Abstract
Cocaine produces its psychoactive and addictive effects primarily by acting on the brain’s limbic system, a set of interconnected regions that regulate pleasure and motivation. An initial, short-term effect—a buildup of the neurochemical dopamine—gives rise to euphoria and a desire to take the drug again. Researchers are seeking to understand how cocaine’s many longer term effects produce addiction’s persistent cravings and risk of relapse. In the author’s laboratory, work has focused on buildup of the genetic transcription factor ΔFosB. Levels of ΔFosB in the limbic system correlate with addiction-like behaviors in mice and may precipitate very long-lasting changes to nerve cell structure. Further pursuit of this and similar leads are first steps toward a complete understanding of the transition from cocaine abuse to addiction—and, ultimately, more effective treatments for those who are addicted.
Some 20 years ago, scientists identified the specific brain mechanisms that underlie the cocaine high. Since then, neurobiologists have focused on the followup questions: What does chronic cocaine abuse do to the brain to cause addiction? In clinical terms, how does repeated cocaine exposure make individuals compulsively continue to take the drug even when they know it may cost them their jobs, possessions, loved ones, freedom, and even their lives? Why do people with every reason and intention to quit for good find it so hard to get away from the drug, and why do they remain vulnerable to relapse after years of abstinence?
We do not yet have complete answers to these questions, but we have learned a great deal. We now know that cocaine affects brain cells in a variety of ways. Some of its effects revert quickly to normal. Others persist for weeks after the drug leaves the brain. With repeated exposure to cocaine, these short- and intermediate-term effects cumulatively give rise to further effects that last for months or years and may be irreversible.
This article presents in broad outline the emerging picture of the neurobiology of cocaine addiction. It begins with a brief review of cocaine’s immediate effects on brain function, then focuses on two more recently discovered types of effects: alterations in genetic activity that last for weeks, and alterations of nerve cell structure that last for months and possibly much longer. A protein called ΔFosB, currently under study by the author, provides an example—we suspect an important one—of how changes in gene activity can promote structural changes during the progression from abuse to addiction. Finally, the article discusses how investigations into the neurobiology of cocaine abuse are providing clues to cocaine vulnerability and the clinical implications of that research.
COCAINE’S INITIAL EFFECT: DOPAMINE BUILDUP
Snorted, smoked, or injected, cocaine rapidly enters the bloodstream and penetrates the brain. The drug achieves its main immediate psychological effect—the high—by causing a buildup of the neurochemical dopamine.
Dopamine acts as a pacesetter for many nerve cells throughout the brain. At every moment of our lives, dopamine is responsible for keeping those cells operating at the appropriate levels of activity to accomplish our needs and aims. Whenever we need to mobilize our muscles or mind to work harder or faster, dopamine drives some of the involved brain cells to step up to the challenge.
Dopamine originates in a set of brain cells, called dopaminergic (dopamine-making) cells, that manufacture dopamine molecules and launch them into their surroundings. Some of the free-floating dopamine molecules latch onto receptor proteins on neighboring (receiving) cells. Once attached, the dopamine stimulates the receptors to alter electrical impulses in the receiving cells and thereby alter the cells’ function.
The more dopamine molecules come into contact with receptors, the more the electrical properties of the receiving cells are altered. To keep the receiving cells in each brain region functioning at appropriate intensities for current demands—neither too high nor too low—the dopaminergic cells continually increase and decrease the number of dopamine molecules they launch. They further regulate the amount of dopamine available to stimulate the receptors by pulling some previously released dopamine molecules back into themselves.
Cocaine interferes with this latter control mechanism: It ties up the dopamine transporter, a protein that the dopaminergic cells use to retrieve dopamine molecules from their surroundings. As a result, with cocaine on board, dopamine molecules that otherwise would be picked up remain in action. Dopamine builds up and overactivates the receiving cells.
Although cocaine also inhibits the transporters for other neurotransmitter chemicals (norepinephrine and serotonin), its actions on the dopamine system are generally thought to be most important. To understand the powerful nature of cocaine’s actions, it is helpful to realize that dopamine pathways in the brain are very old in evolutionary terms. Early rudiments are found in worms and flies, which take us back 2 billion years in evolution. Thus, cocaine alters a neural circuit in the brain that is of fundamental importance to survival. Such alterations affect the individual in profound ways that scientists are still trying to understand.
