Abstract
Background
We present a bivariate twin analysis of anorexia nervosa (AN) and bulimia nervosa (BN) to determine the extent to which shared genetic and environmental factors contribute to liability to these disorders.
Method
Focusing on females from the Swedish Twin study of Adults: Genes and Environment (STAGE) (N=7000), we calculated heritability estimates for narrow and broad AN and BN and estimated their genetic correlation.
Results
In the full model, the heritability estimate for narrow AN was (a2 = .57; 95% CI: .00, .81) and for narrow BN (a2 = .62; 95% CI: .08, .70) with the remaining variance accounted for by unique environmental factors. Shared environmental factors estimates were (c2 = .00; 95% CI: .00, .67) for AN and (c2 = .00; 95% CI: .00, .40) for BN. Moderate additive genetic (.46) and unique environmental (.42) correlations between AN and BN were observed. Heritability estimates for broad AN were lower (a2 = .29; 95% CI: .04, .43) than for narrow AN, but estimates for broad BN were similar to narrow BN. The genetic correlation for broad AN and BN was .79 and the unique environmental correlation was .44.
Conclusions
We highlight the contribution of additive genetic factors to both narrow and broad AN and BN and demonstrate a moderate overlap of both genetic and unique environmental factors that influence the two conditions. Common concurrent and sequential comorbidity of AN and BN can in part be accounted for by shared genetic and environmental influences on liability although independent factors also operative.
INTRODUCTION
Anorexia nervosa (AN) and bulimia nervosa (BN) are separate disorders in the DSM IV-TR, although their diagnostic boundaries are far from stable (1, 2). We apply behavioral genetic methods to determine the extent to which this partially overlapping symptom picture could be attributable to shared genetic or environmental factors.
AN is marked by low weight; however, provisions are made for the presence of bulimic symptoms in the binge-purge subtype (3). No provisions are made in the BN criteria for past AN. Critically, the current classification system is entirely cross-sectional and fails to capture the considerable symptomatic flux observed during the course of both eating disorders. Both clinical and epidemiologic studies demonstrate frequent crossover from AN to BN (8–54%) (1, 2, 4–14), and from BN to AN (4–27%) (1, 2, 13), typically within the first 5 years of illness (2, 4).
Further supporting diagnostic non-independence, AN and BN do not aggregate independently, with family and twin studies revealing considerable heterogeneity in eating disorders presentations in family members (15, 16).
To date, no twin study has applied contemporary behavioral genetic methods to determine the extent to which this complex partially-overlapping diagnostic picture could be accounted for by underlying shared genetic or environmental factors. Although multivariate twin analyses have been conducted with eating disorders and depression (17, 18) and eating disorders with several psychiatric syndromes (19), the low base rate of AN has precluded application of this methodology to AN and BN.
Using data from the Swedish Twin study of Adults: Genes and Environment (STAGE) study of the Swedish Twin Registry (STR) (20), we present the first bivariate twin analysis of AN and BN to determine the extent to which shared genetic and environmental factors contribute to liability to these disorders.
METHOD
Participants
STAGE (STR; http://ki.se/ki/jsp/polopoly.jsp?d=9610&l=en) is a population-based prospective sample of Swedish twins born 1959–1985 (ages 20–47) (20). STAGE participants are part of the larger STR database (∼170,000 individuals from ∼ 85,000 twin pairs). Data were collected on-line in 2005. Approximately 1,300 questions spanned 34 health and demographic topics.
Over 25,000 individuals from a total sample of 43,000 participated (response rate = 59.6%) with 10,510 males and 13,295 females completing the eating disorders section. Due to low prevalences of AN and BN in males, only prevalence data are presented. Of the 13,295 females, 4,099 were MZ, 2,901 were same sex DZ, 2,433 were from opposite sex DZ pairs, 221 could not be assigned zygosity using the algorithm, and 3641 were of unknown zygosity because of cotwin nonparticipation. Our final sample for modeling included 7,000 females from MZ and same sex DZ pairs.
STAGE was approved by the Regional Ethics Committee at the Karolinska Institutet. This study was also approved by the Biomedical Institutional Review Board at the University of North Carolina. STAGE has been extensively described elsewhere (21, 22).
Zygosity
Zygosity was determined from the following questions: (1) During childhood, were you and your twin partner as like as ‘two peas in a pod’ or no more alike than siblings in general? and (2) How often did strangers have difficulty distinguishing between you and your twin partner when you were children? Twin pairs who responded ‘alike as two peas in a pod’ for Q1 and ‘almost always’ or ‘often’ for Q2 were classified MZ. If both twins responded ‘not alike’ for Q1 and ‘seldom,’ ‘almost never’ or ‘never’ for Q2, they were classified DZ. All other twins were classified as ‘not determined.’ The algorithm was validated with a panel of 47 SNPs in a random sample of 198 twin pairs. Ninety five percent (N=188) were correctly classified. Of those misclassified, 8 were MZ and 2 were DZ. This zygosity algorithm has been previously validated (21).
