Abstract
Recent data suggest that ellagitannins (ETs), a class of hydrolyzable tannins found in some fruits and nuts, may have beneficial effects against colon cancer. In the stomach and gut, ETs hydrolyze to release ellagic acid (EA) and are converted by gut microbiota to urolithin-A (UA; 3,8-dihydroxy-6H-dibenzopyran-6-one) type metabolites which may persist in the colon through enterohepatic circulation. However, little is known about the mechanisms of action of either the native compounds or their metabolites on colon carcinogenesis. Components of Wnt signaling pathways are known to play a pivotal role in human colon carcinogenesis and inappropriate activation of the signaling cascade is observed in 90% of colorectal cancers. Here we investigated the effects of UA, EA, and ET rich fruit extracts on Wnt signaling in a human 293T cell line using a luciferase reporter of canonical Wnt pathway-mediated transcriptional activation. The ET extracts were obtained from strawberry (Fragaria annassa), Jamun berry (Eugenia jambolana), and pomegranate (Punica granatum) fruit and were all standardized to phenolic content (as gallic acid equivalents, GAEs, by the Folin Ciocalteau method) and to EA content (by high performance liquid chromatography methods): strawberry=20.5% GAE, 5.0% EA; Jamun berry= 20.5% GAE, 4.2% EA; pomegranate= 55% GAE, 3.5% EA. The ET-extracts (IC50=28.0-30.0 μg/mL), EA (IC50=19.0 μg/mL; 63 μM) and UA (IC50=9.0 μg/mL; 39 μM) inhibited Wnt signaling suggesting that ET-rich foods have potential against colon carcinogenesis and that urolithins are relevant bioactive constituents in the colon.
Keywords: Colon Cancer, Ellagitannin, Urolithins, Pomegranate, Strawberry, Jamun berry
Introduction
Colon cancer is a leading cause of cancer-related deaths in Western societies contributing to 10% of all cancer deaths in the United States alone (1). The World Health Organization estimates that nearly one million people are diagnosed with colorectal cancer worldwide each year. Interestingly, the consumption of a phytochemical rich diet, including fruits and vegetables, has been correlated with a reduced risk of colon cancer incidence (2). Among foods, small fruits and berries have attracted significant attention, and the link between their bioactive components and cancer prevention has received keen scientific interest (3, 4). Compounds present in these foods which have been largely investigated for their cancer preventive properties include anthocyanins, water soluble polyphenolic pigments which impart the red, blue, and purple colors to several fruits and vegetables (4, 5). However, emerging data suggest that ellagitannins (ETs), a class of hydrolysable tannins, may also play an important role in cancer prevention (6). Dietary sources of ETs include fruits and nuts such as berries (strawberries, red raspberry, black raspberries), pomegranates, muscadine grapes, walnuts, almonds and pecans, and oak-aged beverages (for e.g. red wine and whiskey aged in ET-containing oak barrels) (7).
ETs are found naturally in foods as hexahydroxydiphenoyl-glucose esters which on hydrolysis release hexahydroxydiphenic acid which then rapidly rearranges to form ellagic acid (EA). Therefore, EA is commonly found as an artifact during processing, storage, or extraction of ET-rich foods (7). Similarly, on consumption, ETs hydrolyze to release EA which is detected in human plasma (8, and references cited therein). However, of particular importance in the colon, are microbial metabolites such as urolithin A (UA; 3,8-dihydroxy-6H-benzo[b,d]pyran-6-one) which are formed by the action of gut microbiota on ETs (8). Tissue disposition studies reveal that urolithins are enriched in prostate, intestinal, and colon tissues in mouse (9). Urolithins inhibit the proliferation of colon cancer cells, induce cell cycle arrest, and modulate key cellular processes associated with colon cancer development, such as MAPK signaling in vitro (10,11). Furthermore, UA decreases inflammatory markers including inducible nitric oxide synthase, cycloxygenase-2 (COX-2), prostaglandin E synthase and prostaglandin E2, in colonic mucosa in a rat colitis model (12). Therefore, these data suggest that urolithins may be relevant bioactives in the colon and may contribute to the colon cancer chemopreventive properties resulting from the consumption of ET-rich foods.
