Abstract
Classical conditioning models of addiction provide keys to understanding the vexing discrepancy between substance abuse patients’ desire to abstain when they are in therapy sessions and their tendency to relapse. Experiments using these models demonstrate the power of environmental relapse cues and support clinical approaches, including active exposure, aimed at helping patients recognize and withstand them. Internal cues, including emotions and somatic states such as withdrawal, can trigger urges as powerfully as external cues such as people, places, and things associated with prior abuse. The authors describe a cognitive-behavioral therapy approach that focuses on identifying and actively inducing each patient’s high-risk emotions, then helping him or her develop and practice healthy responses. Clinical trials support the approach for patients with panic disorder who have trouble discontinuing benzodiazepines, and early trials suggest it may be useful for patients addicted to other drugs as well.
Clinicians who treat chronic substance abuse disorders often encounter a striking mismatch between patients’ behaviors during sessions and their behaviors in everyday life. In the clinic, the patient may appear to be fully motivated to curb substance abuse. He or she seems to understand the tradeoff between the brief high abuse brings and its myriad negative long-term consequences. Outside the clinic walls, however, abuse continues. In colloquial terms, the patient “talks the talk” but cannot—at least not yet—“walk the walk.”
What accounts for this? One explanation is that patients simply learn to say things in session that they do not really mean. We are convinced, however, that most patients are sincere, at least while they remain in the unique context of the clinic setting. The problem is that their motivation and strategies for change do not “jump the gap” between the clinic and their daily lives. After all, a weekly 50-minute therapy session accounts for less than 1 percent of a patient’s waking life (Otto, 2000). With the goal of having the 1 percent of session time influence the 99 percent of time away from the clinic, special attention has to be placed on helping the motivations and behaviors evident in the clinic extend beyond the clinic walls.
This paper presents strategies for helping patients better apply the skills and motivations they have in session in their daily lives. We emphasize four principles:
Cue-induced cravings are key contributors to substance abuse and relapse;
Exposure therapy can weaken craving responses to cues;
Situational contexts may limit the effects of exposure therapy; and
Internal states constitute powerful cues and contexts for drug abuse.
In discussing these principles, we have two goals. First, we want to encourage clinicians to consider the role of situational cues when planning treatment, and help patients prepare for and become resilient to situations in which they may confront cues for relapse. This goal simply underscores the importance of evaluating high-risk situations for abuse and actively rehearsing alternative responses to these cues—a strategy that is central to many relapse prevention interventions (Carroll and Onken, 2005). Our second, more innovative goal is to encourage clinicians to consider internal states (e.g., emotions and somatic states such as withdrawal sensations) as important contexts and cues for drug abuse and to include evaluation and exposure to emotional cues in treatment.
We first provide a brief review of classical conditioning models of addiction. We then examine the important role of context in classical conditioning research and practice and describe a variant of cognitive-behavioral therapy (CBT) that emphasizes emotional exposure as a strategy to reduce substance abuse linked to emotional cues and contexts. Finally, we review the research history that led us to this perspective on the care of individuals with substance abuse disorders and discuss which patients may be likely to respond to an approach of this kind.
CLASSICAL CONDITIONING AND DRUG CRAVING
Attention to the link between environmental cues and drug abuse is supported by addiction models based on classical conditioning. In classical conditioning, a stimulus—for example, a sight, sound, or smell—may take on a new meaning by being associated with an alternative stimulus. For example, in the animal laboratory, a tone may take on a meaning such as “impending danger” if it is paired repeatedly with a shock. In substance abuse disorders, the meaning of a stimulus is similarly changed by repeated pairings with substance abuse. For example, the sight, sound, or smell of a substance of choice (e.g., the sight of a dealer, syringe, or rolling papers for heroin or marijuana abuse; or the sound of ice tinkling in a glass or the smell of rum in the local bar for alcohol abuse) takes on a new meaning (e.g., “a high is coming”). In addition to the sights or smells of alcohol or drugs, or drug paraphernalia, drug cues may also include specific locations (e.g., the bar, certain friends or houses) where substances are abused. Over time, these cues develop the capacity to elicit alcohol or drug cravings that can be observed reliably in laboratory settings (Carter and Tiffany, 1999; Drummond et al., 1995; Siegel, 1983) and play a role in continued substance abuse. Individuals with substance abuse disorders may feel they have the skills to resist drugs when in the hospital or in the clinic. However, when they are confronted with the cues in daily life, they may feel helpless against urges to take drugs.