Measures
Lifetime eating disorders were assessed using an expanded, on-line Structured Clinical Interview for DSM-IV (SCID)-based instrument. Study criteria and algorithms for narrow and broad AN and BN are listed in Table 1. Diagnoses were derived using the variables listed in Table 1. For both AN and BN, participants were coded ‘1’ if all criteria were present, ‘0’ if only some criteria were met, and ‘missing’ if a diagnosis could not be made.
Table 1.
Criteria used for eating disorder diagnoses.
| Criteria*/Item | Response to Meet Criteria | |
|---|---|---|
| Anorexia Nervosa (AN) | ||
| Narrow | Broad | |
| 1a. Had a period of time when you weighed much less than other people thought you ought to weight? |
1a. Yes | 1a. Yes |
| AND | AND | |
| 1b. BMI calculated from lowest weight and height at lowest weight. | 1b. BMI < 17.55 | 1b. BMI < 18.55 |
| 2. During the time of low weight, how afraid were you that you might gain weight or become fat? (response on 5 point Likert Scale) |
2. (4) Very afraid OR (5) Extremely afraid |
2. (2) Slightly, (3) Somewhat, (4) Very afraid, OR (5) Extremely afraid |
| 3. During the time of low weight, did you feel fat? (response on a 5 point Likert Scale) |
3. (4) Very afraid OR (5) Extremely afraid |
3. (2) Slightly, (3) Somewhat, (4) Very afraid, OR (5) Extremely afraid |
| 4a. Before this time, had your periods started? | 4a. If ‘No,’ then criteria met. | Not Required |
| OR | ||
| If ‘Yes,’ then go to 4b. | ||
| 4b. If yes, did they stop? | 4b. Yes | |
| 4c. For how long did they stop? | 4c. At least 3 months | |
| Bulimia Nervosa (BN) | ||
| Narrow | Broad | |
| 1a. Have you even had eating binges when you ate what most people would regard as an unusually large amount of food in a short period of time? |
1a. Yes | 1a. Yes |
| AND | AND | |
| 1b. When you were having eating binges, did you feel your eating was out of control? (response on 5 point Likert Scale) |
1b.(4) Very much OR (5) Extremely |
1b. (2) Slightly, (3) Somewhat, (4) Very much, OR (5) Extremely |
| 2. Which of these did you use during the same time that you were binge eating? Making yourself vomit? Laxatives? Diuretics? Diet Pills? Exercise more than 2 hours per day? Fast or not eat? Other methods? |
2. A ‘Yes’ response to any of these items meets criteria. |
2. A ‘Yes’ response to any of these items meets criteria. |
| 3a. When you were binging the most, how many binges would you have in a month? |
3a. At least 8 times a month | 3a. At least 8 times a month |
| AND | AND | |
| 3b. For how long did you have binge eating episodes? | 3b. At least 3 months | 3b. At least 1 to 2 months |
| 4. Statements regarding weight and shape on a 5 point Likert scale | 4. Weight or shape are important things that affect how I feel about myself. |
4. Weight or shape play a moderate part in how I feel about myself. |
| OR Weight or shape are the most important things that affect how I feel about myself. |
Weight or shape are important things that affect how I feel about myself. OR |
|
| Weight or shape are the most important things that affect how I feel about myself |
||
| Anorexia and Bulimia Nervosa (ANBN) | ||
| Narrow | Broad | |
| Lifetime diagnosis of AN and BN as defined above | Individuals had to meet criteria for both narrowly defined AN and BN |
Individuals had to meet criteria for both broadly defined AN and BN |
Statistical Analyses
Rationale
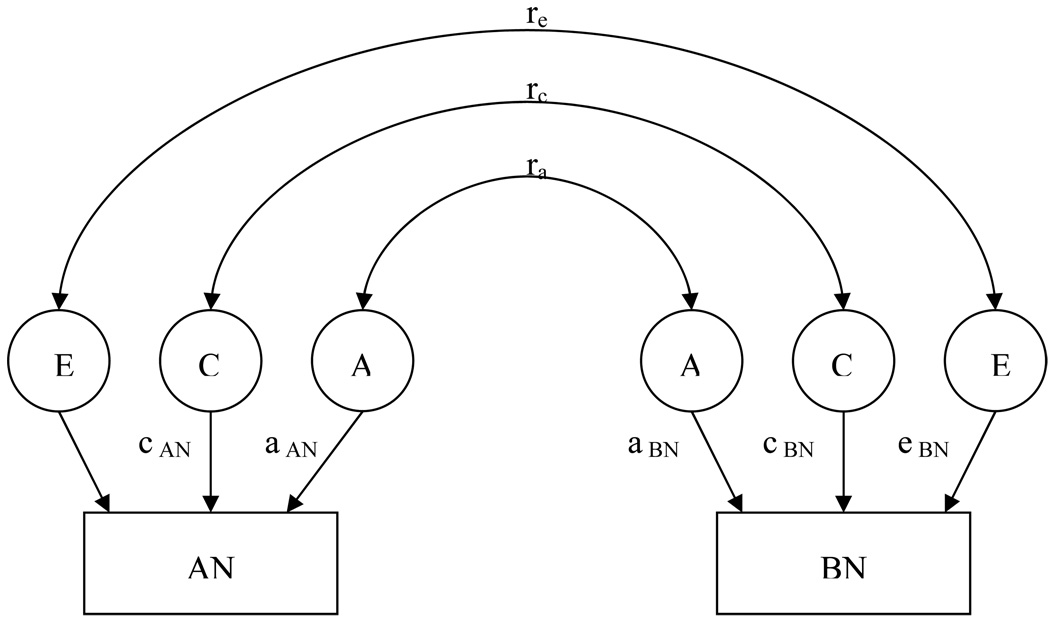
The classic twin study assesses factors influencing liability to a latent phenotype by estimating the proportion of variance due to: (1) additive genetic effects (i.e., heritability, a2); (2) shared (or common) environmental effects (c2); and (3) unique environmental effects (e2). The e2 estimate also includes measurement error. The sum of a2 + c2 + e2 = 1 (total variance). Additive genetic effects (A) represent the cumulative impact of several genes, each resulting in a small effect. Common or shared environmental effects (C) result from etiological influences both members of a twin pair are exposed to regardless of zygosity (e.g., parental income) and contribute equally to the correlations of MZ (rmz) and DZ (rdz) twins. Unique environmental effects (E) impact one twin but not the other (e.g., one twin suffers an injury requiring surgery and the other does not). Structural equation modeling determines the proportion of phenotypic variation attributable to genetic variation among individuals (heritability) and what proportions are due to shared and unique environmental factors.
