).
References
- 1.Centers for Disease Control & Prevention Diabetes Data & Trends September 8; http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm.
- 2.National Center for Health Statistics, Health, United States, 2008 . In Centers for Disease Control. Hyattsville, MD: 2009. [Google Scholar]
- 3.American Diabetes Asociation Total prevalence of diabetes and pre-diabetes September 8; http://www.diabetes.org/diabetes-statistics/prevalence.jsp.
- 4.Story M, Strauss K, Gilbert TJ, Brousard BA. Nutritional health and diet-related conditions. In: Rhoades ER, editor. American Indian Health. The John Hopkins University Press; Baltimore and London: 2000. pp. 201–220. [Google Scholar]
- 5.Mendlein JM, Freedman DS, Peter DG, Allen B, Percy CA, Ballew C, Mokdad AH, White LL. Risk fctors for coronary heart disease among Navajo Indians. J. Nutr. 1997;127:2099S. doi: 10.1093/jn/127.10.2099S. [DOI] [PubMed] [Google Scholar]
- 6.Yurgalevitch SM, Kriska AM, Welty TK, Go O, Robbins DC, Howard BV. Physical activity and lipids and lipoproteins in American Indians ages 45-74. Med. Sci. Sports Ex. 1998;30:543–549. doi: 10.1097/00005768-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Acton KJ, Burrows NR, Geiss LS, Thompson T. Diabetes prevalence among American Indians and Alaska Natives and the overall population--United States, 1994-2002. MMWR Weekly. 2003;52:702–704. [PubMed] [Google Scholar]
- 8.Gahagan S, Silverstein J. Prevention and treatment of type 2 diabetes mellitus in childre, with special emphasis on American Indian and Alaska Native children. Pediatrics. 2003;112:e328–e347. doi: 10.1542/peds.112.4.e328. [DOI] [PubMed] [Google Scholar]
- 9.Murphy NJ, Schraer CD, Thiele MC, Boyko EJ, Bulkow LR, Doty BJ, Lanier AP. Dietary change and obesity associated with glucose intolerance in Alaska Natives. J. Am. Diet. Assoc. 1995;95:676–682. doi: 10.1016/S0002-8223(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 10.Moerman DE. Native American Ethnobotany. Timber Press Inc.; Portland, OR: 1998. p. 927. [Google Scholar]
- 11.Heller CA. Edible and Poisonous Plants of Alaska. Cooperative Agriculture Extension Service; College, AK: 1953. [Google Scholar]
- 12.Viereck EG. Alaska's Wilderness Medicines: Healthful Plants of the Far North. Alaska Northwest Books; Anchorage, AKs: 2007. [Google Scholar]
- 13.Kari PR. Tanaina Plantlore Dena'ina K'et'una. An ethnobotany of the Dena'ina Indians of Southcentral Alaska. Alaska Native Language Center; National Park Service: 1995. p. 205. [Google Scholar]
- 14.Jones A. Nauriat Niginaqtuat = Plants That We Eat. Maniilaq Association Traditional Nutritional Program; Kotzebue: 1983. [Google Scholar]
- 15.Bersamin A, Luick BR, Ruppert E, Stern JS, Zidenberg-Cherr S. Diet quality among Yup'ik Eskimos living in rural communities Is low: The Center for Alaska Native Health Research Pilot Study. J. Am. Diet. Assoc. 2006;106:1055–1063. doi: 10.1016/j.jada.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Al-Awwadi NA, Araiz C, Bornet A, Delbosc S, Cristol JP, Linck N, Away J, Teissedres PL, Gros G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005;53:151–157. doi: 10.1021/jf048919f. [DOI] [PubMed] [Google Scholar]
- 17.Neto CC. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Molecular Nutritional & Food Research. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 18.Dulebohn RV, Weiguang Y, Srivastava A, Akoh CC, Krewer G, Fischer JG. Effects of blueberry (Vaccinium ashei) on DNA damage, lipid peroxidation, and phase II enzyme activities in rats. J. Agric. Food Chem. 2008;56:11700–11706. doi: 10.1021/jf802405y. [DOI] [PubMed] [Google Scholar]
- 19.Schroeter H, Boyd C, Spencer JPE, Williams RJ, Cadenas E, Rice-Evans C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging. 2002;23:861–880. doi: 10.1016/s0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Lee HK, Kim CY, Hong YJ, Choe CM, You TW, Seong GJ. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Brit. J. Nutr. 2005;93:895–899. doi: 10.1079/bjn20051438. [DOI] [PubMed] [Google Scholar]
- 21.Kraft TFB, Schmidt BM, Yousef GG, Knight CT, Cuendet M, Kang Y-H, Pezzuto JM, Siegler DS, Lila MA. Chemopreventive potential of wild lowbush blueberry fruits in multiple stages of carcinogenesis. J. Food Sci. 2005;70:S159–S166. [Google Scholar]