COCAINE, DOPAMINE, AND THE LIMBIC SYSTEM
Cocaine produces dopamine buildup wherever the brain has dopamine transporters. However, its ability to produce pleasure and euphoria, loss of control, and compulsive responses to drug-related cues can all be traced to its impact on the set of interconnected regions in the front part of the brain that make up the limbic system (Hyman and Malenka, 2001; Kalivas and McFarland, 2003; Koob, Sanna, and Bloom, 1998; Nestler, 2001). Dopamine-responsive cells are highly concentrated in this system, which controls emotional responses and links them with memories.
One particular part of the limbic system, the nucleus accumbens (NAc), seems to be the most important site of the cocaine high. When stimulated by dopamine, cells in the NAc produce feelings of pleasure and satisfaction. The natural function of this response is to help keep us focused on activities that promote the basic biological goals of survival and reproduction. When a thirsty person drinks or someone has an orgasm, for example, dopaminergic cells flood the NAc with dopamine molecules. The receiving cells’ response makes us feel good and want to repeat the activity and reexperience that pleasure.
By artificially causing a buildup of dopamine in the NAc, as described above, cocaine yields enormously powerful feelings of pleasure. The amount of dopamine connecting to receptors in the NAc after a dose of cocaine can exceed the amounts associated with natural activities, producing pleasure greater than that which follows thirst-quenching or sex. In fact, some laboratory animals, if given a choice, will ignore food and keep taking cocaine until they starve.
The limbic system also includes important memory centers, located in regions called the hippocampus and amygdala. These memory centers help us remember what we did that led to the pleasures associated with dopamine release in the NAc—for example, where we found water and how we attracted a mate. When someone experiences a cocaine high, these regions imprint memories of the intense pleasure as well as the people, places, and things associated with the drug. From then on, returning to a place where one has taken cocaine or merely seeing images of cocaine-related paraphernalia triggers emotionally loaded memories and desire to repeat the experience. Scientists believe that repeated cocaine exposure, with its associated dopamine jolts, alters these cells in ways that eventually convert conscious memory and desire into a near-compulsion to respond to cues by seeking and taking the drug.
A third limbic region, the frontal cortex, is where the brain integrates information and weighs different courses of action. The frontal cortex acts as a brake on the other regions of the limbic system when we decide to forgo a pleasure in order to avoid its negative consequences. Activity here can help a nonaddicted person heed the disastrous prognosis of continued cocaine abuse and suppress drug-taking urges emanating from the NAc, hippocampus, and amygdala. Once someone becomes addicted, however, the frontal cortex becomes impaired and less likely to prevail over the urges (Nestler and Malenka, 2004; Volkow, Fowler, and Wang, 2003).
COCAINE’S INTERMEDIATE-TERM EFFECTS: CHANGES IN GENE EXPRESSION
Cocaine causes many types of intermediate-term alterations in brain cell functioning. For example, exposure to the drug can alter the amounts of dopamine transporters or dopamine receptors present on the surface of nerve cells. The changes involving genes, however, are particularly intriguing. They occur in the limbic system, the primary site for cocaine effects, and are sufficiently fundamental and long-lasting to contribute significantly to the transition from drug abuse to addiction.
Genes and Gene Expression
Genes determine the shape and function of every cell. Every individual is born with a unique combination of roughly 30,000 genes. Every cell in the body contains all 30,000. One cell differs from another—a liver cell looks and acts differently from a brain cell, for example—because, in each, certain genes are turned on, while others are turned off.
The popular notion that our genes never change is incorrect. It is true that the fundamental pattern of gene activation that gives each of our cells its essential properties is fixed once and for all during development. For example, once a cell develops into a liver cell, it remains a liver cell for life and cannot be converted into a brain cell. However, every cell retains the capacity to change the level of activity (expression) of a portion of its genes in response to the demands we place upon it. An example is weightlifting: Muscle cells respond to repeated exercise by increasing the expression of certain genes, leading to growth and strengthening of the individual cells and, collectively, of the entire muscle. So it is with brain cells: As we use them, they respond with changes in gene expression that, overall, increase their capacity to meet the demands we make upon them. For example, our brains register and store memories by altering gene expression in cells in the hippocampus and amygdala.
Cocaine affects the expression of numerous genes within the NAc, including some that influence the important neurotransmitter chemical glutamate and the brain’s natural opioid-like compounds produced by the body (Kalivas and McFarland, 2003; Nestler, 2001). In the author’s University of Texas laboratory, investigators have been studying cocaine’s effect on one particular genetic component, a protein called ΔFosB.
ΔFosB
Like dopamine, ΔFosB is a pace-setting chemical. However, instead of leaving the cell that produces it and stimulating neighboring cells as dopamine does, ΔFosB remains in its original cell and stimulates certain genes. Chemicals that act this way are called genetic transcription factors. While cocaine affects several transcription factors, its effects on ΔFosB are the most long-lasting.