Current study
A series of bivariate structural equation models using Cholesky’s decomposition was fitted using Mx (23) for narrow and broad AN and BN. We applied the full model including estimates for the three sources of variation a, c, and e for AN and for BN, and correlations indicating the proportion of variance that the two traits share due to genetic (ra), shared environmental (rc), and non-shared environmental (re) factors (Figure 1). We fit seven nested models varying the parameters to be estimated. Model selection was based on a statistical test for the difference in the −2lnL from the nested model and the full model, which is distributed as a chi-square and (24) where degrees of freedom (df) is equal to the difference between the df of the nested model to the full model. A non-significant result suggests no difference, or decrement, in model fit between the two models rendering the parsimonious model preferred. Model selection was also based on Akaike’s Information Criteria [AIC; (25)], a measure of the goodness of fit. The lowest AIC value indicates the best fitting model with regard to precision and complexity. We report the best-fitting model but focus our discussion on the full model as model selection based solely on AIC values can be misleading, especially when sample sizes are small (26). The raw ordinal data option was used incorporating both complete and in complete twin pairs in the analysis. Parameter estimates and 95% confidence intervals are reported.
Figure 1.
Graphical depiction of full bivariate twin model for lifetime history of anorexia nervosa and lifetime history of bulimia nervosa.
RESULTS
Sample Characteristics
In the initial eating disorders sample (10,510 males and 13,295 females), prevalences were: narrow AN (males 0.00% and females 0.70%); narrow BN (males 0.07% and females 1.10%); broad AN (males 0.09% and females 3.59%; broad BN (males 0.22% and females 2.72%).
In females, across zygosity groups, no differences in prevalence of narrow AN (χ2 = 5.62, df = 4, p < .23), narrow BN (χ2 = 1.73, df = 4, p < .79), broad AN (χ2 = 3.50, df = 4, p < .48), or broad BN (χ2 = 1.53, df = 4, p < .82) emerged.
Our final sample for twin modeling included 1,913 MZ and 1,360 DZ pairs with complete data, 51 MZ and 27 DZ pairs with incomplete data, and 172 MZ and 126 DZ individuals without cotwin information. The mean age was 33.0 years (SD = 7.6; range 20–47). BMI differences are reported in Table 2. Forty-one (0.6%) women met lifetime criteria for narrow AN, 78 (1.1%) met lifetime criteria for narrow BN, 237 (3.4%) met lifetime criteria for broad AN, and 190 (2.7%) met lifetime criteria for broad BN. Five individuals (3 MZ and 2 DZ) fulfilled lifetime criteria for both narrow AN and narrow BN and were incorporated into both the AN and the BN groups, corresponding to 12.2% of those with AN and 6.7% of those with BN. For broad AN and broad BN, 50 women (29 MZ and 21 DZ) met criteria for both: 21.1% of those with broad AN and 27.3% of those with broad BN. No significant differences between MZ and DZ twins emerged for the prevalence of narrow AN (χ2 = 2.57, p < .11), narrow BN (χ2 = 1.52, p < .22), broad AN (χ2 = 1.25, p < .27), and broad BN (χ2 = 0.07, p < .79).
Table 2.