- 22.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 23.Foo LY, Lu Y, Howell AB, Vorsa N. A-Type Proanthocyanidin Trimers from Cranberry that Inhibit Adherence of Uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 24.Howell A, Reed JD, Krueger CG, Winterbottom R, Cunningham D, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt BM, Howell AB, McEniry B, Knight CT, Seigler D, Erdman JWJ, Lila MA. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium ait.) fruits. J. Agric. Food Chem. 2004;52:6433–6442. doi: 10.1021/jf049238n. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 27.Hill JO, Peters JC. Biomarkers and functional foods for obesity and diabetes. Brit. J. Nutr. 2002;88:S213–S218. doi: 10.1079/BJN2002685. [DOI] [PubMed] [Google Scholar]
- 28.Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Cory H, Meddah B, Leduc C, Burt A, Vuong T, Le PM, Prentki M, Bennett SA, Arnason JT, Haddad PS. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium antgustifolium Ait. Phytomedicine. 2006;13:612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Jouad H, Maghrani M, Eddouks M. Hypoglycaemic effect of Rubus fructicosis L. and Globularia alypum L. in normal and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2002;81:351–356. doi: 10.1016/s0378-8741(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 30.Leduc C, Coonishish J, Haddad PS, Cuerrier A. Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: A novel approach in quantitative ethnobotany. J. Ethnopharmacol. 2006;105:55–63. doi: 10.1016/j.jep.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Frohne D. Heidelbeerblätter. In: Wichtl M, editor. Teedrogen. Wissenshaftliche Verlagsgesell; Berlin: 1990. pp. 217–219. [Google Scholar]
- 32.McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit a-amylase and a-glucosidase. J. Agric. Food Chem. 2005;53:2760–2766. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- 33.Vuong T, Martineau LC, Ramassamy C, Matar C, Haddad PS. Fermented Canadian lowbush blueberry juice stimulates glucose uptake and AMP-activated protein kinase in insulin-sensitive cultured muscle cells and adipocytes. Can. J. Physiol. Pharmacol. 2007;85:956–965. doi: 10.1139/Y07-090. [DOI] [PubMed] [Google Scholar]
- 34.Tsuda T, Ueno Y, Kojo H, Yoshikawa T, Osawa T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochemica et Biophysica Acta. 2005;1733:137–147. doi: 10.1016/j.bbalip.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Tsuda T, Ueno Y, Aoki H, Koda T, Horio F, Takahashi N, Kawada T, Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Bioph. Res. Co. 2004;316:149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 36.McDougall GJ, Kulkarni NN, Stewart D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009;115:193–199. [Google Scholar]
- 37.Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, Ohtake Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J. Agric. Food Chem. 2007;55:4604–4609. doi: 10.1021/jf070569k. [DOI] [PubMed] [Google Scholar]
- 38.Zern TL, West KL, Fernandez ML. Grape polyphenols decrease plamsa triglycerides and cholesterol accumulation in tha aorta of ovariectomized guinea pigs. J. Nutr. 2003;133:2268–2272. doi: 10.1093/jn/133.7.2268. [DOI] [PubMed] [Google Scholar]
- 39.Tebib K, Besançon P, Rouanet J-M. Dietary grape seed tannins affect lipoproteins, lipoprotein lipases, and tissue lipids in rats fed hypercholesterolemic diets. J. Nutr. 1994;124:2451–2457. doi: 10.1093/jn/124.12.451. [DOI] [PubMed] [Google Scholar]
- 40.Thole JM, Kraft TFB, Suiero L, Kang YH, Gills JJ, Cuendet M, Pezzuto JM, Seigler D, Lila MA. A comparative evaluation of the anticancer properties of European and American elderberry fruits. J. Med. Food. 2006;9:498–504. doi: 10.1089/jmf.2006.9.498. [DOI] [PubMed] [Google Scholar]
- 41.Sueiro L, Yousef GG, Siegler D, de Mejia EG, Grace MH, Lila MA. Chemopreventive potential of flavonoid extracts from plantation-bred and wild Aronia melanocarpa (black chokeberry) fruits. J. Food Sci. 2006;71:480–488. [Google Scholar]
- 42.Lila MA. The nature-versus-nurture debate on bioactive phytochemicals: the genome versus terroir. J. Sci. Food Agric. 2006;86:2510–2515. [Google Scholar]
- 43.Reyes-Carmona J, Yousef GG, Martinez-Peniche RA, Lila MA. Antioxidant capacity of fruit extracts of blackberry (Rubus sp.) produced in different climatic regions. J. Food Sci. 2005;70:S497–S503. [Google Scholar]
- 44.Martel MC, Margolis HA, Coursolle C, Bigras FJ, Heinsch FA, Running SW. Decreasing photosynthesis at different spatial scales during the late growing season on a boreal cutover. Tree Physiol. 2005;25:689–699. doi: 10.1093/treephys/25.6.689. [DOI] [PubMed] [Google Scholar]
- 45.Panta GR, Rieger MW, Rowland LJ. Effect of cold and drought stress on blueberry dehydrin accumulation. J. Hortic. Sci. Biotech. 2007;76:549–556. [Google Scholar]
- 46.Hannemann N, McGinley M. Arctic. May 17th; http://www.eoearth.org/article/Arctic.