A set of cellular signals critical for the development and homeostasis of multicellular animals are those elicited by Wnt proteins, a family of highly conserved secreted signaling molecules. In the canonical Wnt pathway, the signal produced by the binding of Wnt ligands to cell surface receptors is transmitted via a cytoplasmic protein called disheveled (Dvl) to inhibit the activity of a complex of cellular proteins that phosphorylate another protein, β-catenin, and target it for destruction. Thus Dvl-mediated inhibition of the β-catenin destruction complex results in increased levels of cellular β-catenin and translocation of β-catenin into the nucleus. In the nucleus, β-catenin activates transcription factors of the LEF/TCF families and initiates transcription of a broad spectrum of target genes which affect tissue proliferation, differentiation and tumorigenesis. Thus the canonical Wnt signaling pathway plays a pivotal role in cellular developmental processes and human carcinogenesis (13, 14). In colon cancer, a large percentage (∼90%) of the tumor arises from activating mutations in the Wnt pathway (13).
Recent research has shown that several dietary phenolics, such as resveratrol (from red wine and grape) and fisetin (from onion and apple), target Wnt and may have potential for colon cancer prevention and treatment (15, 16). Unfortunately, data on the effects of ETs, EA, and their colonic UA metabolites on Wnt are scarce. Previous work show that an ET-enriched extract of pomegranate, but not EA, suppressed TNF alpha-induced COX-2 protein expression and NFkB in human HT-29 colon cancer cells in vitro (17).
The current study was designed to evaluate the effects of standardized ET-enriched fruit extracts, EA, and their derived colonic metabolite, UA, on canonical Wnt signaling reconstituted in HEK 293T cells, and assayed using a luciferase reporter of canonical Wnt pathway-mediated transcriptional activation. We focused our attention on three ET-rich fruits, namely, pomegranate (Punica granatum) fruit (17), strawberries (Fragaria annassa) (18), and the seed extract of the Indian Jamun berry (Eugenia jambolana) (19). The extracts were evaluated for phenolic content as gallic acid equivalents (GAEs) by the Folin Ciocalteau method. In addition, because EA is released through hydrolysis of native ETs during processing and extraction methods, the samples were also evaluated for EA content using high performance liquid chromatography (HPLC) methods.
The primary finding of this study is that UA, a colonic metabolite of ET rich foods, can inhibit the canonical Wnt signaling pathway at physiologically relevant concentrations.
Materials and Methods
Reagents
All solvents were ACS or high performance liquid chromatography (HPLC) grade and were obtained from Sigma-Aldrich through Wilkem Scientific (Pawcatuck, RI). Amberlite XAD-16 resin was purchased from Sigma Aldrich Co. (St. Louis, MO). Commercial standards of gallic acid and ellagic acid (EA) were purchased from Sigma Aldrich.
Synthesis of Urolithin A (UA)
Chemicals required for the synthesis of UA including 2-bromo-benzoic acid and resorcinol were obtained from Sigma-Aldrich and the synthesis was performed as previously described (20).
Preparation of ET-enriched Extracts
Although all these fruits contain anthocyanins, we followed protocols that enriched the extracts in ET-content, while removing anthocyanins, as previously described for pomegranate peel (17) and strawberry fruit (18). The Indian Jamun (Eugenia jambolana) berry contains anthocyanins in its fleshy edible pulp (21), but its seeds are rich in ETs (19). Therefore, Jamun seeds, and not Jamun pulp, were utilized for the current study. Due to the lack of commercial ET standards it was not possible to standardize the strawberry and Jamun ET-extracts. However, the major ETs in pomegranates are punicalagins (17), and the extract used in this study contained ca. 25% punicalagins (see below). The major ETs in strawberry are sanguiin-H6 but no commercial standard is available. Similarly, for Jamun, our laboratory is currently pursuing the isolation and identification of the native ETs in Jamun seeds for future quantification purposes. However, all of the extracts were standardized to polyphenol content (as gallic acid equivalents, GAEs) and also EA (an artifact/hydrolysis product) content by HPLC methods. A brief description of the preparation of these ET-enriched extracts and their standardizations are provided below.