Mean (std) lifetime lowest BMI and lifetime highest BMI for narrow and broad AN and BN and results comparing affected women to unaffected women.
| AN Mean (std) |
t-valuedf (p- value) |
BN Mean (std) |
t-valuedf (p- value) |
|
|---|---|---|---|---|
| Narrow | ||||
| Lifetime Lowest BMI (kg/m2) |
14.6 (2.0) | 13.06123 (<.001) | 18.6 (2.9) | 13.96150 (<.001) |
| Lifetime Highest BMI (kg/m2) |
23.7 (4.3) | 1.426121 (.16) | 25.8 (5.1) | −2.046150 (.041) |
| Broad | ||||
| Lifetime Lowest BMI (kg/m2) |
16.2 (1.9) | 29.3272 (<.001) | 18.9 (3.4) | 3.89188 (<.001) |
| Lifetime Highest BMI (kg/m2) |
23.4 (4.3) | 4.346099 (<.001) | 25.5 (5.4) | −1.83183 (.07) |
Twin Models
Anorexia Nervosa and Bulimia Nervosa
Polychoric correlation coefficients, 95% confidence intervals (CI), and number of concordant pairs for each correlation for the bivariate models are presented in Table 3. Point estimates should be interpreted in the context of the CIs and number of concordant pairs.
Table 3.
Polychoric correlation coefficients (r) and 95% confidence intervals (95% CI) for anorexia nervosa and bulimia nervosa
| Within Trait | Cross Trait | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anorexia Nervosa | Bulimia Nervosa | Within Twin | Cross Twin | |||||||||
| Zygosity | N* | Concordant/ Discordant Pairs** |
r (95% CI) |
N* | Concordant /Discordant Pairs** |
r (95% CI) |
N* | Concordant /Discordant Pairs** |
r (95% CI) |
N* | Concordant/Di scordant Pairs** |
r (95% CI) |
| NARROW | ||||||||||||
| MZ | 3874 | 2 / 22 | .58 (.29, .87) |
3928 | 6 / 38 | .65 (.45, .85) |
4071 | 3 / 71 | .39 (.15, .63) |
3874 | 1 / 58 | .15 (−.22, .52) |
| DZ | 2768 | 0 / 12 | NE† | 2776 | 0 / 26 | NE† | 2896 | 2 / 35 | .51 (.22, .80) |
2766 | 0 / 36 | NE† |
| BROAD | ||||||||||||
| MZ | 3852 | 9 / 120 | .34 (.16, .48) |
3928 | 15 / 78 | .63 (.49, .77) |
4058 | 29 / 200 | .54 (.44, .64) |
3852 | 13 / 174 | .27 (.11, .43) |
| DZ | 2730 | 0 / 85 | NE† | 2774 | 2 / 70 | .15 (−.16, .46) |
2877 | 21 / 122 | .60 (.48, .72) |
2726 | 1 / 128 | −.21 (−.50, .08) |
N = number of individuals used in the calculation;
number of concordant pairs used in the calculation;
Not Estimable. Within trait correlations reflect the correlation of AN and BN between members of twin pairs by zygosity group. Cross trait-within twin correlations reflect the correlation between AN and BN within individuals. Cross trait-cross-twin correlations reflect the correlations of AN in one twin with BN in the co-twin across zygosity groups.
The full model (i.e., model I) and seven nested models with varying parameters (models II-VIII) were estimated (Tables 4a and 4b). For narrow AN and BN, inspection of the χ2 difference tests suggest that only model VIII, in which all genetic effects were dropped, could be rejected as fitting significantly worse than the full model. Of the remaining models, model V (i.e., AE-AE, ra re) in which additive genetic and unique sources of variation were estimated for both narrow AN and BN (i.e., C pathways were constrained) represented the most parsimonious model as indicated by the lowest AIC value.
Table 4.
| Table 4a. Bivariate model fit statistics for narrow anorexia nervosa and narrow bulimia nervosa. | |||||
|---|---|---|---|---|---|
| Model No. | Model | -2lnL | df | χ2 diff (df) | AIC |
| I | ACE-ACE, ra rc re | 1308.03 | 13957 | --- | −26605.97 |
| II | ACE-ACE, rc re | 1311.80 | 13958 | 3.77 (1) | −26604.20 |
| III | ACE-ACE, ra re | 1308.03 | 13958 | 0.00 (1) | −26607.97 |
| IV | ACE-AE, ra re | 1308.03 | 13959 | 0.00 (2) | −26609.97 |
| V |
AE-AE, ra re
(best fit) |
1308.03 | 13960 | 0.00 (3) | −26611.97 |
| VI | AE-AE, ra | 1310.24 | 13961 | 2.21 (4) | −26611.76 |
| VII | CE-CE, rc re | 1312.55 | 13960 | 4.52 (3) | −26607.45 |
| VIII | E-E, re | 1343.53 | 13963 | 35.50 (6)* | −26582.48 |
| Table 4b. Bivariate model fit statistics for broad anorexia nervosa and broad bulimia nervosa. | |||||
|---|---|---|---|---|---|
| Model No. | Model | -2lnL | df | χ2 diff (df) | AIC |
| I | ACE-ACE, ra rc re | 3610.96 | 13926 | --- | −24241.05 |
| II | ACE-ACE, rc re | 3618.22 | 13927 | 7.26 (1)* | −24235.78 |
| III | ACE-ACE, ra re | 3610.96 | 13927 | 0.00 (1) | −24243.05 |
| IV | ACE-AE, ra re | 3610.96 | 13928 | 0.00 (2) | −24245.05 |
| V | AE-AE, ra re(best fit) | 3610.96 | 13929 | 0.00 (3) | −24247.05 |
| VI | AE-AE, ra | 3634.67 | 13930 | 23.71 (4) * | −24225.33 |
| VII | CE-CE, rc re | 3622.55 | 13929 | 11.59 (3) * | −24235.46 |
| VIII | E-E, re | 3671.23 | 13932 | 60.28 (6) * | −24192.77 |
Note. AIC = Akiake’s Information Criterion (25); −2lnL = −2 log likelihood; df = degrees of freedom; ACE = additive genetic, shared (or common) environment, and unique environmental effects model; AE = additive genetic and unique environmental effects model; CE = shared (or common) and unique environmental effects model; E = unique environmental effects model; ra = genetic correlation between AN and BN; rc = shared environmental correlation between AN and BN; re = unique environmental correlation between AN and BN
p < .05.