CONDITIONED CUES AND EXPOSURE INTERVENTIONS
Efforts to increase individuals’ resistance to drug urges have focused on training patients not to respond to cues. In particular, exposure to drug or alcohol paraphernalia has been used to try to extinguish the link between cues and drug-craving urges. When individuals are repeatedly exposed to the sights or smells of a substance in the absence of actual drug taking, these cues lose some of their power to elicit the expectation of drug effects. They no longer reliably predict that a “high” is coming—that is, they are extinguished. To illustrate this extinction effect, imagine an individual who has abused cocaine consistently when he has seen or had access to it. In an exposure paradigm, he would repeatedly view white powder on a mirror, but not take it; over time, this particular stimulus would lose some of its ability to signal that a high was coming, and cravings would dissipate. As cravings wane, individuals are assumed to be more resilient in high-risk situations—that is, able to say “no” when confronted with these cues.
Cue exposure approaches have yielded promising results as part of comprehensive cognitive-behavioral treatments (e.g., Drummond and Glautier, 1994; Franken et al., 1999; O’Brien et al., 1990; Powell, Gray, and Bradley, 1993). For example, Monti and colleagues (1993) compared two groups of alcohol abusers in an inpatient setting. Both groups engaged in typical inpatient treatment activities; participants in one group also were exposed to the sights and smells of alcohol, and rehearsed coping strategies while imagining themselves in high-risk settings. When outcomes were compared, the patients who received exposure therapy had more days of abstinence and consumed less alcohol on days when they drank. The difference was significant despite evidence that cue-related cravings may affect patients with alcohol abuse disorders relatively less than patients with other substance abuse disorders (Carter and Tiffany, 1999).
The Role of Context
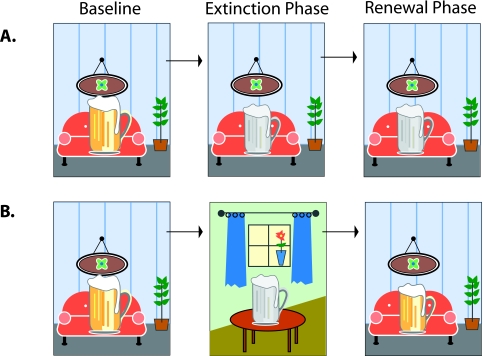
Despite the promise of cue exposure approaches, limitations in their application have emerged. A wealth of animal research indicates that the power of cue exposure varies depending on the context in which it occurs (for a review, see Bouton, 2002). For example, although animals apparently lost drug-seeking behaviors after prolonged exposure (extinction) in one situational context, these behaviors re-emerged when the context was changed (Crombag and Shaham, 2002). The implication of these findings is that cravings in response to drug cues may be successfully weakened in the setting of the clinic, but may resurface when the same cues are encountered away from therapy. Clinical studies support this inference. For example, Collins and Brandon (2002) repeatedly exposed collegiate moderate-to-heavy drinkers to the sight and smell of beer without letting them have any (Figure 1). The intervention successfully reduced participants’ behavioral and self-report measures of urges to drink. However, participants who were later retested in a different context had a greater return of these urges than those who were retested in the extinction context.
FIGURE 1. Impulses extinguished in one context can recur in another.