Strawberry
The ET-enriched extract of strawberry fruit has been previously described (18) and was prepared from freeze-dried whole strawberry fruit powder (SFP), provided by the California Strawberry Commission (Watsonville, CA). Briefly, a portion of SFP (500 g) was exhaustively extracted by cold percolation with methanol (3 L) to yield an extract which was then partitioned in chloroform followed by ethyl acetate. The remaining aqueous portion was further purified by adsorption chromatography on an XAD-16 (Amberlite Resin, Sigma, St. Louis, MO) column and eluted with water followed by acidic methanol. The methanol eluate was dried in vacuo and then further enriched in ET content by suspending in distilled water and filtering to yield a water insoluble fraction enriched in ETs and EA. The extract was standardized by high-performance liquid chromatography (HPLC) methods to 5.0% of EA. The extract contained 20.5% of phenolics measured as gallic acid equivalents (GAEs).
Jamun Seed Extract
Jamun seed powder obtained from the Indian Jamun (Eugenia jambolana) berries, as previously described (21), was provided by Verdure Sciences (Noblesville, IN). A portion of the seed powder (20.2 g) was exhaustively extracted with acetone (200 mL × 3) to yield an acetone (1.1 g) extract after solvent removal in vacuo. The extract was standardized by HPLC to 4.2% of EA. The extract contained 20.5% of phenolics as GAEs.
Pomegranate Extract
The pomegranate fruit extract has been previously described (22) and was provided by Verdure Sciences (Noblesville, IN). The extract is HPLC-standardized using validated standards and methods to the major pomegranate ETs (not less than 25% punicalagin α and punicalagin β) and approximately 3.5% of EA.
Estimation of Total Phenolics
Total phenolics were determined according to the Folin-Ciocalteau method and were measured as gallic acid equivalents (GAEs) as previously reported (23). Briefly, the extracts were diluted 1:100, or as appropriate, with methanol:H2O (1:1, v/v) and 200 μL of sample was incubated with 3 mL of methanol:H2O (1:1, v/v) and 200 μL of Folin-Ciocalteau reagent for 10 min at 25 °C. After this, 600 μL of 20% Na2CO3 solution was added to each tube and vortexed. Tubes were further incubated for 20 min at 40 °C. After incubation, samples were immediately cooled in an ice bath to room temperature. Samples and standards (gallic acid) were processed identically. The absorbance was determined at 755 nm and final results were calculated from the standard curve obtained from a Spectramax Plate Reader.
High Performance Liquid Chromatography (HPLC)
All HPLC analyses for EA were conducted as previously reported (6) but on a Beckman Coulter System Gold 126 Module with a photodiode array (PDA)-UV-VIS 168 Detector and 508 Autosampler and operated by 32 Karat software. All samples (20 μL injection volume; 10 mg/mL concentrations) were filtered (0.22 μM) and analyzed using a Luna C-18 column (Phenomenex; 250 × 4.6 mm i.d., 5μ). The mobile phase, solvent A: Water (0.1% TFA), and solvent B: methanol; Gradient start at 90% A to 40% A over 30 min then to 100% B over 5 min; column was re-equilibrated over 12 min; flow rate 0.75 mL/min; EA was monitored at 360 nm.
Cell Culture
The human 293T cell line was originally obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) were used throughout the study. The 293T cells were grown in high glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL)/streptomycin (100 U/mL). Cells were maintained in a humidified 37 °C incubator in 5% CO2.
T Cell Factor (TCF) Transcription Luciferase Assay
Canonical Wnt-pathway mediated transcriptional activation in 293T cells was produced via transfection and expression of plasmid vectors containing cDNA constructs for either Wnt3a or a point mutant β-catenin and assayed via the cotransfection Super8XTOPFlash(sTOP), a plasmid vector containing a synthetic firefly luciferase reporter of β catenin-mediated transcriptional activation (24). Super8XFOPFlash, a control firefly luciferase reporter with the TCF/LEF sites of Super8XTOPFlash mutated (24) was used to confirm that firefly luciferase expression from Super8XTOPFlash was produced as a result of canonical Wnt-pathway mediated transcriptional activation in this system. The Wnt3a, point mutant β-catenin, Super8XTOPFlash (sTOP) and Super8XFOPFlash cDNA containing plasmid vectors were obtained from Dr. R.T. Moon (Howard Hughes Medical Institute, University of Washington, Seattle, WA).