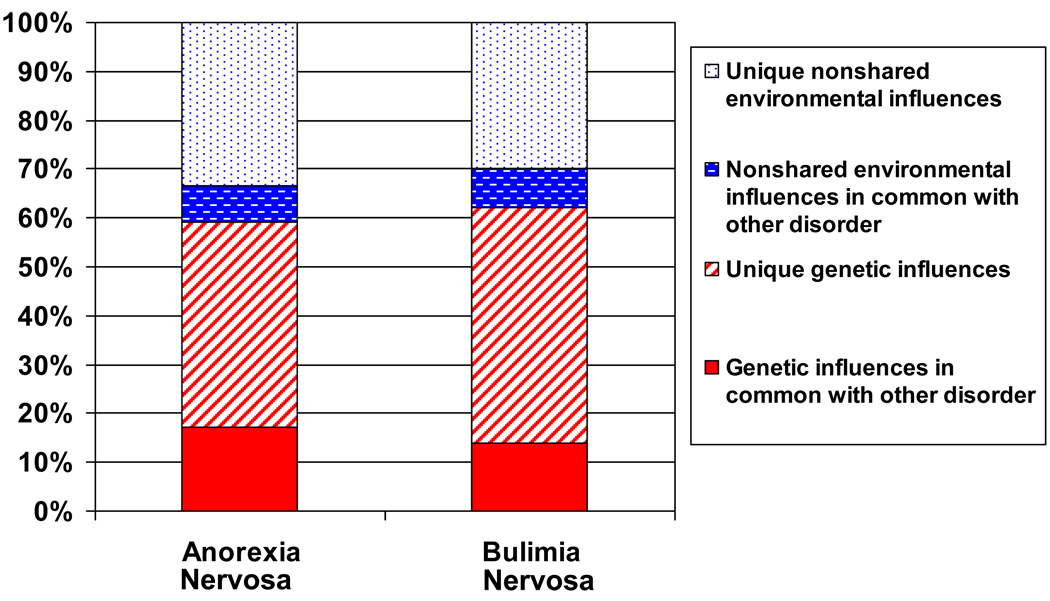
For narrow definitions, in the full model, for AN, a2 = .57; 95% CI: (.00, .81) and c2 = .00; 95% CI: (.00, .67) and for BN a2 = .62; 95% CI: (.08, .70) and c2 = .00; 95% CI: (.00, .40) (Table 5). The correlation between additive genetic factors for narrow AN and BN was ra = .46 and for unique environmental factors re = .43 (Figure 2).
Table 5.
Bivariate model parameter estimates (95% confidence interval) for full and best fit models for both narrow and broad anorexia nervosa and bulimia nervosa.
| Anorexia nervosa | Bulimia nervosa | Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model No. | Model | a2 | c2 | e2 | a2 | c2 | e2 | ra | rc | re |
| NARROW | ||||||||||
| I | ACE-ACE ra rc re |
.57 (.00, .81) |
.00 (.00, .67) |
.43 (.19, .53) |
.62 (.08, .70) |
.00 (.00, .40) |
.38 (.31, .60) |
.46 (.00, 1.00) |
−.67 (−1.00, 1.00) |
.43 (−.16, 1.00) |
| V |
AE-AE, ra re
(best fit) |
.57 (.23, .80) |
--- | .43 (.19, .57) |
.62 (.40, .75) |
--- | .38 (.25, .51) |
.46 (.00, .87) |
--- | .42 (−.16, .86) |
| BROAD | ||||||||||
| I | ACE-ACE ra rc re |
.29 (.04, .43) |
.00 (.00, .23) |
.71 (.57, .88) |
.61 (.24, .73) |
.00 (.00, .32) |
.39 (.27, .54) |
.79 (.46, 1.00) |
.94 (−1.00, 1.00) |
.44 (.20, .65) |
| V |
AE-AE, ra re
(best fit) |
.29 (.12, .42) |
--- | .71 (.58, .88) |
.61 (.46, .73) |
--- | .39 (.27, .54) |
.78 (.51, 1.00) |
--- | .44 (.20, .65) |
Note. For each model, the first set of parameters (ACE) refers to AN and the second set to BN. Values represent standardized parameters estimates with 95% confidence intervals in parentheses below. ACE = additive genetic, shared (or common) environment, and unique environmental effects model; AE = additive genetic and unique environmental effects model; CE = shared (or common) and unique environmental effects model; E = unique environmental effects model; ra = genetic correlation between AN and BN; rc = shared environmental correlation between AN and BN; re = unique environmental correlation between AN and BN.
