Abstract
Background
The over-production of superoxide () derived from NADPH oxidase (NOX) plays a central role in cardiovascular diseases. By contrast, nitric oxide (NO) and prostacyclin (PGI2) are vasculoprotective. The effect of the NO donor, NONOate and iloprost on formation, p47phox and Rac1 activation in human vascular smooth muscle cells (hVSMCs) was investigated.
Methods
hVSMCs were incubated with 10 nM thromboxane A2 analogue, U46619 for 16 h, and then with apocynin (a NOX inhibitor), NONOate or iloprost for 1 h and measured spectrophometrically. The role of cyclic AMP and cyclic GMP was examined by co-incubation of drugs with protein kinase (PK) A and G inhibitors listed above. Rac1 was studied using pull-down assays.
Results
NONOate and iloprost inhibited formation, acutely, effects blocked by inhibition of PKG and PKA, respectively. Rac1 and p47phox activation and translocation to the plasma membrane was completely inhibited by NONOate and iloprost, effects again reversed by co-incubation with PKG or PKA inhibitors.
Conclusions
NO and PGI2 block the acute activity of NOX in hVSMCs via the cGMP–PKG axis (for NO) and by the cAMP–PKA axis (for iloprost) through inhibition of Rac1 and p47phox translocation. These findings have implications in the pathophysiology and treatment of CVD.
1. Introduction
The intravascular over-production of superoxide () derived from NADPH oxidase (NOX) is involved in the pathogenesis of cardiovascular diseases that include diabetic angiopathy, sepsis, hypertension, atherogenesis, thrombosis, vein graft failure, restenosis and ischaemia reperfusion injury [1-4]. Among the vasculopathic effects of is the negation of the vasculoprotective actions of nitric oxide (NO) [5,6] and prostacyclin (PGI2) [7]. Conversely, NO and PGI2 block the expression of NOX subunits including gp91phox and p47phox induced by vasculopathic factors which include thromboxane A2 (TXA2), cytokines, hypoxia, isoprostanes and superoxide [5-11]. It appears, therefore, that NOX is in dynamic balance with endogenous NO and PGI2 such that the over-expression of NOX and augmentation of formation negates the suppressive actions of NO and PGI2 on NOX expression. Drugs that mimic or augment the action of PGI2 and NO (NO-donors, PGI2 mimetics and type-5 phosphodiestase inhibitors) also reduce the expression of NOX subunit proteins (e.g. p47phox and gp91phox) and reduce formation in vascular cells and tissue [11]. It was suggested, therefore, that these drugs alter the balance in favour of inhibition of NOX proteins and formation which would, therefore, restore the vasculoprotection afforded by NO and PGI2.
In these aforementioned studies, however, the acute effects of NO donors and PGI2 mimetics on NOX activity were not studied. This is of importance as acute release of derived from NOX has been implicated in the aetiology of acute syndromes that include myocardial infarction, angina, acute respiratory distress syndrome and ischaemia reperfusion injury [1-5,12]. In vascular smooth muscle cells (VSMCs), acute activation of NOX involves the assembly of subunits of the whole enzyme complex, which is controlled by the activation and translocation of the Rho-like small GTPases, Rac1 [13]. It is possible that PGI2 and NO may block the acute activation of NOX through an inhibition of Rac1 and translocation to the plasma membrane.
In order to test these proposal, the acute effects of NONOate and iloprost on formation in human VSMCs was studied. Human vascular smooth muscle cells (hVSMCs) derived from saphenous veins were incubated with 10 nM thromboxane A2 analogue, U46619 (acutely and over the longer term) and the cells then incubated NONOate or iloprost and measured spectrophometrically. Cells were either incubated acutely with U46619 (i.e. for 1 h) or for 16 h before testing acute effects of NO or iloprost. Priming cells for 16 h with U46619 is of relevance, as NOX activity is low in healthy vasculature. However, cardiovascular disease is associated with an upregulation of NOX by vasculopathic factors, including TXA2 [1-4]. The role of cyclic AMP and cyclic GMP was examined by co-incubation of drugs with protein kinase (PK) A and G inhibitors and effects on Rac1 studied using Rac1 pull-down assays. Effects on the translocation of p47phox were also studied as this is a key event in acute NOX activation.
2. Materials and methods
2.1. Drugs
9,11-Dideoxy-9α,11α-methanoepoxyprostaglandin F2α (U46619), PKA inhibitor, 14–22 amide peptide, and PKG inhibitor, DT-3 peptide, were purchased from Calbiochem (Nottingham, UK). Iloprost was purchased from Schering (Berlin, Germany). Monoclonal antibody to Rac1 was obtained from Upstate (NY, USA) and polyclonal antibody to p47phox was purchased from Santa Cruz (CA, USA). All the other drugs were purchased from Sigma Chemical Co. (Poole, Dorset, UK) unless otherwise stated.
