Abstract
Patients on systemic glucocorticoids for graft-versus-host disease (GVHD) after hematopoietic cell transplant are susceptible to invasive fungal infections (IFI), which greatly contribute to morbidity and mortality. We evaluated the efficacy of prophylactic treatment options (voriconazole versus fluconazole or itraconazole) for IFI by performing a retrospective review of patients on glucocorticoids for GVHD given voriconazole (n = 97), fluconazole (n = 36), or itraconazole (n = 36). IFI developed in 7/72 (10%) patients on fluconazole/itraconazole versus 2/97 (2%) on voriconazole (P = 0.03) within the first 100 days of glucocorticoids. Five patients developed Aspergillus IFI on fluconazole/itraconazole (7%), compared to none on voriconazole (0%) (P = 0.008); Aspergillus IFI resulted in death in all 5 patients. We found that IFI occurred in patients who received an initial dose of at least 2 mg/kg/day of prednisone or equivalent; when the analysis was restricted to these patients, the hazard ratio (0.39; 95% confidence interval: 0.08–1.86) was consistent with a protective effect of voriconazole compared with fluconazole/itraconazole, although this subset analysis did not reach significance. Overall survival at 100 days after start of glucocorticoids was 77% in patients given fluconazole/itraconazole and 85% in those given voriconazole (P = 0.22). Our results suggest that voriconazole is more effective than fluconazole/itraconazole in preventing IFI, especially aspergillosis, in patients receiving glucocorticoids posttransplant.
Keywords: voriconazole, hematopoietic cell transplant, invasive fungal infections, graft-versus-host disease
INTRODUCTION
Recipients of allogeneic hematopoietic cell transplants (HCT) are susceptible to invasive fungal infections (IFI). Mucosal injury, prolonged neutropenia, impaired cell-mediated immunity, and graft-versus-host disease (GVHD) are established risk factors for IFI.1, 2 The incidence of IFI after allogeneic HCT ranges from 19% to 32%.3–5 Because of the significant risk of IFI after allogeneic HCT, several antifungal agents have been studied in the prophylactic setting. Fluconazole prophylaxis reduced the incidence of IFI and improved survival in patients who had allogeneic HCT, as reported in prospective randomized studies.6–9 In other randomized studies, itraconazole failed to show protection from IFI, and many patients discontinued itraconazole early due to poor tolerance.10, 11
Prophylactic strategies have been studied specifically in patients treated with glucocorticoids for GVHD. In one prospective randomized investigation, Ullmann et al12 reported non-inferiority of posaconazole compared to fluconazole. Posaconazole was also superior in preventing invasive aspergillosis and reducing mortality from IFI. However, posaconazole is available only as an oral formulation, and the bioavailability is variable based on whether it is administered in a fasting state. A concern in patients with GVHD is whether oral absorption is compromised due to poor oral intake, vomiting, diarrhea, or changes in intestinal mucosa secondary to GVHD.
Voriconazole is available in both oral and intravenous formulations.13–15 Voriconazole is well-tolerated, with more predictable oral bioavailability. After FDA approval in 2002, we began using voriconazole as standard antifungal prophylaxis in patients receiving glucocorticoid therapy for GVHD, replacing fluconazole or itraconazole in this setting. This change was made because of the superior activity of voriconazole against aspergillus compared to fluconazole, as well as the improved tolerability relative to itraconazole. To evaluate the relative efficacy of voriconazole, we performed a single-center retrospective review and recorded the development of IFI in patients receiving glucocorticoid therapy for GVHD along with antifungal prophylaxis using either voriconazole, itraconazole, or fluconazole.
PATIENTS AND METHODS
This study and corresponding waiver of informed consent were approved by the University of South Florida Institutional Review Board.
Records of consecutive patients who received allogeneic HCT at the Moffitt Cancer Center from 6/1/1996 to 12/31/2006 were reviewed. Patients were included if they received fluconazole beginning the day of transplant, developed GVHD, and were treated with at least 1 mg/kg/day of prednisone (or equivalent). Patients were excluded if they were on systemic antifungal therapies other than fluconazole immediately before glucocorticoid initiation or if they had a history of IFI. Patients were divided into two groups based on antifungal prophylaxis given at the time of glucocorticoid initiation: those receiving either fluconazole (400 mg daily orally or intravenously) or itraconazole (200 mg twice daily orally or 200 mg daily intravenously) or those receiving voriconazole (200 mg twice daily orally or intravenously). For patients who received subsequent infusions of donor cells (including donor lymphocyte infusions and second transplants), only the first transplant period was included for analysis. Data were censored at the time of second donor cell infusion and at the time of relapse.