Baseline: College students handled and smelled beer in a room. The experience triggered an urge to drink the beer. Extinction phase: The students handled and smelled beer repeatedly without being allowed to drink it, some in the same room (Condition A) and some in another room (Condition B). After many such exposures, the experience triggered only a much attenuated urge to drink the beer. Renewal phase: Later, all the students were again exposed to beer in the original room. Those who had experienced the extinction phase in that room again reported a reduced urge, but those who had experienced it in the other room reported an urge as strong as during the baseline exposure. Based on Collins and Brandon, 2002.
The Importance of Internal Contexts
One of the more interesting advances in studies of exposure effects is the finding that they can be dependent on internal contexts as well. For example, renewal effects in animals have been demonstrated to occur with changes in internal contexts as defined by deprivation state (e.g., Davidson, 1993), mood state (e.g., Bower, 1981; Eich, 1995), hormonal state (e.g., Ahlers and Richardson, 1985), and drug state (e.g., Bouton, Kenney, and Rosengard, 1990). Regarding the internal context shifts from the administration of anxiety-modifying drugs, Bouton and colleagues (1990) found that behaviors extinguished during one drug condition (i.e., administration of either saline or a benzodiazepine) re-emerged to a greater degree when the animals were tested under the opposite drug condition (e.g., saline for an animal that received benzodiazepine during the extinction procedure—a switch in internal context).
These effects extend to human learning in CBT. For example, Mystkowski and colleagues (2003) demonstrated the relevance of shifts in internal context during treatment for fear of spiders. The researchers manipulated patients’ internal states by giving them either caffeine or placebo while providing CBT with exposure to spiders. Exposure treatment was effective, and no difference between patient groups was evident immediately after treatment. However, followup testing 1 week later revealed that patients tested under the incongruent condition (e.g., treated while taking caffeine and later tested while taking placebo, or vice versa) had a greater return of fear.
The important role of internal contexts is indicated by the prominence of feeling cues and contexts in drug abuse patterns. Several lines of evidence suggest that many of the cues for drug abuse are internal emotional and physical cues rather than external cues (Lowman, Allen, and Stout, 1996; O’Connell and Martin, 1987; Wikler, 1965). For example, in a retrospective study of opiate abusers, the majority of relapses were triggered by interoceptive rather than environmental cues: 32 percent of relapses occurred after negative emotional states, 32 percent followed negative physical states not characterized by withdrawal-like symptoms, 16 percent followed withdrawal-like states, and 5 percent followed positive emotional states (Chaney, Roszell, and Cummings, 1982). Similarly, drug craving seems to be enhanced by both currently existing (Robbins et al., 2000) and laboratory-induced negative mood states (Childress et al., 1994; Litt et al., 1990; Sherman et al., 1989).
Because an individual’s learning history is not precisely known, it is not clear whether the term “cue” or “context” best describes the role of emotion in increasing drug urges. In laboratory studies, cues and contexts can be precisely controlled; cues are reliably linked with drug administration, while contexts are the surroundings in which the linkage is established. Outside the laboratory, the distinction between what has been, over time, a direct cue versus a context is not known. Nonetheless, as reviewed earlier, both cue and context can play a role in increasing cravings and risk for relapse. Accordingly, attention to these factors in treatment is encouraged.
CBT WITH INTERNAL CUE EXPOSURE
Attention to internal cues and contexts is the central focus of our recent modification of CBT (Otto, Safren, and Pollack, 2004; Pollack et al., 2002). The centerpiece of our program is a “drug abuse protocol” that uses standard features of CBT (e.g., structured sessions providing motivational, cognitive-restructuring, problem-solving, and relaxation interventions), but emphasizes the role of emotional cues with interventions designed to:
Repeatedly expose patients to the emotional states and bodily sensations that serve as cues and contexts for drug craving and abuse, and
Help patients develop alternative responses.
The goal is to weaken the link between emotions and drug craving and abuse, while helping patients select and develop adaptive, nondrug responses to feelings of stress and distress (Otto, Safren, and Pollack, 2004; Pollack et al., 2002). Our work is similar to that of other researchers (e.g., Monti et al., 1993; Powell, Gray, and Bradley, 1993), except that we focus on internal rather than external cues.