2.2. Culture and incubation of vascular smooth muscle cells
Saphenous veins were obtained from patients undergoing coronary artery bypass graft surgery CABG for which ethical approval and patient consent had been obtained. Veins were placed in medium RPMI 1640 (Gibco BRL; Paisley, Scotland) containing 2% amphotericin (Gibco BRL and 0.4% heparin; Sigma Chemical Co.). hVSMC were then grown in Dulbecco’s minimum essential medium—Glutamax without sodium pyruvate (DMEM; Gibco BRL), containing 100 units/ml penicillin (Sigma), 100 μg/ml streptomycin (Sigma) and 10% foetal calf serum (Gibco BRL). After passage 4, hVSMCs were seeded in a 6-well or 24-well plates at a density of 6 × 104 cells per well and cultured for 2 days in DMEM/fetal calf serum. Cells were then rendered quiescent for 3 days in serum-free medium (Gibco BRL) before commencing experiment. Under these conditions, there was no loss of cell numbers over this time course. Cells were incubated with thromboxane A2 analogue, U46619 (100 nM) for either 1 or 16 h in the continual presence of either iloprost (100 ng/ml) or Deta-NONOate (10 μM) or NADPH oxidase inhibitor, apocynin (10 μM). Acute effects of iloprost and Deta-NONOate were studied by exposing cells to these compounds for 1 h following overnight incubation with U46619. To assess the role of PKA and PKG in mediating the effects of iloprost and Deta-NONOate, in some experiments, cells were pre-incubated for 30 min with the heat-stable and cell-permeable inhibitors of PKA, 14-22 amide peptide sequence [14], or PKG, DT-3 peptide [15], before the addition of iloprost or Deta-NONOate. These are highly specific inhibitors of PKA and PKG [14,15]. Following incubation, cells were rinsed in PBS and superoxide release was measured by ferricytochrome c assay and protein expression was measured by the Western analysis and Rac1 by pull-down assay.
2.3. Measurement of superoxide
The measurement of superoxide formation and release by cultured cells was performed by detection of ferricytochrome c reduction, as previously described [6]. We opted for this method rather than the lucigenin method as even though more sensitive lucigenin itself undergoes auto-oxidation and thereby act as a source of superoxide generation. We also found that cytochrome c was adequate as sensitive in detecting superoxide release in our system. Following incubation, cells were washed three times with phosphate buffered saline (PBS) and equilibrated in DMEM without phenol red for 10 min at 37 °C in a 95% air–5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Germany). Twenty micromolar horseradish cytochrome c with or without 500 U/ml copper–zinc SOD was added to the cells and incubated at 37 °C in a 95% air–5% CO2 incubator for 1 h. The final volume of the reaction mixture was 0.5 ml per well. After 1 h, the reaction medium was removed and maximum rate of reduction of cytochrome c was determined at 550 nM on a temperature-controlled Anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, UK) and converted to micromoles of superoxide, using ΔE550 nM = 21.1 mM/cm/min as the extinction coefficient for (reduced–oxidised) cytochrome c. The reduction of cytochrome c that was inhibitable with SOD reflected actual formation release. Cells were rinsed in PBS, lysed with 0.1% (v/v) Triton X-100 and total protein content measured using BCA-protein assay kit.
2.4. Western blotting
For Western analysis of NOX1, HVSMC were washed and lysed with Tris buffer (100 mM, pH 6.8) containing 10% glycerol and 1% sodium dodecyl sulphate (SDS) [16-19]. Extracts were boiled at a 1:1 ratio with the loading buffer containing Tris (125 mM, pH 6.8), 4% (w/v) SDS, 10% (v/v) glycerol, 4% (v/v) 2-mercaptoethanol and 2 mg/ml bromophenol blue. Total cell lysates of equal protein (50 μg) were loaded onto 10% Tris–glycine sodium dodecyl sulphate gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed overnight with the primary antibodies to Rac1 (1:1000) and p47phox (1:500). The blots were then incubated with either goat anti-mouse (1:3000; Dako, UK; for Rac1) or goat anti-rabbit (1:100,000; for p47phox) conjugated to horseradish peroxidase for 1 h and developed by enhanced chemiluminescence (Amersham International, UK). Rainbow markers (10–250 kDa; Amersham International) were used for molecular weight determination.