ΔFosB is naturally present in small quantities in the cells of the NAc, but chronic cocaine exposure causes it to accumulate to high levels (Nestler, Barrot, and Self, 2001). Researchers believe ΔFosB may constitute an important molecular “switch” in the transition from drug abuse to addiction, mainly for three reasons:
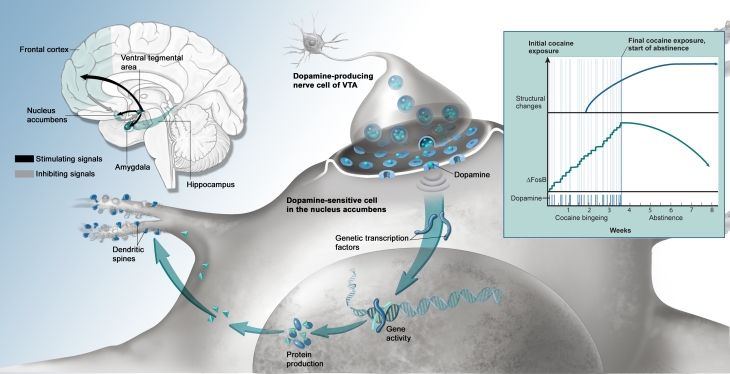
FROM THE RUSH TO THE ADDICTION, COCAINE'S EFFECTS IN THE BRAIN.
(Brain inset) Cocaine causes euphoria in the short term and addiction in the long term via its effects on the brain’s limbic system, which consists of numerous regions, including the ventral tegmental area (VTA) and nucleus accumbens (NAc), centers for pleasure and feelings of reward; the amygdala and hippocampus, centers for memory; and the frontal cortex, a center for weighing options and restraint.
(Main panel) Cocaine causes the neurotransmitter dopamine to build up at the interface between VTA cells and NAc cells, triggering pleasurable feelings and NAc cellular activities that sensitize the brain to future exposures to the drug. Among the activities are increased production of genetic transcription factors, including ΔFosB; altered gene activity; altered production of potentially many proteins; and sprouting of new dendrites and dendritic spines.
(Graph inset) The time courses of cocaine-induced buildup of ΔFosB and cocaine-related structural changes (dendrite sprouting) suggest that these neurobiological effects may underlie some of the drug’s short-term, medium-term, and long-term behavioral effects.
Once created, a molecule of ΔFosB lasts for 6 to 8 weeks before breaking apart chemically (Nestler, Barrot, and Self, 2001). Therefore, each new episode of cocaine abuse exacerbates the buildup of ΔFosB that has accumulated from all previous episodes during roughly 2 months. If someone is abusing cocaine daily, the levels of ΔFosB will be extremely elevated all the time.
Mice with elevated ΔFosB exhibit a set of behaviors that correspond to human addictive behaviors, while mice with normal levels do not. Conversely, blocking the buildup of ΔFosB in mice during a regimen of cocaine exposure reduces these behaviors.
ΔFosB plays a role in the genetic machinery that determines very basic properties of a cell, including very long-term or permanent ones such as its structure and interface with other cells.
Experimental Results With ΔFosB
The author’s research team hypothesized that increasing ΔFosB levels might promote addictive behaviors independently of cocaine’s other effects in the brain. To investigate this idea, we needed to find a way to control levels of ΔFosB in animals independently of cocaine exposure. Molecular biology gave us the tools to accomplish this. We bred a strain of mice that are genetically normal with one key difference: We can turn production of ΔFosB within the mouse NAc on and off, at will, by giving or withholding a chemical that is completely inert in normal animals.
We tested the animals’ response to cocaine while varying their ΔFosB levels. When we elevated levels of ΔFosB in the NAc, the mice exhibited behaviors that are considered reliable indicators that exposing people to the same conditions would cause addiction: They showed more sensitivity to the drug (responded to doses one-third those required to produce a response in normal animals), self-administered more drug, and displayed greater drive (or craving) for cocaine (they worked two to three times as hard to get the drug) (McClung et al., 2004; Nestler, Barrot, and Self, 2001). Conversely, when we blocked the activity of ΔFosB, we saw the opposite effects, an overall blunting of the animals’ response to the drug. These results suggest that cocaine’s buildup of ΔFosB is both necessary and sufficient for some of the drug’s behavioral effects and, in particular, its ability to increase drug craving and drug taking (Nestler, 2001).