The predefined primary study endpoint was the proportion of proven or probable IFI that occurred within 100 days from initiation of glucocorticoid therapy. A study period of 100 days was chosen to ensure consistent follow-up, as many of our patients return to local health care providers after that period. Proven or probable IFI was defined per European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria.16 Secondary endpoints were cumulative incidence of IFI (with relapse or death due to other causes considered competing risks), IFI-related mortality, and overall survival at 100 days after initiation of glucocorticoids.
We hypothesized that prophylaxis with voriconazole would reduce the incidence of IFI from 20% to 5%. Using a two-sided test of equal proportions with alpha = 0.05, we required a sample size of 76 patients in each group to have a power of 80%. To assess statistical significance of the differences in patient characteristics, the exact unconditional test was used for 2 × 2 tables; for larger contingency tables, the exact Fisher exact test was used. Ninety-five percent confidence intervals for differences in various proportions were tested by the unconditional test, and all calculations were performed using StatXact version 7 (Cytel, Cambridge, MA). Probabilities for overall survival and IFI-free survival were calculated using Kaplan-Meier estimation. Survival curves were compared using the log-rank test, and Cox regression was used for multivariate analysis. Proportional hazard regression modeling by Fine and Gray was used in the univariate analyses. Cumulative incidence curves were generated based on the life-table method17 and compared using the method of Gray.18
RESULTS
Four hundred and forty-eight patients received allogeneic HCT at the Moffitt Cancer Center from 7/1/1996 to 12/31/2006. Of these, 169 patients were eligible for this study (Table 1). Seventy-two patients received either fluconazole (n = 36) or itraconazole (n = 36), and 97 patients received voriconazole. Patients on voriconazole were older, more likely to have received peripheral blood hematopoietic cells from unrelated donors, and were treated more recently with fludarabine-based conditioning regimens. The initial glucocorticoid dose was lower in the patients who received voriconazole, but there was no difference in the initial grade of GVHD between the two groups.
Table 1.
Characteristics of patients in the study
| Characteristic | Fluconazole/Itraconazole Group | Voriconazole Group | P |
|---|---|---|---|
| Number of patients | 72 | 97 | |
| Sex | 0.9 | ||
| Male | 40 (56%) | 53 (55%) | |
| Female | 32 (44%) | 44 (45%) | |
| Age | 0.008 | ||
| <45 years | 43 (60%) | 37 (38%) | |
| ≥45 years | 29 (40%) | 60 (62%) | |
| Date of transplant | 7/96 to 4/02 | 5/02 to 12/06 | |
| Diagnosis | 0.046 | ||
| Acute lymphoblastic leukemia | 8 (11%) | 9 (9%) | |
| Acute myelogenous leukemia | 21 (29%) | 35 (36%) | |
| Chronic myelogenous leukemia | 20 (28%) | 9 (9%) | |
| MDS/MF/MPD | 7 (10%) | 14 (15%) | |
| Multiple myeloma | 4 (6%) | 4 (4%) | |
| NHL/HD/CLL | 11 (15%) | 25 (26%) | |
| Aplastic anemia | 1 (1%) | 1 (1%) | |
| Disease risk per CIBMTR criteria | 0.19 | ||
| Low | 30 (42%) | 30 (31%) | |
| Intermediate | 13 (18%) | 30 (31%) | |
| High | 28 (39%) | 36 (37%) | |
| Not classified | 1 (1%) | 1 (1%) | |
| Conditioning Regimen | <0.0001 | ||
| Cyclophosphamide or TBI-based myeloablative | 70 (97%) | 19 (20%) | |
| Fludarabine-based reduced intensity | 2 (3%) | 78 (80%) | |
| Donor source | 0.0003 | ||
| Matched related | 46 (64%) | 38 (39%) | |
| Mismatched related | 5 (7%) | 1 (1%) | |
| Matched unrelated | 18 (25%) | 45 (46%) | |
| Mismatched unrelated | 3 (4%) | 13 (14%) | |
| Hematopoietic cell source | <0.0001 | ||
| Bone marrow | 52 (72%) | 3 (3%) | |
| T cell-depleted bone marrow | 5 (7%) | 0 (0%) | |
| Peripheral blood | 15 (21%) | 94 (97%) | |
| Absolute neutrophil count at start of glucocorticoid therapy, count/mm3 | 0.39 | ||
| <2500 | 30 (42%) | 47 (48%) | |
| >2500 | 42 (58%) | 50 (52%) | |
| Initial GVHD grade | 0.41 | ||
| 1–2 | 54 (75%) | 67 (69%) | |
| 3–4 | 18 (25%) | 30 (31%) | |
| Initial daily dose of glucocorticoids | <0.0001 | ||
| 1 mg/kg | 27 (38%) | 66 (68%) | |
| 2 mg/kg | 45 (62%) | 31 (32%) |
MDS/MF/MPD, myelodysplastic syndrome/myelofibrosis/myeloproliferative disease; NHL/HD/CLL, Non-Hodgkin/Hodgkin/chronic lymphocytic leukemia; CIBMTR, Center for International Blood and Marrow Transplant Research; TBI, total body irradiation; GVHD, graft-versus-host disease.