2.5. Rac1-activation and translocation assays
In order to investigate the involvement of Rac1 in U46619-stimulated generating system and the effect of iloprost and NONOate on Rac1 activation, hVSMCs were treated for 1 h with a Rho–GTPase inhibitor, Clostridium difficile toxin B, following a 16-h incubation with U46619. Activation of Rac1 was analysed by GST-PAK-CRIB pull down assays. Briefly, cells were washed in cold PBS and lysed in 800 μl of lysis buffer: 50 mM Tris–HCl, pH 7.2, 1% Triton X-100, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 1 mM AEBSF, 10 μg/ml aprotinin and 10 μg/ml leupeptin. Insoluble cell debris was removed by centrifugation and the supernatant mixed with GST-tagged CRIB domain of PAK (bound to glutathione-sepharose) at 4 °C for 1 h. Resin was washed four times in 50 mM Tris–HCl, pH 7.2, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 1 mM AEBSF, 10 μg/ml aprotinin and 10 μg/ml leupeptin. Bound proteins were eluted in SDS sample buffer and analysed by immunoblotting for Rac1.
Membrane and cytosolic localisation of Rac1 and p47phox was quantified by cell fractionation. Briefly, cells were washed in PBS and lysed by sonication (5-7 Hz; 3 × 10 s bursts) in pre-chilled hypotonic lysis buffer (10 mM Tris–HCl (pH 7.4), 1.5 mM MgCl2, 5 mM KCl, 1 mM DTT, 0.2 mM NaVO4, 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1 mM AEBSF). Nuclei and intact cells were removed by centrifugation at 3000 rpm at 4 °C for 5 min. Supernatants were subjected to ultra-centrifugation at 100,000 × g at 4 °C for 45 min using Beckman Optima L-90K Ultracentrifuge (Beckman Instruments Inc., USA). The pellet was defined as the membrane fraction and the supernatant as cytosolic fraction. The membrane pellets were dissolved in 80 μl of SDS-lysis buffer and 200 μl of cytosolic fractions were also mixed with 50 μl of SDS-lysis buffer. Equal protein amounts of membrane and cytosolic fractions were subjected to western blot analysis for p47phox and Rac1. Membranes were re-probed with anti-α/β tubulin antibody (Upstate, USA) to assess the efficiency of cell fractionation.
2.6. Data analysis
The data were tested for normality by inspecting histograms and by applying the Kolmogorov–Smirnov test (automatically applied by Sigma Stat™ as part of the procedure for producing ANOVA results). In all cases, the data did not deviate sufficiently from normality to warrant non-parametric statistics. All data represent the means of triplicate determinations; each experiment was repeated at least six times. Both one-way and two-way analysis of variance (ANOVA) was used to determine statistical significance. Two-way ANOVA tests were employed where two conditions existed and one-way ANOVA was used when comparing effects of drug treatments with untreated controls. Statistical significance was assumed at a value of P<0.05.
3. Results
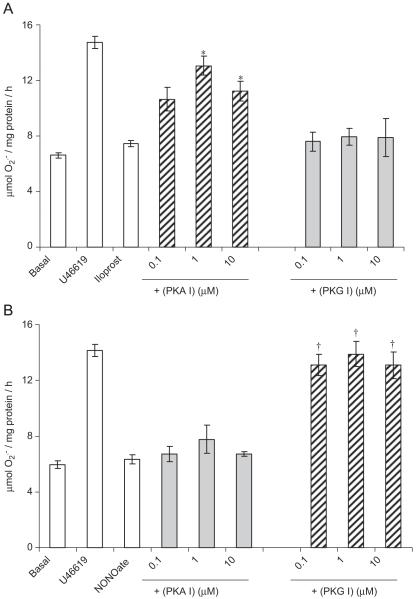
Sixteen-hour incubation of hVSMCs with the thromboxane A2 analogue, U46619 elicited an increase in formation (Fig. 1). Incubation with iloprost or NONOate for 1 h, following a 16 h incubation with U46619, completely blocked U46619-stimulated formation in concentration-dependent manners (Fig. 1). The inhibitory effect of iloprost on U46619-stimulated formation was blocked by PKA peptide inhibitor but not by PKG peptide inhibitor (Fig. 2A). By contrast, the inhibitory effect of NONOate was blocked by the PKG peptide inhibitor and not by PKA peptide inhibitor (Fig. 2B). Acutely, incubation with U46619 for 1 h augmented formation from hVSMCs, an effect blocked by iloprost, NONOate and apocynin (Fig. 3).
Fig. 1.
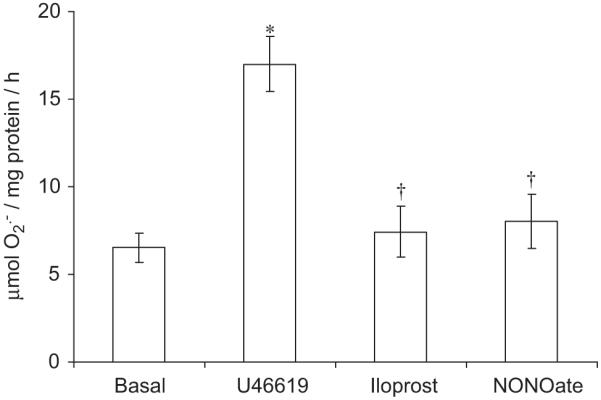
Acute effect (1 h) of iloprost (100 ng/ml) and NONOate (10 μM) following a 16-h incubation with U46619 (100 nM) on superoxide () formation by human isolated vascular smooth muscle cells (hVSMCs). Each point represents the mean (±S.E.M.), n = 6. *P<0.05, significantly greater than basal. †P<0.01, significantly reduced compared to U46619.