Further Clues About ΔFosB’s Significance
The NAc is the only brain region where ΔFosB is found in normal animals. However, chronic administration of cocaine has recently been shown to increase ΔFosB in several additional brain regions, such as the frontal cortex and amygdala (McClung et al., 2004). The accumulations of ΔFosB are much smaller in these regions than those that cocaine causes in the NAc, and their behavioral consequences are still unknown. It is tempting to speculate, though, that the presence of ΔFosB in the frontal cortex may contribute to the loss of frontal cortex control over cocaine urges that is seen in addiction. Although we do not yet have direct evidence of this possibility, it represents an additional mechanism by which ΔFosB may contribute to a state of addiction.
Scientists currently are working to identify which specific genes ΔFosB stimulates to produce its effects. Comparisons of genes expressed in NAc nerve cells in mice that make ΔFosB versus mice that lack the transcription factor have revealed more than a hundred ΔFosB-mediated changes in gene expression (McClung and Nestler, 2003). This work has also indicated that ΔFosB causes more than 25 percent of all chronic cocaine-induced changes in gene expression in the NAc—a finding that highlights the dominant role of this transcription factor in mediating cocaine’s genetic effects in the brain. One of the genes stimulated by ΔFosB is an enzyme, cyclin-dependent kinase-5 (CDK5), which promotes nerve cell growth. This finding has shed new light on mechanisms underlying cocaine’s very long-lasting effects on the brain (Nestler, 2001).
COCAINE’S LONG-TERM EFFECTS: CHANGES IN NERVE CELL STRUCTURE
With its 2-month lifespan, ΔFosB does not last long enough to explain why former cocaine abusers continue to experience cravings and relapse after months and years of abstinence. The extreme persistence of those features of addiction indicates that cocaine must cause some equally long-lasting neuro-biological effects. Scientists have identified one potentially key type of cocaine-related change that appears to last for many months after the last cocaine exposure, and perhaps longer: an alteration in the physical structure of nerve cells in the NAc. Chronic cocaine exposure causes these cells to extend and sprout new offshoots on their dendrites (Nestler, 2001; Robinson and Berridge, 2001). Dendrites are the branch-like fibers that grow out from nerve cell bodies and collect incoming signals from other nerve cells. Just as a bigger antenna picks up more radio waves, more dendrite branches in the NAc theoretically will collect a greater volume of nerve signals coming from other regions—for example, the hippocampus, amygdala, and frontal cortex. This will give those other regions an enhanced influence over the NAc, which could drive some of the very long-lived behavioral changes associated with addiction. For example, enhanced inputs from the hippocampus and amygdala could be responsible for the intense craving that occurs when drug-associated memories are stimulated (e.g., by drug paraphernalia).
While we do not yet know how cocaine triggers NAc nerve cells to grow and sprout new offshoots, ΔFosB appears to be involved. Recall that one of the genes stimulated by ΔFosB is CDK5, a known regulator of nerve cell growth. When laboratory animals are treated with a compound that deactivates CDK5 in the NAc and then are given cocaine, the nerve cell growth normally associated with exposure to the drug does not occur.
INDIVIDUAL RISK FOR COCAINE ADDICTION
What makes certain individuals particularly vulnerable to addiction and others relatively resistant? Extensive epidemiological studies show that roughly half of a person’s risk for addiction to cocaine or other drugs is genetic (Goldstein, 2001; Nestler and Malenka, 2004). This degree of heritability exceeds that of many other conditions that are considered highly heritable, such as type 2 (non-insulin-dependent) diabetes, hypertension, and breast cancer.
The specific genes that confer risk for cocaine addiction remain unknown. One possibility is that at least some of them are the same genes that are affected by cocaine exposure. For example, variations in the genes encoding ΔFosB or any of hundreds of other genes affected by cocaine could conceivably contribute to the genetic risk for addiction. It is easy to imagine, by way of illustration, that an individual with a gene that expresses ΔFosB at high levels might be more prone to addiction; such a person would be analogous to the experimental mice that are engineered to produce more ΔFosB and are, consequently, more addiction prone. It is also possible that other genes—genes not affected by cocaine exposure—are responsible. Work is now under way to examine these alternatives.
Finding addiction vulnerability genes will enable us to identify individuals who are at particular risk for an addictive disorder and target them for educational and other preventive measures. It will also help us understand how factors other than genetics contribute to the development of addiction. For example, it has long been known that stress can increase an individual’s risk for addiction, but how stress produces this effect, and why it does so in some individuals but not others, remains a mystery.