Proven or probable IFI developed in 7 of 72 patients (10%) on fluconazole/itraconazole versus 2 of 97 (2%) on voriconazole (unconditional test P =0.03; 95% confidence interval for the difference in proportions: 1% to 17%). All infections occurred while the patients were still receiving the designated antifungal prophylactic medication. The cumulative incidence of IFI from start of glucocorticoid therapy is shown in Figure 1; at 100 days after start of glucocorticoid therapy, the cumulative incidence was 10% for the fluconazole/itraconazole group and 2% for the voriconazole group (P = 0.029). Two patients on fluconazole and three patients on itraconazole developed Aspergillus IFI (7%): 4 with pneumonia (A. flavus, A. terreus, A. fungoides and 1 not speciated) and 1 with skin abscess (A. ustus). However, no patients on voriconazole developed Aspergillus IFI (P=0.008; confidence interval for the difference in proportions: 3% to 16%). Two patients (3%) developed candidemia (Candida krusei and C. glabrata) on fluconazole, and 2 patients (2%) developed candidemia (C. glabrata) on voriconazole (P = 0.83; confidence interval for the difference in proportions:–5% to 8%). No patient developed an infection due to Zygomycoses within the study period. Treatment for IFI was chosen by the individual treating physician, and this information was not collected as part of this study.
Figure 1.
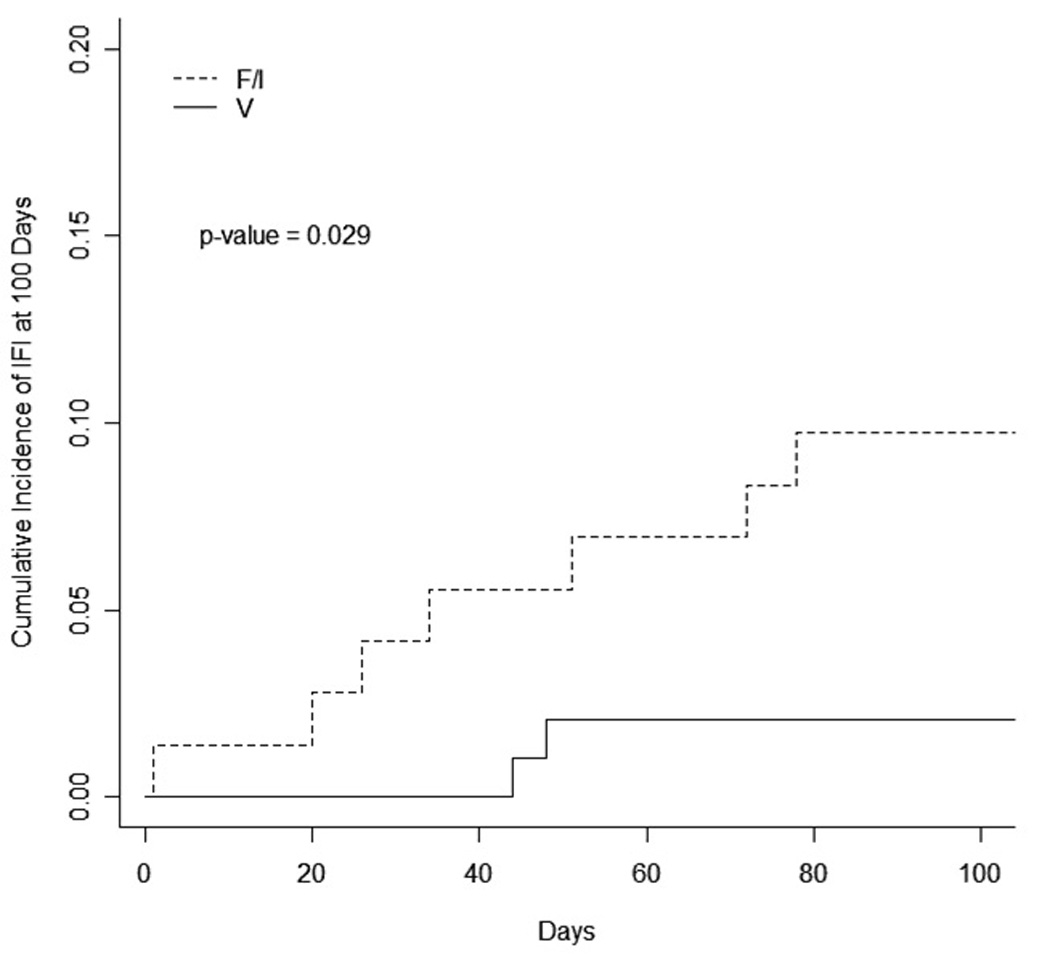
Cumulative incidence of invasive fungal infection (IFI) from start of glucocorticoid therapy in patients treated with fluconazole or itraconazole (F/I; n = 72) compared to patients treated with voriconazole (V; n = 97).
Mortality from IFI within 100 days of the start of glucocorticoid therapy was 5 of 72 (7%) on fluconazole/itraconazole and 0 of 97 (0%) on voriconazole (P = 0.008; confidence interval for the difference in proportions: 3% to 16%). All 5 patients who developed aspergillosis died as a result of the infection. Survival without IFI at 100 days after start of glucocorticoid therapy was 75% in the fluconazole/itraconazole group and 85% in the voriconazole group (P = 0.10). Overall survival at 100 days after start of glucocorticoid therapy was 77% for patients on fluconazole/itraconazole and 85% for patients on voriconazole (P = 0.22).
