Abstract
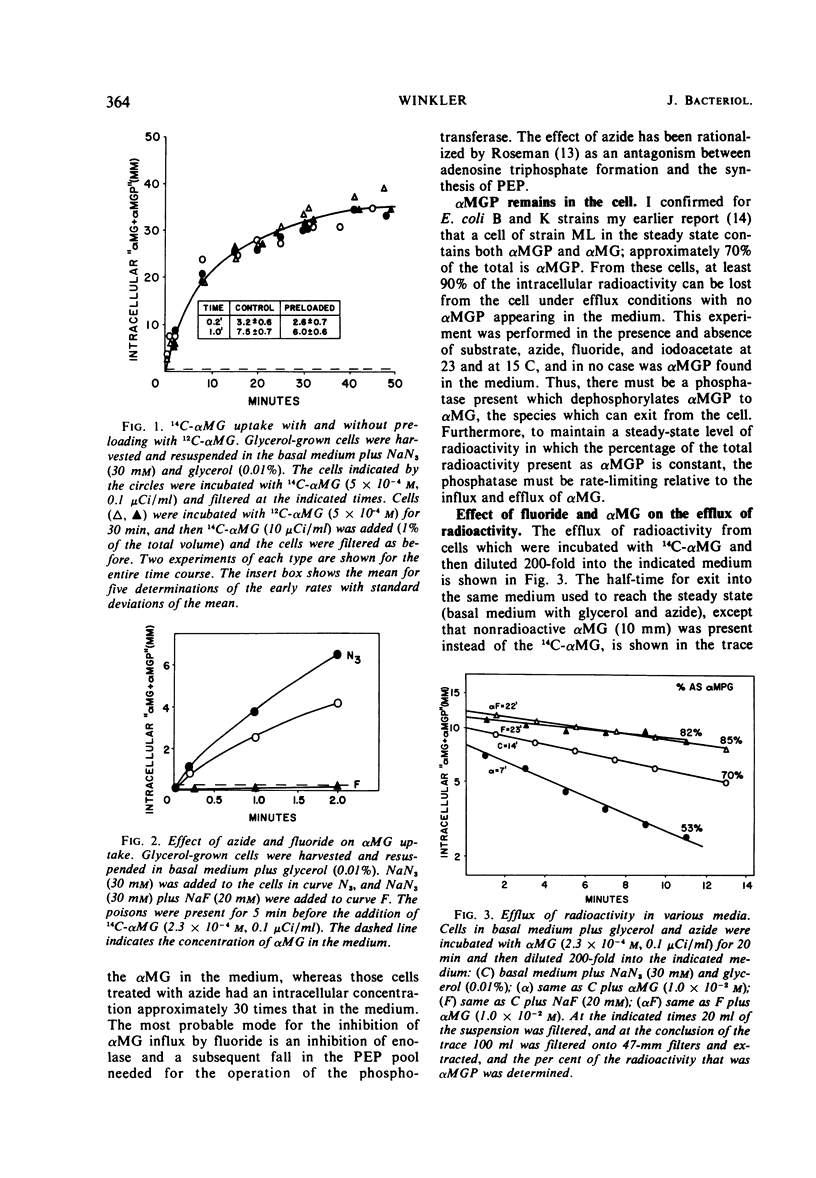
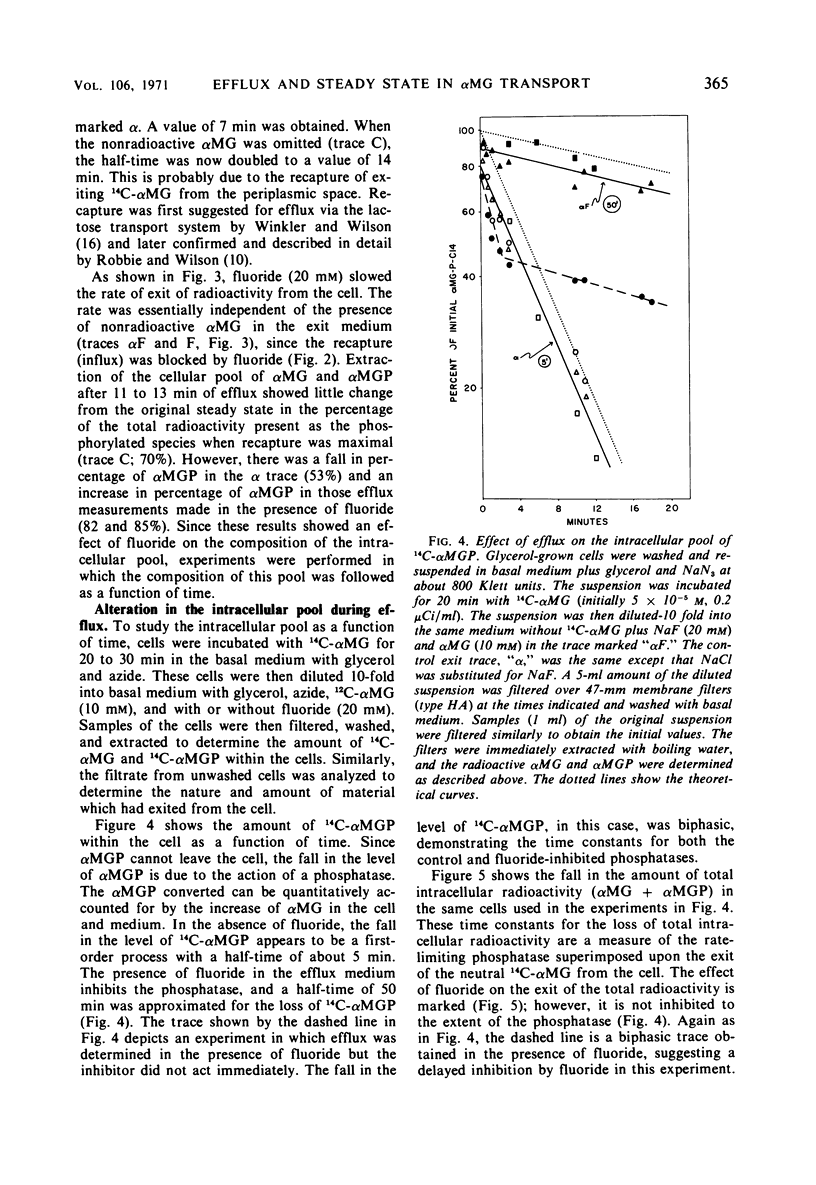
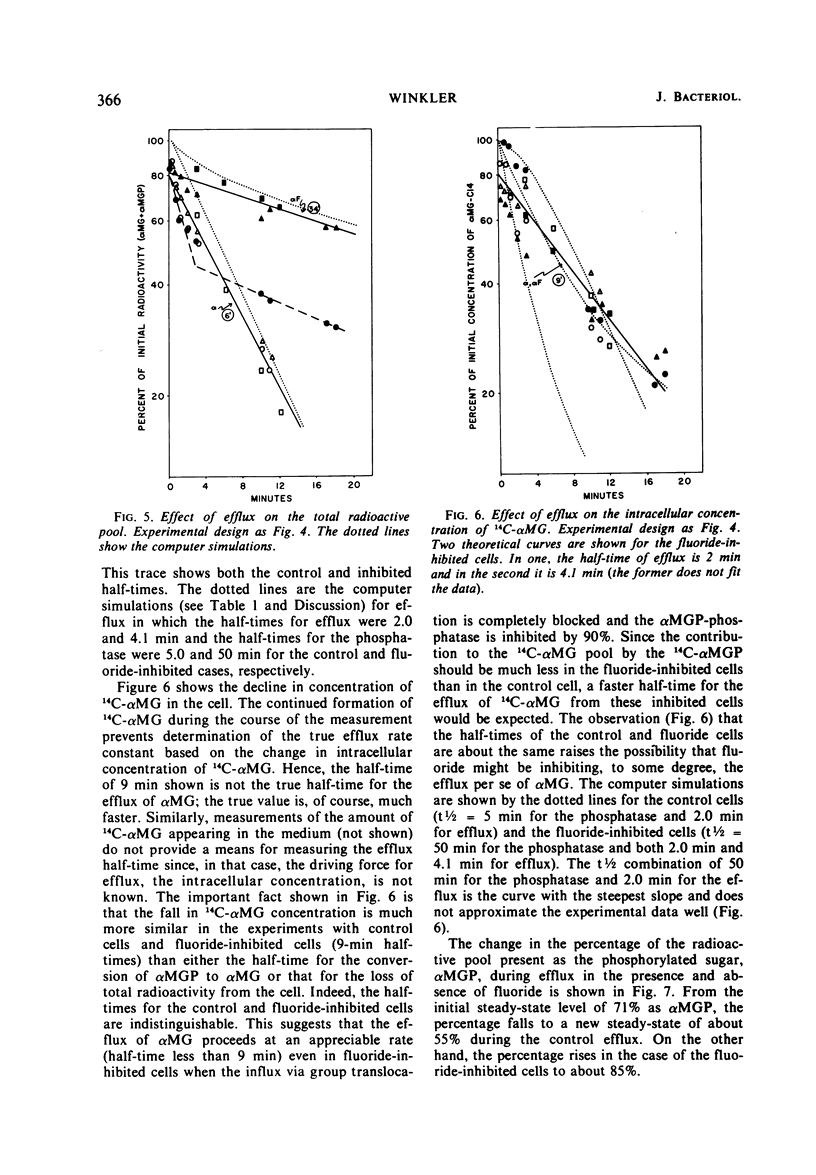
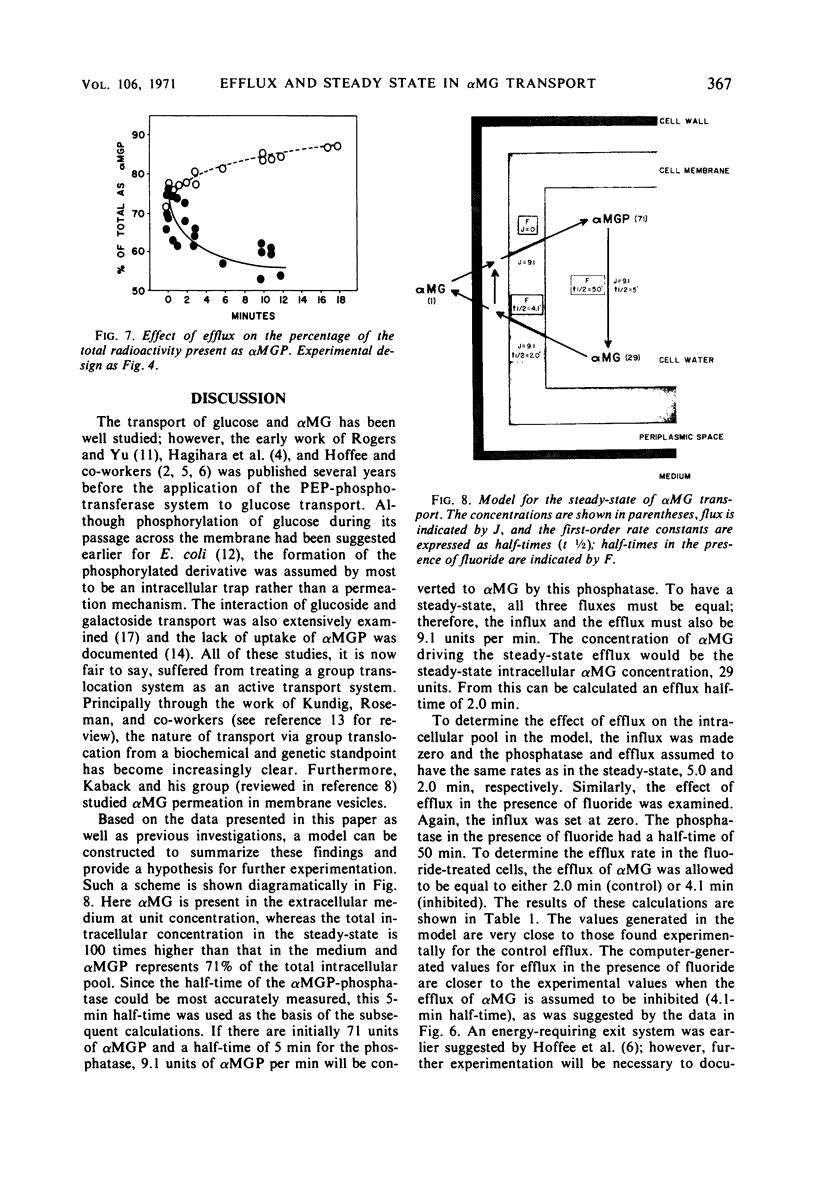
Efflux and the steady state in a group translocation system, the α-methylglucoside (αMG) transport system, were investigated. The maximum intracellular level of α-methylglucoside is a function of a steady state. There is no inhibition of αMG influx as the intracellular pool of αMG, and α-methylglucoside-6-phosphate (αMGP) rises. This steady state has three components: αMG influx, action of an αMGP phosphatase, and αMG efflux. The phosphatase is the rate-limiting step (half-time = 5.0 min); thus, the true efflux rate (half-time = 2.0 min) cannot be simply measured from the kinetics of αMG loss from the cell. Under our steady-state conditions the percentage of intracellular radioactivity present as αMGP was 71%. Under conditions of zero influx, after