We initiate the drug-abuse protocol by the third treatment session. Each 50-minute session targets drug-related activities during the past week and includes the following elements:
Identify external and internal cues that preceded drug abuse during the past week;
Discuss alternative responses to the cues;
Induce the most relevant (primarily emotional) cues;
Practice labeling and accepting the emotional states;
Rehearse one or more nondrug responses (e.g., in-the-moment activities, behavioral self-control strategies, acceptance, and relaxing with the emotions); and
Assign home practice of the exposure and alternative responses during the subsequent week.
The clinician induces the patient’s emotional cues by using imagery (e.g., imaginatively reviewing events in the patient’s past to induce sadness or envy) or behavioral strategies (e.g., staring at a blank wall for boredom or using mild hyperventilation to induce anxiety-like sensations; see Table 1; Otto et al., 2003; see also Otto, Safren, and Pollack, 2004). Once the patient gets into the mood, he or she and the clinician identify and challenge high-risk thoughts (e.g., “I can’t stand this feeling!”). The clinician asks the patient to note the feeling without doing anything to manage it. Finally, the clinician and the patient work together to select adaptive alternative behaviors to drug abuse, and the patient rehearses them in the presence of the cues.
TABLE 1.
Inducing emotions and sensations
Many emotions are induced by the therapist describing and asking the patient to imagine a past or potential future emotionally challenging event. All such imaginal inductions use realistic scenarios, often actual recent experiences of the patient.
Sensations, particularly those associated with anxiety or withdrawal, often are induced with interoceptive exposure exercises (e.g., hyperventilation).
No induction is undertaken without consideration of the patient’s physical and emotional health in relation to the content, intensity, and duration of exposures (e.g., no headrolling for patients with preexisting neck pain).
| EMOTION OR SENSATION | SAMPLE METHOD OF INDUCTION |
|---|---|
| Sadness or Grief | Imaginal review of the death of a loved one or pet |
| Guilt | Imaginal review of child-rearing regrets |
| Frustration/Anger | Imaginal review of disputes with roommates, spouses, etc. |
| Envy/Frustration | Imaginal review of other people having more advantages (e.g., better cars, money in the bank, etc.) |
| Boredom | 1 minute of staring at a blank space on the office wall, thinking “I am so bored” |
| Embarrassment/Frustration | Working simple math problems aloud or spelling aloud |
| Other Emotional Distress | Reading a personally distressing account from the newspaper |
| Dizziness/Disorientation | Rolling the head in a circle while seated |
| Anxiety, Dizziness, Lightheadedness, Numbness, Tingling, Hot Flushes, Visual Distortions | Hyperventilation (1 minute of 1-per-second breaths) |
| Anxiety, Agitation, Trembling, Muscle Heaviness, or Numbness | Full body tension (1 minute of clenching the jaw, shoulders, abdominal muscles, arms, legs, feet) |
The clinician introduces the patient to cognitive self-control procedures and cognitive-restructuring techniques to aid the transition from unhealthy to healthy responses to emotional cues. For example, the patient learns to think, “This feeling won’t last forever, and there are useful things I can do while I’m feeling this way.” Clinicians guide patients in thinking through high-risk situations, comparing the cost-benefit ratio of different courses of action.
Patients also receive training in diaphragmatic breathing (Garssen, de Ruiter, and van Dyck, 1992) and muscle relaxation (Klajner, Hartman, and Sobell, 1984). These procedures reduce anxiety, muscle tension, and other physical sensations associated with craving cues, including drug withdrawal symptoms. Patients rehearse them in the context of exposure to emotional craving cues, not as a means to avoid the emotions, but rather to enhance internal awareness and serve as in-the-moment recourses to avoid drug-taking responses.