Briefly, 293Tcells (30,000 cells/well) were plated in poly-D-lysine (Sigma Aldrich, St. Louis, MO) treated 96-well tissue culture plates (n= 5/treatment) using standard growth conditions. The cells were allowed to attach to the plate for several hours then were transfected with, 0.030 μg of sTOP and either 0.005 μg of Wnt plasmid vector, 0.010 μg of Dvl plasmid or 0.005 μg of β-catenin plasmid. The levels of baseline firefly luciferase expression (in the absence of exogenously driven Wnt, Dvl or β-catenin expression) derived from the sTOP receptor was determined by transfection of the sTOP alone. Cotransfection of all wells with 0.010 μg of pRL-CMV of the plasmid vector, pRL-CMV (Promega, Madison, WI) encoding Renilla luciferase under the control of a constitutively active CMV promoter was used to normalize for variations in transfection efficiency and cell number between wells. The total amount of plasmid DNA used to transfect each well of cells was kept constant by the addition of an appropriate amount of pCDNA3.1 plasmid vector (Invitrogen, Carlsbad, CA). The transfections were performed using lipofectamine LTX transfection reagent (Invitrogen) and serum free media according to the manufacturer's instructions. Following transfection, the transfection media was replaced with fresh growth media and the ET extracts, EA and UA samples (dissolved in DMSO) were immediately added at the indicated concentrations. The stock solutions of the samples prepared such that the final concentration of DMSO in the cell wells was 0.1% v/v. DMSO at this concentration had no effect on the activity of the luciferase reporters (data not shown) in any of the experiments. After 48 h, the relative firefly luciferase and Renialla luciferase protein levels were measured via the luminescence generated by separately by each of these proteins. The luminescence was generated using the Promega Dual-Luciferase assay system, according to the manufacturer's instructions, and measured with an Lmax Luminometer (Molecular Devices). The luminescence from the firefly luciferase expressed from sTOP was normalized for transfection efficiency and variations in numbers of cells/well by dividing against the luminescence produced from the Renilla luciferase expressed from pRL-CMV plasmid. The IC50 values were determined by fitting the normalized Wnt pathway activation versus ET, EA or UA concentration plots to the function y=100/(1+10(x-logIC50)), where “y” is the percent ratio of the normalized Wnt activated transcriptional response upon treatment with 0.1% DMSO (vehicle) versus the normalized Wnt-activated transcriptions response upon treatment with ET extracts, EA or UA and “x” represents the concentrations of the latter. The fits were performed using GraphPad Prism software.
Results and Discussion
In the current study, standardization of the strawberry and Jamun fruit extracts to their individual ET constituents was not possible due to the lack of authentic commercial ET marker standards. However, the pomegranate extract used here has been previously described and contained ca. 25% punicalagins, the widely accepted ET phytochemical marker of pomegranate fruit (17, 22). In lieu of this lack of standardization to individual ET constituents, all of the fruit extracts were evaluated for total phenolic content using the Folin-Ciocalteau method. It should be noted that the Folin-Ciocalteau method has its limitations, lacks specificity, and is not ideal to standardize fruit matrices to their native ET content. Nevertheless, according to the Folin-Ciocalteau method, the strawberry, Jamun and pomegranate fruit extracts contained 20.5, 20.5 and 55 % phenolics (as gallic acid equivalents, GAEs), respectively. In addition, because ETs are known to hydrolyse to release EA in the gut and during commercial processing, the extracts were also evaluated for presence and levels of EA using HPLC methods. Indeed, free EA was present in all three extracts ranging between 3-5%.
All of the fruit ET-enriched extracts were evaluated for effects on canonical Wnt signaling and they showed similar IC50 values ranging between 28.0-30.0 μg/mL. The dose-dependent Wnt inhibition response for the ET extract obtained from strawberry fruit is shown in Fig. 1A. In the current study, the abilities of the extracts to inhibit Wnt could not be correlated with their total phenolic content which is not surprising given the limitations of the Folin-Ciocalteau method as discussed above. Future work should include correlation of Wnt signaling activity of ET rich fruits to their individual ET contents.
Figure 1.