Figure 2.
Graphical depiction of best-fitting narrowAN-BN bivariate model indicating common and specific genetic and environmental effects.
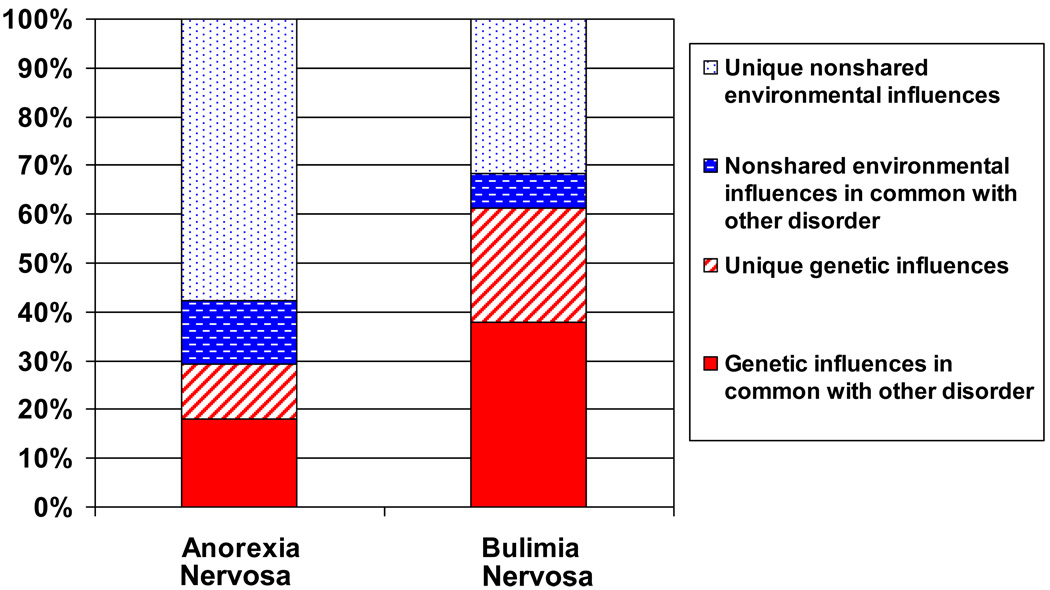
For broadly defined AN and BN, the χ2 difference tests indicate that models II, VI, VII and VIII could all be rejected as fitting significantly worse than the full model. The best fitting model was model V (i.e., AE-AE, ra re), where the C parameters were constrained to be zero. The genetic and environmental estimates for the full model were: for AN, a2 = .29; 95% CI: (.04, .43), c2 = .00; 95% CI: (.00, .23) and e2 = .71; 95% CI: (.57, .88), and for BN a2 = .61; 95% CI: (.24, .73), c2 = .00; 95% CI: (.00, .32) and e2 = .39; 95% CI: (.27, .54). The correlation between additive genetic factors for AN and BN was ra = .79 and for unique environmental factors re = .44 (Figure 3).
Figure 3.
Graphical depiction of best-fitting broad AN-BN bivariate model indicating common and specific genetic and environmental effects.
DISCUSSION
We applied bivariate twin modeling to determine the extent to which correlated genetic and environmental factors account for the concurrent and sequential comorbidity between AN and BN. The full models for both definitions of AN and BN indicated a substantial contribution of genetic factors to both disorders. In addition, the genetic correlation between narrow AN and BN was estimated to be .46 suggesting considerable, but not complete overlap in genetic liability to both syndromes.
Clinically, pure forms of AN (i.e. restricting subtype) and BN (i.e., no history of AN) exist. Yet, the majority of individuals with AN develop features of BN during the course of illness. Likewise, up to 27% of individuals with BN report a history of AN (1, 2) and 20–50% develop AN subsequent to meeting full criteria for BN (1). Our results suggest that about half of the genetic factors contribute to liability to both disorders while the remaining genetic factors contribute independently to AN and BN.
Our heritability estimates are broadly similar to those reported from other twin samples (17, 27–30). Using the full models, we found an additive genetic effect for narrow AN of .57 (95% CI: .00, .81) and for broad AN of .29 (95% CI: .04, .43). The estimate for narrow AN is consistent with previously published estimates from an independent Swedish cohort [.56 (95% CI; .00, .87)] (30). The fact that the heritability estimate decreases with broadening definition of illness suggests stronger genetic influences on narrower case definitions and is consistent with molecular genetic studies in which stronger linkage peaks were reported in more homogeneously defined AN samples (31). The observed parameter estimates for both narrow BN [.62 (95% CI: .08, .70] and broad BN [.61 (95% CI: .24, .73] were consistent with reports from other cohorts—.55 for narrow BN (32) and .60 for broad BN (33).