When patients come to sessions not having abused drugs in the past week, the therapist applies the drug abuse protocol in a relapse prevention format. The therapist and patient together select internal and external scenarios that are likely to create risk in the coming week and address them through exposure rehearsal.
The drug abuse protocol’s informational, exposure, cognitive, and somatic skill interventions together are designed to weaken cue-induced drug cravings and equip the patient with skills for resilience. In addition, beginning early and continuing throughout treatment, clinicians train patients to develop pleasurable alternatives to chronic drug abuse. Such training is important, we believe, because without it the program would put patients into the difficult situation of trying to maintain abstinence while having time on their hands and lacking nondrug sources of enjoyment. The training consists of:
Problem solving: Clinicians introduce patients to longer term goal setting and to the technique of breaking larger goals into manageable steps (for a review, see Nezu et al., 1998). Progress on the steps is evaluated each week, and the clinician teaches the patient new problem-solving skills to practice at home.
Activity scheduling: Designed to complement problem solving, this therapy component provides patients with a set of activities to help structure satisfying, healthy lives (for a review, see Hopko et al., 2003). Interventions include the identification of positive events, identification of the problem-solving activities required to make these events accessible, and regular scheduling and monitoring of these activities.
The “Feel” of Treatment
Our therapy sessions follow a format that resembles those used in many cognitive-behavioral protocols. Each session includes, in order: a review of the previous week’s learning, formulation of an agenda for the session, completion of the agenda with attention to in-session rehearsal of concepts (including exposure practice), review of the session content itself, and assignment of home practice. This format maintains a consistent focus on the step-by-step, goal-oriented, skill acquisition process that is at the heart of cognitive-behavioral interventions (e.g., Beck, 1995).
Our intervention shares with other CBT approaches a focus on teaching patients to recognize the self-perpetuating chains of thoughts, feelings, and behaviors that characterize substance abuse disorders, along with behavioral strategies to change these patterns. Clinicians instill this learning through giving verbal instruction, as in informational interventions, and by using didactic or Socratic methods, as in many cognitive-restructuring interventions. Also, as in other CBT protocols, we employ self-monitoring, role playing, and exposure, and assign behavioral experiments. The goal is to make sure patients have alternative responses immediately accessible when they encounter situations or emotions or sensations that in the past have led them to abuse substances.
We maintain a consistent focus throughout our therapy manual on appropriate styles for the introduction and practice of interventions. One formal feature of our intervention, as of other manualized treatments (e.g., Hayes, Strosahl, and Wilson, 1999; Otto et al., 1996) is the active use of stories and metaphors to aid the acquisition of therapeutic concepts. These strategies cast therapeutic information in a format designed to be easily recalled in critical moments (see Otto, 2000).
Clinical Trials of the Intervention
Our intervention model grew out of our experiences helping patients withdraw from benzodiazepines (BZs) following treatment for panic disorder. Many patients fail to complete BZ tapers because they cannot tolerate the anxiety and panic that occur and reportedly rival or exceed in intensity the sensations that motivated the use of the medications in the first place (Fyer et al., 1987; Mellman and Uhde, 1986; Noyes et al., 1991). In a series of clinical trials, we showed that learning and practicing alternative responses in a context of repeated controlled exposures to these sensations can improve the chances of success.
In our first study (Otto et al., 1993), we treated 33 outpatients who had used high-potency BZs (alprazolam or clonazepam) for at least 6 months and were seeking help to stop taking the medication. We assigned them all to a slow BZ taper schedule and weekly supportive/informational meetings with a physician. Half also received an additional intervention consisting of 10 weekly 1-hour sessions delivered in a group therapy format (for a treatment manual, see Otto et al., 1996). This intervention aimed to eliminate catastrophic beliefs about the meaning and consequences of adverse sensations, regardless of their source, but with specific preparation for those that occur with BZ taper (e.g., muscle tension, anxiety, dizziness). In sessions starting 3 weeks prior to initiating the BZ taper, the patients assigned to the intervention performed simple exercises to induce these sensations: for example, moving the head rapidly back and forth to induce dizziness or hyper-ventilating to induce dizziness, lightheadedness, and numbness and tingling (Otto, Hong, and Safren, 2002). Along with exposure and cognitive restructuring, the patients received training in arousal management using diaphragmatic breathing, muscle relaxation, and similar techniques.