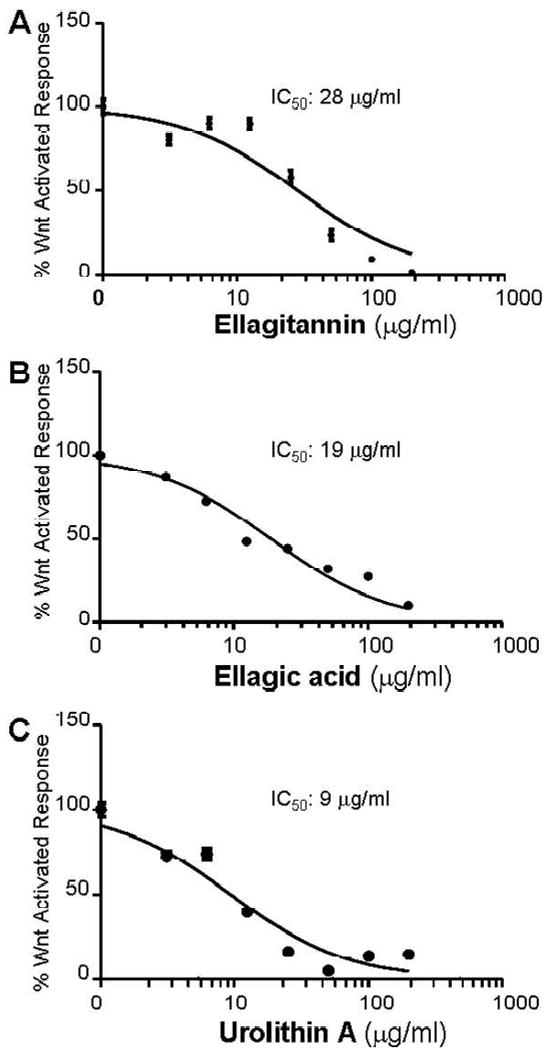
Effects of ET-enriched strawberry fruit extract (1A), ellagic acid (1B), and urolithin A (1C) on Wnt signaling reconstituted in 293T cells and assayed using luciferase reporters. Each point represents the mean (±SEM, n=5) % Wnt-activated transcriptional response at the different concentrations of the indicated compounds.
Both EA and UA also inhibited Wnt signaling with IC50 of 19 μg/mL (63 μM) and 9 μg/mL (39 μM) as shown in Figs. 1B and C, respectively. Unfortunately, based on current knowledge of ET bioavailability and metabolism (8, 25), the levels of ET extracts or EA required to significantly inhibit Wnt signaling may be near impossible to achieve through regular dietary intake of ET rich foods. Thus the inhibition of canonical Wnt signaling produced by these compounds may not be physiologically relevant. However, the inhibition of Wnt signaling achieved by urolithin A (IC50 of 39 μM) is indeed interesting and physiologically relevant. Previous studies estimate that urolithins (and their related conjugates formed by phase-2 metabolism) may reach and even exceed these levels in the colon lumen due to enterohepatic circulation (8, 12, 26). Many dietary polyphenols are known to be transformed in the colon by the intestinal microbiota before absorption and the resulting metabolite (s) may act as substrates for several phase-2 enzymes including both hydrolyzing and conjugating enzymes (8).
To corroborate that the measured firefly luciferase expression was indeed a reporter of Wnt signaling we used Super8XFOPFlash, a control firefly luciferase reporter with the TCF/LEF sites of Super8XTOPflash mutated. We found that Wnt coexpression failed to drive firefly luciferase expression from Super8FOPFlash confirming that firefly luciferase expression from Super8XTOPFlash was produced as a result of canonical Wnt-pathway mediated transcriptional activation.
In the in vitro Wnt assays described above, the Wnt pathway is activated by inducing expression of the secreted Wnt ligand. The 293T cells endogenously express the Wnt receptors, Dvl, the members of the β-catenin destruction complex, β-catenin and the β-catenin-activated TCF/LEF transcription factors required for transcriptional activation through TCF/LEF promoters. However, the pathway can also be activated by the exogenous cellular expression (i.e. through the transient transfection and promoter driven expression of corresponding cDNA) of down-stream components such as β-catenin. To further confirm that these ET-related compounds acted on the canonical Wnt pathway, we tested their effects on TCF/LEF-dependent transcriptional activation that is produced by a mutant β-catenin construct. This construct has four point mutations introduced into the sites that are phosphorylated by the β-catenin destruction complex and which when phosphorylated target β-catenin for cellular destruction (27). This mutant β-catenin construct can elicit TCF/LEF-dependent transcriptional activation but since the cellular levels of this mutant version is insensitive to canonical Wnt signals, the TCF/LEF-dependent transcriptional activation is also insensitive to canonical Wnt signals. Our data shows that the fruit ET extracts and related compounds did not inhibit TCF/LEF-dependent transcription activated produced by the the mutant β-catenin construct (Figure 2).