Our results must be appraised within the context of several limitations. First, diagnoses of AN and BN were based on computer-administered self-report. Although sensitive information may be more reliably reported via computer (34), added precision afforded by interview-based diagnosis may be lost. Second, although a 56% response rate is respectable for large population-based studies, it may have introduced bias. The prevalences of AN and BN in this sample fall within the confidence intervals of estimates from other Scandinavian population-based samples supporting the representativeness of the sample (35–37). Third, as the age of respondents was 20–47, all individuals might yet not have attained their ultimate diagnostic profile and crossover could still occur. Fourth, the power to provide estimates of common environmental factors is limited by the small number of concordant pairs leading to an inability to detect C in the presence of A. Fifth, the genetic and environmental correlations in these analyses is limited so the confidence intervals are broad. In the full and best fitting models for narrow phenotypes, the confidence interval for the genetic correlation includes zero, suggesting that the estimate may not be statistically significant. Sixth, our results apply only to females. Seventh, these results are specific to Sweden and generalization to other ancestry groups cannot be made.
Our study also has several strengths. We used a large population-based sample with sufficient numbers of cases of AN and BN to apply threshold DSM IV diagnostic criteria in addition to the broader case definitions. Although broadening of criteria is entirely defensible given the absence of evidence that individuals with amenorrhea differ from individuals without amenorrhea (AN) (38–40), and given no clear rationale for the frequency criterion of twice per week for BN (41); the impact of broadening case definitions is an important area of inquiry. This comparison yielded few differences for BN; however, broadening of the AN definition did impact heritability estimates suggesting caution when making AN definitions more inclusive.
Our results have explanatory power for the commonly observed concurrent presentations of AN and BN as well as the phenomenon of diagnostic crossover and may inform subsequent revisions eating disorders nosology. Currently, the only way to account for the presence of bulimic features in an individual with AN is if they currently meet diagnostic criteria for AN and warrant the inclusion of the binge/purge subtype indicator. If they currently meet criteria for AN restricting subtype, there is no mechanism to report a history of BN or binge/purge AN—a transition, which although less common, does indeed occur (2). An optimal diagnostic schema for eating disorders could account for both concurrent symptoms expression of AN- and BN-related traits as well as the historical presentation of one or the other eating disorder. Such a system is in line with a general trend in psychiatry to acknowledge and seek evidence for cross-disorder etiological overlap (42–44) while acknowledging and reflecting the existence of both shared and independent genetic risk factors for AN and BN and providing richer description for clinical utility.
DISCLOSURES AND ACKNOWLEDGEMENTS
Dr. Root was supported by National Institute of Health grant T32MH076694. This study was supported by grants CA-085739 (P.I.: P.F. Sullivan) and AI-056014 (P.I.: P.F. Sullivan) from the National Institutes of Health. The Swedish Twin Registry is supported by grants from the Swedish Department of Higher Education and the Swedish Research Council. All authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165:245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tozzi F, Thornton L, Klump K, Bulik C, Fichter M, Halmi K, et al. Symptom fluctuation in eating disorders: correlates of diagnostic crossover. Am J Psychiatry. 2005;162:732–740. doi: 10.1176/appi.ajp.162.4.732. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition Text Revision. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 4.Bulik C, PF S, Fear J, Pickering A. Predictors of the development of bulimia nervosa in women with anorexia nervosa. J Nerv Ment Disease. 1997;185:704–707. doi: 10.1097/00005053-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychol Med. 1995;25:143–156. doi: 10.1017/s0033291700028166. [DOI] [PubMed] [Google Scholar]
- 6.Fichter M, Quadflieg N, Hedlund S. Twelve-year course and outcome predictors of anorexia nervosa. Int J Eat Disord. 2006;39:87–100. doi: 10.1002/eat.20215. [DOI] [PubMed] [Google Scholar]
- 7.Gillberg IC, Rastam M, Gillberg C. Anorexia nervosa outcome: Six-year controlled longitudinal study of 51 cases including a population cohort. J Am Acad Child Adol Psychiatry. 1994;33:729–739. doi: 10.1097/00004583-199406000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Keel P, Dorer D, Franko D, Jackson S, Herzog D. Postremission predictors of relapse in women with eating disorders. Am J Psychiatry. 2005;162:2263–2268. doi: 10.1176/appi.ajp.162.12.2263. [DOI] [PubMed] [Google Scholar]
- 9.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10– 15 years in a prospective study. Int J Eat Disord. 1997;22:339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.van der Ham T, van Strien D, van Engeland H. A four-year prospective follow-up study of 49 eating-disordered adolescents: Differences in course of illness. Acta Psych Scand. 1994;90:229–235. doi: 10.1111/j.1600-0447.1994.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 11.Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry. 2005;187:573–578. doi: 10.1192/bjp.187.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderluh M, Tchanturia K, Rabe-Hesketh S, Collier D, Treasure J. Lifetime course of eating disorders: design and validity testing of a new strategy to define the eating disorders phenotype. Psychol Med. 2009;39:1–10. doi: 10.1017/S0033291708003292. [DOI] [PubMed] [Google Scholar]