Fig. 2.
Effect of PKA inhibitor (PKA I; 14-22 amide peptide sequence) or PKG inhibitor (PKG I; DT-3 peptide) on the acute inhibition of formation by hVSMCs pre-incubated for 16 h with 100 nM U46619 (100 nM) by (A) iloprost (100 ng/ml) and (B) NONOate (10 μM). Each point = mean±S.E.M., n = 6. *P<0.01, significantly increased compared to iloprost alone. †P<0.01, significantly increased compared to NONOate alone.
Fig. 3.
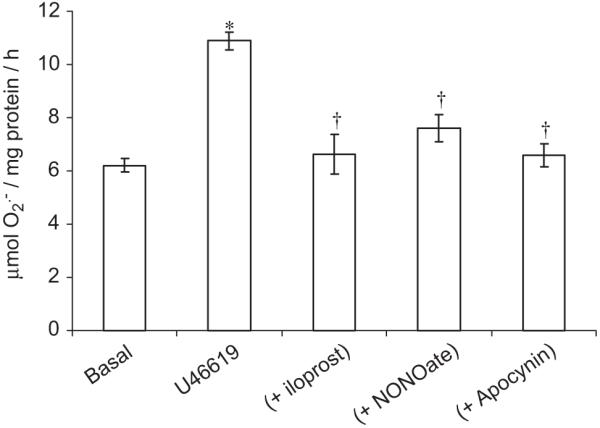
Acute (1-h incubation) effect of U46619 (100 nM) [±iloprost (100 ng/ml), NONOate (10 μM) or apocynin (10 μM)] on superoxide () formation by human isolated vascular smooth muscle cells (hVSMCs). Each point represents the mean (±S.E.M.), n = 6. *P<0.01, significantly increased compared to basal. †P<0.01, significantly reduced compared to U46619 alone.
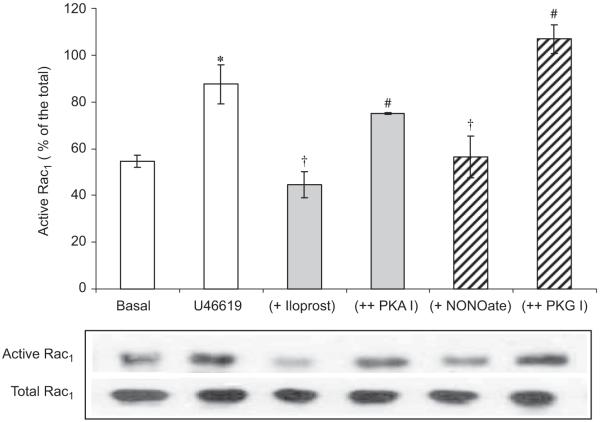
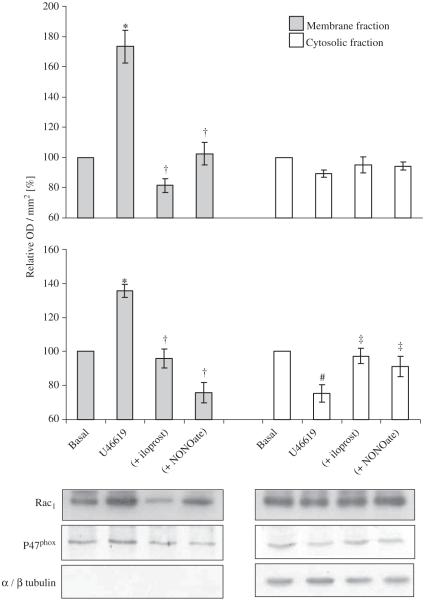
Acute incubation with C. difficile toxin B, a Rho-GTPase inhibitor, for 1 h completely inhibited the increase in release from hVSMCs following a 16-h incubation with U46619 (Fig. 4). Incubation with U46619 for 16 h also increased Rac1 activity in hVSMCs (Fig. 5). Acute incubation with iloprost and NONOate for 1 h reversed increased Rac1 activity to basal level (Fig. 5). These inhibitory effects of iloprost or NONOate on Rac1 activity were blocked by PKA or PKG peptide inhibitor, respectively (Fig. 5). Acute incubation with iloprost and NONOate for 1 h also inhibited p47phox and Rac1 translocation to the plasma membrane in hVSMCs when co-incubated with U46619 for 16 h (Fig. 6).