Figure 2.
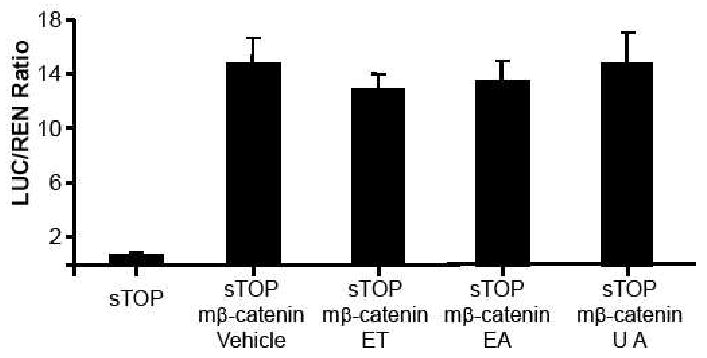
Effects of ET-enriched strawberry fruit extract (ET), ellagic acid (EA), and urolithin A (UA) on TCF/LEF-dependent transcriptional activation that is produced by a mutant β-catenin construct (m-β-catenin) that is insensitive to canonical Wnt signaling. sTOP (Super8XTOPFlash) is the plasmid vector containing a synthetic luciferase reporter of LEF/TCF-dependent transcriptional activation. Vehicle is 0.1% DMSO. The concentration of ET, EA, and UA was 200 μg/mL, respectively. The value represented by each of the bars is the mean normalized luciferase luminescence (±SEM, n=5).
Further support for our data suggesting a role for these ET-related compounds in inhibiting canonical Wnt signaling and colon carcinogenesis is provided by previous studies showing that EA is an inhibitor of the protein kinase CK2 (28, 29). Previous studies have shown that casein kinase CK2 is a positive regulator of Wnt signaling and can modulate Dvl function as well as the interaction of β-catenin with members of the β-catenin destruction complex (30). Therefore, whether the effects of ET derived colonic metabolites on Wnt are indeed mediated through inhibition of CK2 deserves further investigation.
It should be noted that like many other bioactive dietary agents, ETs have been shown to exert multi-mechanistic effects on multiple targets and pathways implicated in carcinogenesis (6). Therefore ETs and their colonic derived metabolites may be most relevant in relation to colon cancer prevention rather than treatment. Whether these compounds affect β-catenin nuclear localization in some cells, and the expression of genes encoding proteins involved in this process, should be investigated. Further studies on definitively identifying the cellular target/s of UA and examinations of the influence of urolithins on the Wnt pathway in animal models are warranted. Future in vivo studies are necessary since there are many limitations to in vitro studies as reported here. For example, although native ETs may be water soluble compounds, both EA and UA are poorly water soluble, and therefore their real concentrations in cell culture media is questionable. Further, it has been shown that ETs are unstable in cell culture media and hydrolyze to release EA, which enters cells and are subsequently metabolised to produce dimethyl-EA derivatives (31). Therefore studies to determine what are the actual forms of compounds (and their concentrations) present in the cell media and cells at the incubation time of various in vitro experiments should be addressed. Nevertheless, the current study adds to the growing body of data elucidating the potential mechanisms of cancer chemopreventive actions of ETs and more importantly, their derived in vivo metabolites.
In summary, the data presented here suggests that UA metabolites produced in the colon from ETs present in small fruits and berries is an inhibitor of the canonical Wnt signaling pathway at physiologically relevant concentrations. Furthermore, the colon, rather than serving simply as an excretory organ, is also an active site for the production of physiologically relevant metabolites through microbiota transformation of dietary components.
Acknowledgments
Funding for this project was provided by a grant from the URI Council for Research Proposal Development Program and from New Faculty Startup Funds from the URI College of Pharmacy, to NS, and a RI-INBRE Grant # P20RR016457 from the National Center for Research Resources, a component of the National Institutes of Health, to MS and AK.