At the study’s end, 76 percent of the patients who received the adjunctive CBT intervention had successfully discontinued BZs, compared with just 25 percent of those who received only support and general information. Of the CBT-treated patients who completed the taper, 77 percent remained BZ-free for at least 3 months. In addition, two of three patients who crossed over from the no-CBT to the CBT group achieved successful BZ discontinuation on schedule.
Subsequently, two other research teams studied the use of CBT to enhance outcomes of BZ tapering following treatment for panic disorder. In an open trial, Hegel, Ravaris, and Ahles (1994) documented a successful discontinuation rate of 80 percent with a 12-week CBT program similar to ours, using information, exposure to panic sensations, and cognitive restructuring. In a randomized trial of 21 patients, Spiegel and colleagues (1994) treated patients with a panic-suppressing dose of alprazolam, then initiated a very slow taper program with or without adjunctive CBT. Most patients in both groups (80 percent of the taper alone group and 90 percent of the taper plus CBT group) achieved acute BZ discontinuation. However, after 6 months, only half the patients in the taper alone group were able to stay BZ-free, compared with all the patients who received the CBT program.
Although these studies supported the hypothesis that CBT can facilitate discontinuation of BZs, they did not control for the additional therapy time and support received by patients in CBT, raising questions as to the active elements in treatment. Therefore, our research group (Otto et al., 1999) performed a second study in which an additional control group received equal time in therapy, but focused on reducing withdrawal and anxiety symptoms with relaxation techniques. We found that our manualized CBT emphasizing exposure to interoceptive cues was significantly more effective for BZ discontinuation than the relaxation interventions.
In sum, research conducted to date supports the hypothesis that modifying responses to emotional and other internal cues crucially facilitates patients’ tolerance of BZ withdrawal symptoms. The results are consistent with the hypothesis that brief CBT can be used to reduce these cues and aid BZ discontinuation (Otto, Hong, and Safren, 2002).
Application to Treatment-Resistant Illicit Drug Abuse
We next evaluated our treatment model with opiate-dependent patients who continued to abuse multiple substances despite comprehensive therapy that included methadone, counseling, and contingency management (i.e., providing take-home methadone contingent upon drug-free weeks). We pilot-tested a 15-session program using the drug abuse protocol described.
In our first empirical test of this approach, we randomized 23 patients to either a control condition or our modified CBT delivered in 12 individual weekly sessions and three booster sessions scheduled for 2, 4, and 8 weeks following completion of the treatment. A detailed manual guided the treatment protocol (Otto et al., 2003). Patients in the control condition received the same amount of therapist time as those in the experimental group, along with an intensification of their normal therapy.
Compared with the doubling of contact with the drug counselor, our modified CBT was associated with significantly greater reductions in illicit drug abuse (documented by negative urine screens) for women, but not for men (Pollack et al., 2002). To try to understand this apparent sex difference in treatment response, we examined the literature for differences in drug abuse patterns between women and men. We found evidence that negative mood induction elicits stronger urges to abuse substances in women than in men (Monti et al., 1995; Rubonis et al., 1994). For example, Zywiak and colleagues (1996) reported that on the “Reasons for Drug Abuse” questionnaire, women score higher than men on the negative emotions factor, while men score higher than women on the positive emotions and social pressure factors. The hypothesis that women may be more responsive to treatments that enhance distress tolerance skills is consistent with our clinical observations during our study. In many cases, women reported drug abuse as a strategy to dull emotional pain (emotional avoidance), whereas it was more common for men to report drug abuse as a reward or as a way to enhance other tasks (e.g., “I only like playing pool when I’m high.”). These uncontrolled observations correspond well to findings suggesting that sensation seeking (pursuing adventure and relief from boredom) may play a more important role in men’s substance abuse behavior than women’s (Beck et al., 1995).