Fig. 4.
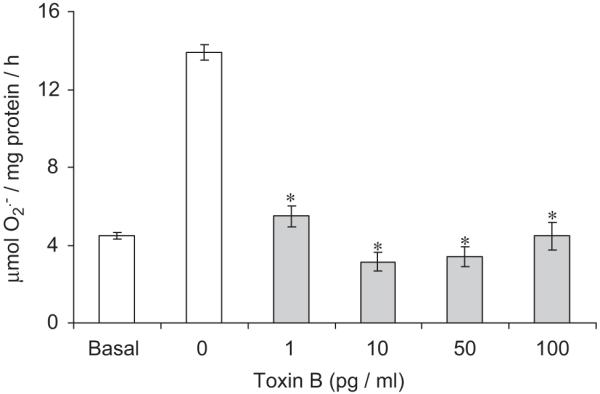
Acute effect (1-h incubation) of a Rho–GTPase inhibitor, Clostridium difficile toxin B, on U46619 (100 nM)-induced superoxide () formation (16 h) in human isolated vascular smooth muscle cells (hVSMCs). Each point represents the mean (±S.E.M.), n = 6. *P<0.01, significantly reduced compared to U46619.
Fig. 5.
Acute effect (1-h incubation) of iloprost (100 ng/ml) or Deta-NONOate (10 μM)±PKA or PKG inhibitors (100 nmol) on Rac1 activity following a 16-h incubation of human VSMC with U46619 (100 nmol). Rac1 activity was quantified by Rac1 pull down assays. Blots are representative of six independent experiments. *P<0.05, significantly increased compares to basal value. #P<0.05, significantly inhibited compared to U46619-treated value. †P<0.01, significantly increased compared to iloprost or Deta-NONOate only values.
Fig. 6.
Acute effect (1-h incubation) of iloprost (100 ng/ml) or Deta-NONOate (10 μM) on Rac1 and p47phox membrane localisation following a 16-h incubation of human VSMC with 100 nmol U46619. Total cell lysates were fractionated and the membrane and cytosolic levels of (A) Rac1 and (B) p47phox quantified by Western blotting. Blots are representative of at least six independent experiments. *P<0.05, significantly increased compares to basal value. †P<0.05, significantly inhibited compared to U46619-treated membrane fractions. #P<0.01, significantly reduced compares to basal value. ‡P<0.01, significantly increased compared to U46619-treated cytosolic fractions.
4. Discussion and conclusions
The present study demonstrates firstly that U46619 (a stable analogue of thromboxane A2) augments formation in human VSMCs following a 16-h incubation. This effect of U46619 was blocked by the NOX inhibitor, apocynin, indicating that U46619 up-regulates NOX, which is the source of under these conditions. This is consistent with previous studies in porcine and rabbit vascular cells, in which U46619 was shown to upregulate the expression of NOX subunits, including gp91phox and p47phox [7-9,11]. In subsequent experiments, cells were primed by incubating with U46619 and acute effects of NONOate and iloprost were then studied. This model is of relevance as acute cardiovascular events are associated with an a priori upregulation of NOX by vasculopathic factors [1-4].
U46619 also acutely promoted the formation of , an effect again blocked by apocynin indicating that TXA2 acutely activates NOX in human VSMCs. This too is consistent with a previous study in isolated rabbit cavernosal smooth muscle cells in which it was demonstrated that U46619 increases formation through activation of NOX [11]. In this latter study, however, the acute effects of iloprost, NO donor, the role of PKA or PKG, Rac1 or the translocation of p47phox was not studied.
In the present study, both NONOate and iloprost inhibited the acute formation of following priming and upregulation of NOX activity with U46619 as well as acutely (over 1 h without priming). It should be stressed that direct quenching could not account for these effects as NONOate and iloprost were washed clear of the system before measuring . Furthermore, the effect of iloprost was blocked by PKA inhibitors but not by inhibitors of PKG and the effect of NONOate was blocked by inhibition of PKG but not by inhibition of PKA. This demonstrates that the inhibition of formation is mediated by classic pathways: adenylyl cyclase–cAMP–PKA for iloprost and guanylyl cyclase–cGMP–PKG pathway for NONOate. These novel effects adds to the repertoire of vasculoprotective attributes of NO and PGI2.