CLINICAL RAMIFICATIONS
Research to understand the neurobiology of cocaine addiction is essential because the available treatments do not work for everyone, and the surest path toward definitive treatments and even cures, as well as prevention, is through greater appreciation of the underlying neurobiological mechanisms (Goldstein, 2001; O’Brien, 2003). The identification of underlying biological mechanisms has been crucial for all major advances in treatment of other medical disorders, and there is no reason to think addiction will be any different.
To date, most efforts to develop new medications for treatment of cocaine addiction have focused on preventing or suppressing the drug’s acute effects. Cocaine “vaccines,” for example, are designed to bind cocaine molecules in the blood with antibodies and so keep them from getting into the brain. A related approach seeks to develop a medication that keeps cocaine from tying up the dopamine transporter without itself interfering with the transporter’s normal function of dopamine retrieval. Still other approaches attempt to take advantage of the fact that cocaine’s acute effects on the brain involve increased activation of dopamine receptors. NAc nerve cells make five types of dopamine receptors; drugs that affect the functioning of one or more of them could, in theory, produce a palliative effect on cocaine addiction. Efforts are under way in each of these areas, including clinical trials, but so far no clear breakthrough has been reported.
A potential limitation of these approaches is that they focus on cocaine’s initial actions, not on the long-lasting changes that are present in the brain once addiction has been established. A medication aimed at preventing or reversing such changes might be an effective approach for treating cocaine addiction. There are literally hundreds of proteins that could be targeted in development of such a medication. For example, ΔFosB, or any of the hundred or so proteins it regulates, represent possible drug targets. The same is true for numerous additional molecular changes that have been implicated in cocaine addiction. Glutamate receptors and receptors for the brain’s natural opioid-like substances (e.g., κ opioid receptors) are two examples.
Effective medications for treating cocaine addiction will eventually be developed, and the best strategy for progress in this area is to target neurobio-logical mechanisms, such as those described above. Although the process takes a very long time—it can take 10 to 20 years to advance from identification of a disease mechanism to development of a new treatment—this work is in progress and represents the best hope for those who are addicted.
People often ask: Is it possible to treat a drug addiction with another drug? Isn’t addiction a complex psychological and social phenomenon that requires psychological and social treatments? The answer to both questions is “yes.”
Even though psychological and social factors predominate in the presentation and diagnosis of addiction, the disease is at its core biological: changes that a physical substance (drug) causes in vulnerable body tissue (brain). Today’s treatments do not effectively control the biology of addiction, leaving the addicted individual with a dramatically altered limbic system. He or she must then work against powerful biological forces to recover from addiction; those who succeed often do so only after many attempts, and many do not succeed.
While a medication that counters the powerful biological forces of addiction is essential, it will not be a “magic bullet.” People in recovery from addiction will always need support and rehabilitation to rebuild their lives. Presumably, effective psychosocial treatments for addiction work by causing changes in the brain, perhaps even some of the same changes that will be produced by effective medications. While very little information is currently available on the neurobiological mechanisms underlying psychosocial treatments, this is a topic of great interest.
CONCLUSION
In the last two decades, scientists have determined how cocaine produces intoxication through its initial effects in the brain’s limbic system, and we are beginning to understand the neurobiological mechanisms underlying the drug’s later developing and longer lasting effects of craving and relapse vulnerability. Among the most intriguing of these mechanisms is elevation of the genetic transcription factor ΔFosB, a molecule that lasts for approximately 2 months and theoretically can promote neuron structural changes that have potentially lifelong persistence. The most important goal for the next decade is to translate the knowledge we have already gained, along with any future advances we make, into better treatments for addiction.
ACKNOWLEDGMENTS
This work was supported by NIDA grants R37-DA-07359, P01-DA-08227, and K05-DA-00404.
REFERENCES
- Goldstein A. Addiction: From Biology to Drug Policy. 2nd ed. Oxford: Oxford University Press; 2001. [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nature Reviews. Neuroscience. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berlin) 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nature Neuroscience. 2003;6(11):1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McClung CA, et al. ΔFosB: A molecular switch for long-term adaptation. Molecular Brain Research. 2004;132(2):146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of neural plasticity underlying addiction. Nature Reviews. Neuroscience. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. ΔFosB: A molecular switch for addiction. Proceedings of the National Academy of Sciences of the USA. 2001;98(20):11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Malenka RC. The addicted brain. Scientific American. 2004;290(3):78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Research advances in the understanding and treatment of addiction. American Journal on Addictions. 2003;12(Suppl 2):S36–S47. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]