All occurrences of IFI were in patients who received an initial dose of glucocorticoids of ≥2 mg/kg/day. Among this subset, 7 of 45 patients (16%) on fluconazole/itraconazole developed IFI compared to 2 of 31 patients (6%) on voriconazole (P = 0.27; confidence interval for the difference in proportions: –9% to 24%). Aspergillosis occurred in 5 of 45 patients (11%) on fluconazole/itraconazole compared to 0 of 31 patients on voriconazole (P = 0.06; confidence interval for the difference in proportions: –1 to 24%). In our evaluation of the differences between characteristics in this subgroup of patients, patients on voriconazole were still more likely to have received peripheral blood (90% versus 18% in the fluconazole/itraconazole group) from unrelated donors (66% versus 29% in the fluconazole/itraconazole group). The voriconazole-treated patients were also more likely to have had more severe GVHD at initial diagnosis (data not shown). The combination of these characteristics would have put the patients at higher risk for IFI.
Table 2 shows univariate analyses of IFI for all patients, and Table 3 shows results limited to those patients who received at least 2 mg/kg/day of glucocorticoid therapy. Prophylaxis with voriconazole was significantly protective in all patients (hazard ratio: 0.2; 95% confidence interval: 0.04−0.97). There was suggestion of a similar protective effect when the analysis was restricted to those patients who received higher doses of glucocorticoids (hazard ratio: 0.39; 95% confidence interval: 0.08−1.86), but the comparison with smaller numbers of patients was not significant. The relative impact of other patient characteristics could not be assessed in a multivariate analysis due to the small number of informative cases.
Table 2.