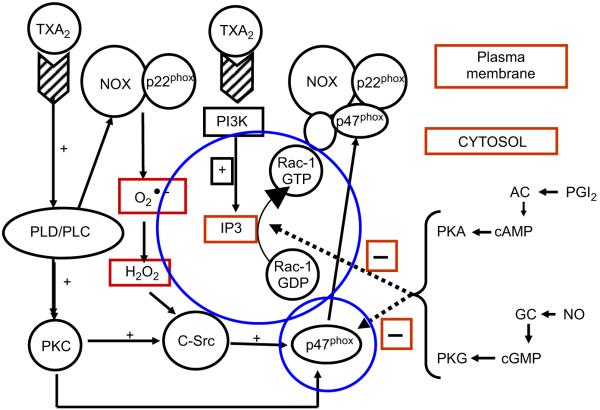
The acute activation of NOX involves a complex sequence of events that is controlled by activation and translocation of both Rac1 and p47phox to the plasma membrane [13]. In the present study, pharmacological inhibition of Rac1 with toxin B completely inhibited formation, consolidating that Rac1 plays an obligatory role in mediating acute NOX activity in hVSMCs. Using adenoviruses, we have also previously demonstrated that over-expression of Rac1 in hVSMCs promotes NOX activity but infection with a dominant negative adenovirus blocks NOX activity [20]. In the present study, iloprost and NONOate also blocked the translocation of both Rac1 and p47phox to the plasma membrane, effects that were again blocked by PKA inhibition (for iloprost) and PKG inhibition (for NONOate) as for formation above (Fig. 7; [42-44]).
Fig. 7.
Working model for the acute activation thromboxane A2 (TXA2) of NADPH oxidase (NOX) activity and the inhibitory action of prostacyclin (PGI2) and nitric oxide (NO) in human vascular smooth muscle cells. TXA2 activates both phospholipase C (PLC) and D (PLD) and PI3 kinase (PI3 K) activity [42-44]. This has two consequences: (i) activation of protein kinase C (PKC) and c-Src which in turn triggers the translocation of 47phox, a crucial catalytic subunit of the NOX complex to the plasma membrane and (ii) generation of inositol trisphosphate (IP3) which activates Rac1 which then associates with the NOX complex, including NOX1 and 22phox at the plasma membrane. These key events trigger the activation of NOX which then releases superoxide () which further augments NOX activation. By contrast, PGI2 and NO, through activation of the cyclic GMP–PKG and the cyclic AMP–PKA systems, respectively, inhibit both the translocation of p47phox and the activation and translocation of Rac1, thereby acutely blocking the activity of NOX and release.
These data are consistent with another study in which it was demonstrated that cAMP inhibits Rac1 activity, which in turn inhibits the replication and migration of VSMCs [18,19]. Other functions associated with vasculopathy that are elicited by Rac1 activation but inhibited by both NO and PGI2 are adhesion molecule expression [21-23], calcium mobilisation [24-26], metalloproteinase expression [27-29] and VSMC migration [30-32]. In turn, derived from NOX also augments these diverse vascular events, suggesting that a common denominator for NOX–NO and PGI2 interactions is Rac1.
The mechanisms underlying the regulation of Rac1 activity by PKA and or PKG are unknown but may involve phosphorylation of one or more of the large family of guanine nucleotide exchange factors (GEFs) and/or GTPase activating proteins (GAPs) that govern functional status of all GTPases in the cell [33]. The GTPases are activated by GEFs, which catalyse loading with GTP, and inactivated by GAPs, which accelerate hydrolysis of the bound nucleotide to form GDP [34]. Exploring this area is beyond the scope of the present study but warrants further investigation.
From a clinical perspective, the acute activation of intravascular NOX and the resultant burst in endogenous formation promotes events that include thrombosis, vasoconstriction, adhesion molecule expression and ischaemia reperfusion injury [1-4]. These events are central to the aetiology of diverse disorders that include angina pectoris, myocardial infarction and sepsis. In turn, both PGI2 and NO donors have been shown to prevent these events in pre-clinical models and in man [35,36]. It is not unreasonable to suggest, therefore, that the acute therapeutic action of NO and iloprost are mediated, at least in part, through inhibition of acute NOX activation. The corollary to this is that over-expression of NOX and excess formation leads to a negation of the endogenous vasculoprotective actions of NO and PGI2 which in turn leads to a greater expression of NOX [4-7,37,38]. Therapeutically, PGI2 mimetics and NO donors may act by breaking this cycle. Furthermore, drugs that augment the biological actions of NO and PGI2, namely phosphodiesterase inhibitors, have been shown to block the upregulation of NOX in vascular cells through protection of PKA and PKG activation driven by NO and PGI2 [11,39-41]. Thus, it is reasonable to suggest that co administration of PDE inhibitors may augment the therapeutic potential of NO donors and PGI2 mimetics through enhanced suppression of intravascular oxidative stress.
Acknowledgement
This research was funded by the British Heart Foundation (RFLS.RJ4313).