- 13.Fichter MM, Quadflieg N. Long-term stability of eating disorder diagnoses. Int J Eat Disord. 2007;(40 Suppl):S61–S66. doi: 10.1002/eat.20443. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura H, Komaki G, Ando T, Nakahara T, Oka T, Kawai K, et al. Psychological and weight-related characteristics of patients with anorexia nervosa-restricting type who later develop bulimia nervosa. BioPsychoSoc Med. 2008;2:5. doi: 10.1186/1751-0759-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilenfeld L, Kaye W, Greeno C, Merikangas K, Plotnikov K, Pollice C, et al. A controlled family study of restricting anorexia and bulimia nervosa: comorbidity in probands and disorders in first-degree relatives. Arch Gen Psychiatry. 1998;55:603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 16.Walters EE, Kendler KS. Anorexia nervosa and anorexic-like syndromes in a population-based female twin sample. Am J Psychiatry. 1995;152:64–71. doi: 10.1176/ajp.152.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: shared genetic and environmental risk factors. Am J Psychiatry. 2000;157:469–471. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 18.Walters EE, Neale MC, Eaves LJ, Heath AC, Kessler RC, Kendler KS. Bulimia nervosa and major depression: a study of common genetic and environmental factors. Psychol Med. 1992;22:617–622. doi: 10.1017/s0033291700038071. [DOI] [PubMed] [Google Scholar]
- 19.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: phobia, generalized anxiety disorder, panic disorder, bulimia, major depression and alcoholism. Arch Gen Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen N. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen N, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the Third Millenium. Twin Research. 2002;5:427–432. doi: 10.1375/136905202320906219. [DOI] [PubMed] [Google Scholar]
- 23.Neale MC, Boker S, Xie G, Maes H. Mx: Statistical Modeling. 5th ed. Richmond, VA: Medical College of Virginia, Department of Psychiatry; 1999. [Google Scholar]
- 24.Neale M, Cardon L. Methodology for the Study of Twins and Families. Dordrecht, the Netherlands: Kluwer Academic Publisher Group; 1992. [Google Scholar]
- 25.Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- 26.Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behav Genet. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- 27.Kortegaard LS, Hoerder K, Joergensen J, Gillberg C, Kyvik KO. A preliminary population-based twin study of self-reported eating disorder. Psychol Med. 2001;31:361–365. doi: 10.1017/s0033291701003087. [DOI] [PubMed] [Google Scholar]
- 28.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 29.Mazzeo SE, Mitchell KS, Bulik CM, Reichborn-Kjennerud T, Kendler KS, Neale MC. Assessing the heritability of anorexia nervosa symptoms using a marginal maximal likelihood approach. Psychol Med. 2009;39:463–473. doi: 10.1017/S0033291708003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulik C, Sullivan P, Tozzi F, Furberg H, Lichtenstein P, Pedersen N. Prevalence, heritability and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 31.Grice DE, Halmi KA, Fichter MM, Strober M, Woodside DB, Treasure JT, et al. Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. Am J Hum Genet. 2002;70:787–792. doi: 10.1086/339250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of bulimia nervosa. Am J Psychiatry. 1991;148:1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- 33.Bulik C, Sullivan P, Kendler K. Heritability of binge-eating and broadly defined bulimia nervosa. Biol Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 34.Tourangeau R, Smith T. Collecting Sensitive Information with Different Modes of Data Collection. In: Couper M, Baker R, Bethlehem J, Clark C, Martin J, Nicholls W II, et al., editors. Computer Assisted Survey Information Collection. New York: Wiley; 1998. [Google Scholar]
- 35.Götestam K, Agras W. General population-based epidemiology study of eating disorders in Norway. Int J Eat Disord. 1995;18:119–126. doi: 10.1002/1098-108x(199509)18:2<119::aid-eat2260180203>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–1265. doi: 10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]
- 37.Keski-Rahkonen A, Hoek HW, Linna MS, Raevuori A, Sihvola E, Bulik CM, et al. Incidence and outcomes of bulimia nervosa: a nationwide population-based study. Psychol Med. 2008:1–9. doi: 10.1017/S0033291708003942. [DOI] [PubMed] [Google Scholar]
- 38.Gendall K, Joyce P, Carter F, McIntosh V, Jordan J, Bulik C. The psychobiology and diagnostic significance of amenorrhea in patients with anorexia nervosa. Fertil Steril. 2006;85:1531–1535. doi: 10.1016/j.fertnstert.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 39.Poyastro Pinheiro A, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. Patterns of menstrual disturbance in eating disorders. Int J Eat Disord. 2007;40:424–434. doi: 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- 40.Roberto CA, Steinglass J, Mayer LE, Attia E, Walsh BT. The clinical significance of amenorrhea as a diagnostic criterion for anorexia nervosa. Int J Eat Disord. 2008;41:559–563. doi: 10.1002/eat.20534. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan PF, Bulik CM, Kendler KS. The epidemiology and classification of bulimia nervosa. Psychol Med. 1998;28:599–610. doi: 10.1017/s0033291798006576. [DOI] [PubMed] [Google Scholar]
- 42.Psychiatric GWAS Consortium (PGC) Genomewide Association Studies: History, Rationale, and Prospects for Psychiatric Disorders. Am J Psychiatry. 2009;14:10–17. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- 44.Kas MJ, Fernandes C, Schalkwyk LC, Collier DA. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol Psychiatry. 2007;12:324–330. doi: 10.1038/sj.mp.4001979. [DOI] [PubMed] [Google Scholar]