Results of univariate analyses for all patients
| Univariate analysis |
|||
|---|---|---|---|
| HR | 95% CI | P | |
| Antifungal prophylaxis | 0.046 | ||
| Fluconazole/Itraconazole | 1 | ||
| Voriconazole | 0.20 | 0.04–0.97 | |
| Donor source | 0.06 | ||
| Related | 1 | ||
| Unrelated | 0.14 | 0.02–1.09 | |
| Age | 0.23 | ||
| < 50 | 1 | 0.03–2.22 | |
| > 50 | 0.28 | ||
| Disease risk | 0.37 | ||
| Low | 1 | ||
| Intermediate | 0.69 | 0.30–1.56 | |
| High | 0.47 | 0.09–2.43 | |
| Regimen | 0.15 | ||
| Cyclophosphamide or TBI based | 1 | ||
| Fludarabine-based reduced intensity | 0.31 | 0.06–1.51 | |
| Hematopoietic cell source | 0.06 | ||
| Bone marrow | 1 | ||
| Peripheral blood | 0.27 | 0.07–1.08 | |
CI, confidence interval; HR, hazard ratio.
Table 3.
Results of univariate analyses for patients receiving glucocorticoids of ≥2 mg/kg/day
| Univariate Analysis |
|||
|---|---|---|---|
| HR | 95% CI | P | |
| Antifungal prophylaxis | 0.24 | ||
| Fluconazole/itraconazole | 1 | ||
| Voriconazole | 0.39 | 0.08–1.86 | |
| Donor source* | 0.07 | ||
| Related | 1 | ||
| Unrelated | 0.14 | 0.02–1.13 | |
| Age | 0.58 | ||
| ≤50 years | 1 | ||
| >50 years | 0.55 | 0.07–4.47 | |
| Disease risk | 0.34 | ||
| Low | 1 | ||
| Intermediate | 0.68 | 0.30–1.50 | |
| High | 0.46 | 0.09–2.25 | |
| Regimen* | 0.79 | ||
| Cyclophosphamide or TBI based | 1 | ||
| Fludarabine-based reduced intensity | 0.81 | 0.17–3.91 | |
| Hematopoietic cell source* | 0.41 | ||
| Bone marrow | 1 | ||
| Peripheral blood | 0.56 | 0.14–2.22 | |
Voriconazole patients receiving at least 2 mg/kg/day of prednisone-equivalent dose of glucocorticoids were significantly more likely to have received peripheral blood from an unrelated donor following a fludarabine-based regimen; they were also more likely to have grade 3 or 4 GVHD at initial diagnosis
DISCUSSION
These results suggest that voriconazole is effective antifungal prophylaxis in patients receiving at least 1 mg/kg/day of glucocorticoids for treatment of GVHD. The incidence of proven or probable IFI in our series is comparable to that shown in the report by Ullmann et al in a similar population.12 In that study, the incidence of any IFI in patients receiving posaconazole was 5.3% (incidence of invasive aspergillosis = 2.3%). This compares to 2% and 0%, respectively, in our series. Our results in the comparator group (fluconazole/itraconazole) are also consistent with the findings of Ullmann et al in their fluconazole group: 9% and 7% for IFI and aspergillosis, respectively. In addition, mortality associated with IFI was also comparable to that seen by Ullmann et al. This suggests that voriconazole may be as effective as posaconazole in this setting, a hypothesis that would require a prospective evaluation in order to confirm.
A limitation of retrospective evaluations is the change in practice patterns over time. The two groups differed with respect to age, conditioning regimen intensity, donor source, hematopoietic stem cell source, and initial dose of glucocorticoids used to treat acute GVHD. These differences are reflective of the changes that have occurred in the field of hematopoietic cell transplantation with the use of less intensive conditioning, especially in older patients, peripheral blood becoming the more common source of hematopoietic cells for patients with advanced disease, the increasing availability of HLA-compatible unrelated donors, and the use of lower doses of glucocorticoids for patients with acute GVHD. Three of these factors (age, disease risk, and unrelated donor grafts) are risk factors for IFI,19–21 which would bias treatment effect in favor of the fluconazole/itraconazole group because they were younger, had lower risk disease, and were more likely to have received transplants from related donors. Novel antifungal therapies have also become available over time that could have influenced the comparison of IFI-related mortality. All of the incidences of IFI in this series occurred in patients who received an initial glucocorticoid dose of at least 2 mg/kg/day, suggesting an increased risk of IFI with higher doses of glucocorticoids. Other investigators have also shown this association.19–21 The voriconazole group was more likely to have received lower doses of glucocorticoids, which would bias the treatment effect in its favor. However, when the analysis was restricted to those patients who received at least 2 mg/kg/day of glucocorticoids, the hazard ratio (0.39; 95% confidence interval: 0.08−1.86) was consistent with a protective effect of voriconazole compared to fluconazole/itraconazole, although the subset analysis did not reach significance. The relative impact of other patient characteristics could not be assessed in a multivariate analysis due to the small number of informative cases.