BRIDGING THE GAP
A central contribution of our approach to treatment—and the approach of some others (e.g., Collins and Brandon, 2002; Zvolensky et al., 2001)—is to emphasize the important role of context in drug abuse behaviors and the specific role of emotional cues and contexts in chronic substance abuse and relapse. We currently are engaged in NIDA-supported research to further investigate the importance of emotions in substance abuse disorders and the potential benefit of our treatment for individuals whose drug taking is characterized by emotion avoidance motives.
Our research investigating novel treatment options for substance abuse disorders has been influenced by the following principles:
In therapy, clinicians should consider strategies for helping patients bridge the gap between the adaptive attitudes and behaviors elicited in session and the chronic maladaptive patterns that may continue to dominate their lives out of session.
Among such strategies, clinicians should consider integrating therapeutic rehearsals and exposure procedures with the relevant internal and external cues and contexts experienced by their patients. By practicing adaptive behaviors in the context of these cues, clinicians may be able to enhance their patients’ ability to exhibit adaptive behaviors out of session.
ACKNOWLEDGMENTS
Support for some of the projects described herein was provided by NIDA grants to Dr. Otto (R10 DA 09692) and Dr. Pollack (R01 DA 10040); the writing of this manuscript was supported in part by grant R01 DA 017904 to Dr. Otto.
REFERENCES
- Ahlers ST, Richardson R. Administration of dexamethasone prior to training blocks ACTH-induced recovery of an extinguished avoidance response. Behavioral Neuroscience. 1985;99(4):760–764. doi: 10.1037//0735-7044.99.4.760. [DOI] [PubMed] [Google Scholar]
- Beck JS. Cognitive Therapy: Basics and Beyond. New York: Guilford Press; 1995. [Google Scholar]
- Beck KH, et al. Social context and sensation seeking: Gender differences in college student drinking motivations. International Journal of Addictions. 1995;30(9):1101–1115. doi: 10.3109/10826089509055830. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behavioral Neuroscience. 1990;104(1):44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36(2):129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. American Journal of Psychiatry. 2005;162(8):1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Chaney EF, Roszell DK, Cummings C. Relapse in opiate addicts: A behavioral analysis. Addictive Behaviors. 1982;7(3):291–297. doi: 10.1016/0306-4603(82)90058-2. [DOI] [PubMed] [Google Scholar]
- Childress AR, et al. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11(1):17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. Journal of Consulting and Clinical Psychology. 2002;70(2):390–397. [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behavioral Neuroscience. 2002;116(1):169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: Toward integration of physiological and learning perspectives. Psychology Review. 1993;100(4):640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier SP. A controlled trial of cue exposure treatment in alcohol dependence. Journal of Consulting and Clinical Psychology. 1994;62(4):809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Drummond DC, et al., editors. Addictive Behaviour: Cue Exposure Theory and Practice. New York: Wiley; 1995. [Google Scholar]
- Eich E. Searching for mood dependent memory. Psychological Science. 1995;6(2):67–75. doi: 10.1111/1467-9280.00249. [DOI] [PubMed] [Google Scholar]
- Franken IHA, et al. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. Journal of Substance Abuse Treatment. 1999;16(1):81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, et al. Discontinuation of alprazolam treatment in panic patients. American Journal of Psychiatry. 1987;144(3):303–308. doi: 10.1176/ajp.144.3.303. [DOI] [PubMed] [Google Scholar]
- Garssen B, de Ruiter C, van Dyck R. Breathing retraining: A rational placebo? Clinical Psychology Review. 1992;12(2):141–153. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy. New York: Guilford Press; 1999. [Google Scholar]