- 47.Serreze MC, Walsh JE, Chapin FS, III, Osterkamp T, Dyurgerov M, Romanovsky V, Oechel WC, Morison J, Zhang T, Barry RG. Observational evidence of recent change in the northern high-lattitude environment. Climatic Change. 2000;46:159–207. [Google Scholar]
- 48.Keyser AR, Kimball JS, Nemani RR, Running SW. Simulating the effects of climate change on the carbon balance of North American high-latitude forests. Glob. Change Biol. 2000;6:185–195. doi: 10.1046/j.1365-2486.2000.06020.x. [DOI] [PubMed] [Google Scholar]
- 49.Simpson JJ, Hufford GL, Fleming MD, Berg JS, Ashton JB. Long-term climate patterns in Alaskan surface temperature and preceipitation and their biological consequences. IEEE T. Geosci. Remote. 2002;40:1164–1184. [Google Scholar]
- 50.Sturm M, Racine C, Tape K. Increasing shrub abundance in the Arctic. Nature. 2001;411:546–547. doi: 10.1038/35079180. [DOI] [PubMed] [Google Scholar]
- 51.Van Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, III, Cornelissen JHC, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen A, Press MC, Richardson SJ, Rueth H. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Glob. Change Biol. 2003;10:105–123. [Google Scholar]
- 52.Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J. Agric. Food Chem. 2008;56:642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- 53.Alaska Department of Commerce, C., and Economic Development Alaska Community Database: Community Information Summaries. May 29; http://www.commerce.state.ak.us/dca/commdb/CF_CIS.htm.
- 54.National Geographic. Fund WW. Wild World Terrestrial Ecoregions of the World. May 27; http://www.nationalgeographic.com/wildworld/terrestrial.html.
- 55.Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007;53:287–292. doi: 10.3177/jnsv.53.287. [DOI] [PubMed] [Google Scholar]
- 56.Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Villaluenga C, Bringe NA, Berhow MA, De Mejia EG. b-Conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro. J. Agric. Food Chem. 2008;56:10533–10543. doi: 10.1021/jf802216b. [DOI] [PubMed] [Google Scholar]
- 58.Wu X, Prior RL. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 59.Lee J, Finn CE, Wrolstad RE. Anthocyanin pigment and total phenolic content of three Vaccinium species native to the Pacific Northwest of North America. Hortscience. 2004;39:959–964. doi: 10.1021/jf049108e. [DOI] [PubMed] [Google Scholar]
- 60.Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 61.Taruscio TG, Barney DL, Exon J. Content and profile of flavonoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of Northwest Vaccinium berries. J. Agric. Food Chem. 2004;52:3169–3176. doi: 10.1021/jf0307595. [DOI] [PubMed] [Google Scholar]
- 62.Määttä-Riihinen K,R, Kamal-Eldin A, Mattila PH, González-Paramás AM, Törrönen AR. Distribution and contents of phenolic compounds in eighteen Scandanavian berry species. J. Agric. Food Chem. 2004;52:4477–4486. doi: 10.1021/jf049595y. [DOI] [PubMed] [Google Scholar]
- 63.Andersen ØM. Anthocyanins in fruits of Vaccinium uliginosum L. (bog whortleberry) J. Food Sci. 1987;52:665–666. [Google Scholar]
- 64.Li Y, Meng F, Zheng Y. Study of anthocyanins in fruit of different Vaccinium genotypes. Acta. Hortic. 2006;715:589–594. [Google Scholar]
- 65.Ogawa K, Sakakibara K, Iwata R, Ishii T, Sato T, Goda T, Shimoi K, Kumazawa S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008;56:4457–4462. doi: 10.1021/jf800406v. [DOI] [PubMed] [Google Scholar]
- 66.Kallio H, Pallasaho S, Kärppä J, Linko RR. Comparison of the half-lives of the anthocyanins in the juice of crowberry, Empetrum nigrum. J. Food Sci. 1986;51:408–410. [Google Scholar]
- 67.Kärppä J, Kallio H, Petltonen I, Linko RR. Anthocyanins of crowberry, Empetrum nigrum coll. J. Food Sci. 1984;49:634–636. [Google Scholar]