Voriconazole has been compared to fluconazole as prophylaxis beginning in the immediate posttransplant setting in a large, prospective, randomized, double-blind trial.22 In that trial, the cumulative rates of proven, probable, and presumptive IFI were not statistically different in the two arms at 6 months: 10.6% in the fluconazole arm and 6.6% in the voriconazole arm. These proportions are higher than those that we observed, likely due to the inclusion of presumptive infections and longer follow up (6 months versus 100 days) in the prospective study. Microbiologically documented Aspergillus infections were significantly less frequent in the voriconazole-treated patients, as seen in our series.
In this series, more recently transplanted patients being treated with high-dose glucocorticoid therapy for GVHD appeared to be at a lower risk of developing IFI than in the past. Many factors may have contributed to this lower risk, including the use of reduced intensity conditioning and lower doses of corticosteroids. This retrospective analysis also suggested that giving voriconazole as prophylaxis in this setting had a beneficial effect compared to fluconazole or itraconazole in preventing IFI, especially aspergillosis. A randomized prospective trial would be required to confirm these results.
ACKNOWLEDGMENTS
Financial support. Pfizer, Inc. (to J.P. and U.G.); National Cancer Institute (grant 3 P30-CA7692-09 to C.A).
Potential conflicts of interest.: Consulting/speakers bureau for both Pfizer and Schering Plough and research funding from Pfizer for separate project (G.W.)
REFERENCES
- 1.Wingard JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant. 1999;5:55–68. doi: 10.1053/bbmt.1999.v5.pm10371357. [DOI] [PubMed] [Google Scholar]
- 2.Wald A, Leisenring W, van Burik J-A, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 3.Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91:986–989. [PubMed] [Google Scholar]
- 4.Hagen EA, Stern H, Porter D, Duffy K, Foley K, Luger S, et al. High rate of invasive fungal infections following nonmyeloablative allogeneic transplantation. Clin Infect Dis. 2003;36:9–15. doi: 10.1086/344906. [DOI] [PubMed] [Google Scholar]
- 5.Parody R, Martino R, Rovira M, Vazquez L, Vázquez MJ, de la Cámara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Eng J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 7.Rotstein C, Bow EJ, Laverdiere M, Ioannou S, Carr D, Moghaddam N. Randomized placebocontrolled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis. 1999;28:331–340. doi: 10.1086/515128. [DOI] [PubMed] [Google Scholar]
- 8.Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation - a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 9.Marr KA, Seidel K, Slavin M, Bowden RA, Schoch HG, Flowers ME, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–2061. [PubMed] [Google Scholar]
- 10.Winston DJ, Maziarz RT, Chandrasekar PH, Lazarus HM, Goldman M, Blumer JL, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med. 2003;138:705–713. doi: 10.7326/0003-4819-138-9-200305060-00006. [DOI] [PubMed] [Google Scholar]
- 11.Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 12.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 13.Marco F, Pfaller MA, Messer S, Jones RN. In vitro activities of voriconazole (UK-109–496) and four other antifungal agents against 394 clinical isolates of Candida sp. Antimicrob Agents Chemother. 1998;42:161–163. doi: 10.1128/aac.42.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purkins L, Wood N, Ghahramani P, Greenleigh K, Allen MJ, Kleinermans D. Pharmacokinetics and safety of voriconazole following intravenous to oral-dose escalation regimens. Antimicrob Agents Chemother. 2002;46:2546–2553. doi: 10.1128/AAC.46.8.2546-2553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarus HM, Blumer JL, Yanovich S, Schlamm H, Romero A. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J Clin Pharmacol. 2002;42:395–402. [PubMed] [Google Scholar]
- 16.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 17.Marubini E, Valsecchi MG. Analyzing survival data from clinical trials and observational studies. Chichester, United Kingdom: John Wiley & Sons Ltd; 1995. [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1140–1154. [Google Scholar]
- 19.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplantation after nonmyeloablative conditioning. Blood. 2003;102:823–833. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Gersten ID, et al. Results of a randomized, double-blind trial of fluconazole vs. voriconazole for the prevention of invasive fungal infections in 600 allogeneic blood and marrow transplant patients. Blood. 2007;110:163. doi: 10.1182/blood-2010-02-268151. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]