Literature Cited
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, Thorlacius-Ussing O, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Kaaks R, Linseisen J, Boeing H, Nöthlings U, Trichopoulou A, Trichopoulos D, Misirli G, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, van Gils CH, Ocké MC, Lund E, Engeset D, Skeie G, Suárez LR, González CA, Sánchez MJ, Dorronsoro M, Navarro C, Barricarte A, Berglund G, Manjer J, Hallmans G, Palmqvist R, Bingham SA, Khaw KT, Key TJ, Allen NE, Boffetta P, Slimani N, Rinaldi S, Gallo V, Norat T, Riboli E. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 3.Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;55:630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- 4.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomasset S, Teller N, Cai H, Marko D, Berry DP, Steward WP, Gescher AJ. Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother Pharmacol. 2009;64:201–211. doi: 10.1007/s00280-009-0976-y. [DOI] [PubMed] [Google Scholar]
- 6.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Bakkalbaşi E, Menteş O, Artik N. Food ellagitannins-occurrence, effects of processing and storage. Crit Rev Food Sci Nutr. 2009;49:283–298. doi: 10.1080/10408390802064404. [DOI] [PubMed] [Google Scholar]
- 8.Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: Role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 9.Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 10.Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, Espín JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–20. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Sarrias A, Espin JC, Tomas-Barberan FA, Garcia-Conesa MT. Comparative transcriptional analysis reveals key cell cycle and MAPK signaling genes involved in the S-G2/M-phase arrest of Caco-2 cells exposed to ellagic acid and its colonic derivatives, urolithins. Mol Nut Food Res. 2009;53:686–698. doi: 10.1002/mnfr.200800150. [DOI] [PubMed] [Google Scholar]
- 12.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2009 Jul 17; doi: 10.1016/j.jnutbio.2009.04.012. [Epub ahead of print] PubMed PMID: 19616930. [DOI] [PubMed] [Google Scholar]
- 13.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 14.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 15.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, Santoso C, Hanson JA, Holcombe RF. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52:S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- 19.Helmstädter A. Syzygium cumini (L.) SKEELS (Myrtaceae) against diabetes-125 years of research. Pharmazie. 2008;63:91–101. [PubMed] [Google Scholar]
- 20.Ghosal L, Lal J, Singh SKl, Shilajiy FS. Chemistry of two bioactive benzopyrone metabolites. Part 4. J Chem Res Synop. 1989;11:350–351. [Google Scholar]
- 21.Li L, Zhang Y, Seeram NP. Structure of anthocyanins from Eugenia jambolana fruit. Nat Prod Commun. 2009;4:217–219. [PubMed] [Google Scholar]
- 22.Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J Agric Food Chem. 2008;56:8434–41. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- 23.Singleton VL, Esau P. Phenolic substances in grapes and wine, and their significance. Adv Food Res Suppl. 1969;1:1–261. [PubMed] [Google Scholar]
- 24.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, amodulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 25.Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás- Barberán FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–85. doi: 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- 26.González-Sarrías A, Azorín-Ortuño M, Yáñez-Gascón MJ, Tomás-Barberán FA, García-Conesa MT, Espín JC. Dissimilar in vitro and in vivo effects of ellagic acid and its derived microbial metabolites, urolithins, on the expression of CYP1A1. J Agric Food Chem. 2009;57:5623–32. doi: 10.1021/jf900725e. [DOI] [PubMed] [Google Scholar]
- 27.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 28.Cozza G, Bonvini P, Zorzi E, Poletto G, Pagano MA, Sarno S, Donella-Deana A, Zagotto G, Rosolen A, Pinna LA, Meggio F, Moro S. Identification of ellagic acid as potent inhibitor of protein kinase CK2: a successful example of a virtual screening application. J Med Chem. 2006;49:2363–2366. doi: 10.1021/jm060112m. [DOI] [PubMed] [Google Scholar]
- 29.Sekiguchi Y, Nakaniwa T, Kinoshita T, Nakanishi I, Kitaura K, Hirasawa A, Tsujimoto G, Tada T. Structural insight into human CK2alpha in complex with the potent inhibitor ellagic acid. Bioorg Med Chem Lett. 2009;19:2920–2923. doi: 10.1016/j.bmcl.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 30.Dominguez I, Sonenshein GE, Seldin DC. Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NF-kappaB signaling: linking development and cancer. Cell Mol Life Sci. 2009;66:1850–1857. doi: 10.1007/s00018-009-9153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrosa M, Tomás-Barberán FA, Espín JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611–625. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]