References
- [1].Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- [2].Shah AM, Channon KM. Free radicals and redox signalling in cardiovascular disease. Heart. 2004;90:486–487. doi: 10.1136/hrt.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- [4].Muzaffar S, Shukla N, Jeremy JY. Nicotinamide adenine dinucleotide phosphate oxidase: a promiscuous therapeutic target for cardiovascular drugs? Trends Cardiovasc. Med. 2005;15:278–282. doi: 10.1016/j.tcm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [5].Muzaffar S, Jeremy JY, Angelini GD, Stuart Smith K, Shukla N. The role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Nitroaspirins and SIN-1, but not aspirin, inhibit the expression of endotoxin- and cytokine-induced NADPH oxidase in vascular smooth muscle cells from pig pulmonary arteries. Circulation. 2004;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- [7].Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits superoxide formation and NADPH oxidase expression induced by the thromboxane A2 analogue, U46619, and isoprostane F2α in cultured porcine pulmonary artery vascular smooth muscle cells. Br. J. Pharmacol. 2004;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide “auto-augments” the formation of superoxide through upregulation of NADPH oxidase expression in porcine pulmonary artery endothellial cells: inhibition with iloprost. Eur. J. Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- [9].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Hypoxia and the expression of gp91phox and endothelial nitric oxide synthase in the pulmonary artery. Thorax. 2005;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate (NCX 911) are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary endothelial cells. Br. J. Pharmacol. 2005;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kouparris AJ, Jeremy JY, Muzaffar S, Angelini GD, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47phox NADPH oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. Br. J. Urol. Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- [12].Jeremy JY, Yim AP, Wan S, Angelini GD. Oxidative stress, nitric oxide and vascular disease. Cardiovasc. Surg. 2002;17:324–327. doi: 10.1111/j.1540-8191.2001.tb01151.x. [DOI] [PubMed] [Google Scholar]
- [13].Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ. Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- [14].Rimon G, Rubin M. Regulation of a common, low-affinity binding site for primary prostanoids on bovine aortic endothelial cells. Biochim. Biophys. Acta. 1998;10:289–296. doi: 10.1016/s0304-4165(97)00153-0. [DOI] [PubMed] [Google Scholar]
- [15].Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc. Natl. Acad. Sci. USA. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu YJ, Bond M, Sala-Newby G, Newby AC. A novel mechanism underlying the regulation of smooth muscle cell proliferation by the small GTPase Rac-1. Eur. Heart J. 2006;27(Suppl. 1):966. [Google Scholar]
- [17].Bond M, Sala-Newby GB, Newby AC. Focal adhesion kinase (FAK)-dependent regulation of S-phase kinase-associated protein-2 (Skp-2) stability. A novel mechanism regulating smooth muscle cell proliferation. J. Biol. Chem. 2004;279:37304–37310. doi: 10.1074/jbc.M404307200. [DOI] [PubMed] [Google Scholar]
- [18].Wu YJ, Bond M, Sala-Newby G, Newby AC. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ. Res. 2006;98:1141–1150. doi: 10.1161/01.RES.0000219905.16312.28. [DOI] [PubMed] [Google Scholar]
- [19].Pelletier S, Julien C, Popoff MR, Lamarche-Vane N, Meloche S. Cyclic AMP induces morphological changes of vascular smooth muscle cells by inhibiting a Rac-dependent signaling pathway. J. Cell Physiol. 2005;204:412–422. doi: 10.1002/jcp.20308. [DOI] [PubMed] [Google Scholar]
- [20].Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Acute inhibition of NADPH oxidase activity in vascular smooth muscle cells by nitric oxide, iloprost and hydrogen sulfide. Br. J. Pharmacol. 2007 in press (abstract) [Google Scholar]
- [21].Wung RS, Ni CW, Wang DL. ICAM-1 induction by TNFα and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J. Biomed. Sci. 2005;12:91–101. doi: 10.1007/s11373-004-8170-z. [DOI] [PubMed] [Google Scholar]
- [22].Goya K, Otsuki M, Xu X, Kasayama S. Effects of the prostaglandin I2 analogue, beraprost sodium, on vascular cell adhesion molecule-1 expression in human vascular endothelial cells and circulating vascular cell adhesion molecule-1 level in patients with type 2 diabetes mellitus. Metabolism. 2003;52:192–198. doi: 10.1053/meta.2003.50025. [DOI] [PubMed] [Google Scholar]
- [23].Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J. Clin. Endocrinol. Metab. 2000;85:2572–2575. doi: 10.1210/jcem.85.7.6677. [DOI] [PubMed] [Google Scholar]