an efflux of 12 min the percentage present as αMGP fell to 55%. However, when fluoride was present during the efflux period, the percentage of the sugar as αMGP increased to about 85%. Fluoride greatly inhibits both influx and phosphatase activity (half-time = 50 min). The efflux of αMG from the cell is apparently also fluoride-sensitive but to a lesser extent (half-time = 4.1 min). These data are summarized in a model describing the three components of the steady-state and effect of fluoride.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Regulation of sugar transport in isolated bacterial membrane preparations from Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):724–731. doi: 10.1073/pnas.63.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D., YU S. H. Substrate specificity of a glucose permease of Escherichia coli. J Bacteriol. 1962 Nov;84:877–881. doi: 10.1128/jb.84.5.877-881.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D., YU S. H. TURBIDITY CHANGE DURING GLUCOSE PERMEATION IN ESCHERICHIA COLI. J Bacteriol. 1963 May;85:1141–1149. doi: 10.1128/jb.85.5.1141-1149.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie J. P., Wilson T. H. Transmembrane effects of beta-galactosides on thiomethyl-beta-galactoside transport in Escherichia coli. Biochim Biophys Acta. 1969 Mar 11;173(2):234–244. doi: 10.1016/0005-2736(69)90107-2. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Compartmentation in the induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1970 Feb;101(2):470–475. doi: 10.1128/jb.101.2.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. Inhibition of beta-galactoside transport by substrates of the glucose transport system in Escherichia coli. Biochim Biophys Acta. 1967;135(5):1030–1051. doi: 10.1016/0005-2736(67)90073-9. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]