- 68.Jennings DL, Carmichael E. Anthocyanin variation in the genus Rubus. New Phytol. 1979;84:505–513. [Google Scholar]
- 69.Prior RL, Gu L. Occurrence and biological significance of proanthocyanins in the American diet. Phytochemistry. 2005;66:2264–2280. doi: 10.1016/j.phytochem.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 70.Gu L, Kelm MA, Hammerstone JF, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 71.Li H-J, Deinzer ML. The mass spectral analysis of isolated hops A-type proanthocyanidins by electrospray ionization tandem mass spectrometry. J. Mass. Spectrom. 2008;43:1353–1363. doi: 10.1002/jms.1411. [DOI] [PubMed] [Google Scholar]
- 72.Määttä-Riihinen K,R, Kähkönen MP, Törrönen AR, Heinonen M. Catechins and procyanidins in berries of Vaccinium species and their antioxidant activity. J. Agric. Food Chem. 2005;53:8485–8491. doi: 10.1021/jf050408l. [DOI] [PubMed] [Google Scholar]
- 73.Puupponen-Pimiä R, Nohynek L, Hartmann-Schmidlin S, Kähkönen M, Heinonen M, Määttä-Riihinen K, Oksman-Caldentey K-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005;98:991–1000. doi: 10.1111/j.1365-2672.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 74.Laaksonen DE, Linström J, Lakka TA, Eriksson JG, Niskanen L, Wikström K, Aunola S, Keinänen-Kiukaanniemi S, Laakso M, Valle TT, Ilanne-Parikka P, Louheranta A, Hämäläinen H, Rastas M, Salminen V, Cepaitis Z, Hakumäki M, Kaikkonen H, Härkönen P, Sundvall J, Tuomilehto J, Uusitupa M. Physical activity in the prevention of type 2 diabetes: The Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 75.Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue and Cell. 2005;37:335–338. doi: 10.1016/j.tice.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Nakae J, Kitamura T, Kitamura Y, Biggs WHI, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 77.Ntambi JM, Kim Y-C. Adipocyte differentiation and gene expression. J. Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 78.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 79.Moon YS, Smas CM, Lee K, Villena JA, Kim K-H, Yun EJ, Sul HS. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinent M, Bladé MC, Salvado MJ, Arola L, Hackl H, Quackenbush J, Trajanoski Z, Ardévol A. Grape-seed derived procyanidins interfere with adipogenesis of 3T3-L1 cells at the onset of differentiation. Int. J. Obesity. 2005;29:934–941. doi: 10.1038/sj.ijo.0802988. [DOI] [PubMed] [Google Scholar]
- 81.Vasco C, Riihinen K,R, Ruales J, Kamal-Eldin A. Phenolic compounds in Rosaceae fruits from Ecuador. J. Agric. Food Chem. 2009;57:1204–1212. doi: 10.1021/jf802656r. [DOI] [PubMed] [Google Scholar]
- 82.Mullen W, Yokota T, Lean MEJ, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 83.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Bas JM, Ricketts ML, Vaque M, Sala E, Quesada H, Ardevol A, Salvado MJ, Blay M, Arola L, Moore DD, Pujadas G, Fernandez-Larrea J, Blade C. Dietary procyanidins enhance transcriptional activity of bile acid-activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol. Nutr. Food Res. 2009;53:805–814. doi: 10.1002/mnfr.200800364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pinent M, Bladé MC, Salvado MJ, Arola L, Ardévol A. Intracellular mediators of procyanidin-induced lipolysis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2005;53:262–266. doi: 10.1021/jf048947y. [DOI] [PubMed] [Google Scholar]
- 86.Hinzman LD, Bettez ND, Bolton WR, Chapin FS, Dyurgerov MB, Fastie CL, Friffith B, Hollister RD, Hope A, Huntington HP, Jensen AM, Jia GJ, Jorgenson T, Kane DL, Klein DR, Kofinas G, Lynch AH, Lloyd AH, McGuire AD, Nelson FE, Oechel WC, Osterkamp TE, Racine CH, Romanovsky VE, Stone RS, Stow DA, Strurm M, Tweedie CE, Vourlitis GL, Walker MD, Walker DA, Webber PJ, Welker JM, Winkler KS, Yoshikawa K. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climactic Change. 2005;72:251–298. [Google Scholar]