- [24].Christensen EN, Mendelsohn ME. Cyclic GMP-dependent protein kinase Iα inhibits thrombin receptor-mediated calcium mobilization in vascular smooth muscle cells. J. Biol. Chem. 2006;281:8409–8416. doi: 10.1074/jbc.M512770200. [DOI] [PubMed] [Google Scholar]
- [25].Purdy KE, Arendshorst WJ. Prostaglandins buffer ANG II-mediated increases in cytosolic calcium in preglomerular VSMC. Am. J. Physiol. Renal Physiol. 1999;277:F850–F858. doi: 10.1152/ajprenal.1999.277.6.F850. [DOI] [PubMed] [Google Scholar]
- [26].Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc. Res. 2006;71:236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [27].Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. Role in cell invasion across collagen barrier. J. Biol. Chem. 2001;276:16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- [28].Kitahara M, Ichikawa M, Kinoshita T, Shiozawa S, Shigematsu H, Komiyama A. Prostacyclin inhibits the production of MMP-9 induced by phorbol ester through protein kinase A activation, but does not affect the production of MMP-2 in Human cultured mesangial cells. Kidney Blood Press. Res. 2001;24:18–26. doi: 10.1159/000054201. [DOI] [PubMed] [Google Scholar]
- [29].Eagleton MJ, Peterson DA, Sullivan VV, Roelofs KJ, Ford JA, Stanley JC, Upchurch GR., Jr. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J. Surg. Res. 2002;104:15–24. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- [30].Jacob A, Molkentin JD, Smolenski A, Lohmann SM, Begum N. Insulin inhibits PDGF-directed VSMC migration via NO/cGMP increase of MKP-1 and its inactivation of MAPKs. Am. J. Physiol. Cell Physiol. 2002;283:C704–C713. doi: 10.1152/ajpcell.00110.2002. [DOI] [PubMed] [Google Scholar]
- [31].Liu D, Hou J, Hu X, Wang X, Xiao Y, Mou Y, De Leon H. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circ. Res. 2006;98:480–489. doi: 10.1161/01.RES.0000205764.85931.4b. [DOI] [PubMed] [Google Scholar]
- [32].Bulin C, Albrecht U, Bode JG, Weber AA, Schrör K, Levkau B, Fischer JW. Differential effects of vasodilatory prostaglandins on focal adhesions, cytoskeletal architecture, and migration in human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:84–92. doi: 10.1161/01.ATV.0000146814.81581.68. [DOI] [PubMed] [Google Scholar]
- [33].Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J. Biol. Chem. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- [34].Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ. Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- [35].Fink AN, Frishman WH, Azizad M, Agarwal Y. Use of prostacyclin and its analogues in the treatment of cardiovascular disease. Heart Dis. 1999;1:29–40. [PubMed] [Google Scholar]
- [36].Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jeremy JY, Angelini GD, Khan M, Mikhailidis DP, Morgan RJ, Thompson CS, Bruckdorfer KR, Naseem KM. Platelets, oxidant stress and erectile dysfunction: an hypothesis. Cardiovasc. Res. 2000;46:50–54. doi: 10.1016/s0008-6363(00)00009-2. [DOI] [PubMed] [Google Scholar]
- [38].Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and vascular smooth muscle cell proliferation. Cardiovasc. Res. 1999;43:658–665. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- [39].Jeremy JY, Jones RA, Kouparris A, Hotston M, Persad R, Angelini GD, Shukla N. Oxidative stress and erectile dysfunction: possible role of NADPH oxidase. Int. J. Impot. Res. 2007;19:265–280. doi: 10.1038/sj.ijir.3901523. [DOI] [PubMed] [Google Scholar]
- [40].Jeremy JY, Shukla N, Muzaffar S, Angelini GD. Reactive oxygen species, vascular disease and cardiovascular surgery. Curr. Vasc. Pharmacol. 20004;2:229–236. doi: 10.2174/1570161043385691. [DOI] [PubMed] [Google Scholar]
- [41].Hotston M, Jeremy JY, Kouparris A, Persad N, Angelini GD, Shukla N. Sildenafil (Viagra™) inhibits the upregulation of type 5 phosphodiesterase elicited with nicotine and tumour necrosing factor-α in cavernosal vascular smooth muscle cells: mediation by superoxide. Br. J. Urol. Int. 2007;99:612–618. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]
- [42].Martinson EA, Scheible S, Greinacher A, Presek P. Platelet phospholipase D is activated by protein kinase C via an integrin alpha IIb beta 3-independent mechanism. Biochem. J. 1995;310:623–628. doi: 10.1042/bj3100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jeremy JY, Mikhailidis DP, Dandona P. Excitatory receptor-prostanoid synthesis coupling in smooth muscle: mediation by calcium, protein kinase C and G proteins, Prostaglandins Leukot. Essent. Fatty Acids. 1988;34:215–227. [PubMed] [Google Scholar]
- [44].Dangelmaier C, Jin J, Smith JB, Kunalpi SP. Potentiation of thromboxane A2-induced platelet secretion by Gi signaling through the phosphoinositide-3 kinase pathway. Thromb. Haemost. 2001;85:341–348. [PubMed] [Google Scholar]