- Hegel MT, Ravaris CL, Ahles TA. Combined cognitive-behavioral and time-limited alprazolam treatment of panic disorder. Behavior Therapy. 1994;25(2):183–195. [Google Scholar]
- Hopko DR, et al. Contemporary behavioral activation treatments for depression: Procedures, principles, and progress. Clinical Psychology Review. 2003;23(5):699–717. doi: 10.1016/s0272-7358(03)00070-9. [DOI] [PubMed] [Google Scholar]
- Klajner F, Hartman LM, Sobell MB. Treatment of substance abuse by relaxation training: A review of its rationale, efficacy, and mechanisms. Addictive Behaviors. 1984;9(1):41–55. doi: 10.1016/0306-4603(84)90006-6. [DOI] [PubMed] [Google Scholar]
- Litt MD, et al. Reactivity to alcohol cues and induced moods in alcoholics. Addictive Behaviors. 1990;15(2):137–146. doi: 10.1016/0306-4603(90)90017-r. [DOI] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Section II. Marlatt’s taxonomy of high-risk situations for relapse: Replication and extension. Addiction. 1996;91(Suppl):s51–s171. [PubMed] [Google Scholar]
- Mellman TA, Uhde TW. Withdrawal syndrome with gradual tapering of alprazolam. American Journal of Psychiatry. 1986;143(11):1464–1466. doi: 10.1176/ajp.143.11.1464. [DOI] [PubMed] [Google Scholar]
- Monti PM, et al. Alcohol cue reactivity: Effects of detoxification and extended exposure. Journal of Studies on Alcohol. 1993;54(2):235–245. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Monti PM, et al. Smoking among alcoholics during and after treatment: Implications for models, treatment strategies, and policy. In: Ferig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Policy. Washington, DC: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- Mystkowski JL, et al. Changes in caffeine states enhance return of fear in spider phobia. Journal of Consulting and Clinical Psychology. 2003;71(2):243–250. doi: 10.1037/0022-006x.71.2.243. [DOI] [PubMed] [Google Scholar]
- Nezu AM, et al. Treatment maintenance for unipolar depression: Relevant issues, literature review, and recommendations for research and clinical practice. Clinical Psychology: Science and Practice. 1998;5(4):496–512. [Google Scholar]
- Noyes R, et al. Controlled discontinuation of benzodiazepine treatment for patients with panic disorder. American Journal of Psychiatry. 1991;148(4):517–523. doi: 10.1176/ajp.148.4.517. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, et al. Integrating systematic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behaviors. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1987;55(3):367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Otto MW. Stories and metaphors in cognitive-behavior therapy. Cognitive and Behavioral Practice. 2000;7(2):166–172. [Google Scholar]
- Otto MW, et al. Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavior therapy for patients with panic disorder. American Journal of Psychiatry. 1993;150(10):1485–1490. doi: 10.1176/ajp.150.10.1485. [DOI] [PubMed] [Google Scholar]
- Otto MW, et al. Stopping anxiety medication: Panic control therapy for benzodiazepine discontinuation (Therapist Guide) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Otto MW, et al. Further evaluation of panic control therapy for benzodiazepine discontinuation. Paper presented at the 33rd Association for Advancement of Behavior Therapy convention in Toronto; Canada. November 11–14.1999. [Google Scholar]
- Otto MW, et al. Therapist Manual for Cognitive-Behavior Therapy for Interoceptive Cues (CBT-IC) Boston, MA: Massachusetts General Hospital; 2003. Unpublished manuscript, WACC-812. [Google Scholar]
- Otto MW, Hong JJ, Safren SA. Benzodiazepine discontinuation difficulties in panic disorder: Conceptual model and outcome for cognitive-behavior therapy. Current Pharmaceutical Design. 2002;8(1):75–80. doi: 10.2174/1381612023396726. [DOI] [PubMed] [Google Scholar]
- Otto MW, Safren SA, Pollack MH. Internal cue exposure and the treatment of substance use disorders: Lessons from the treatment of panic disorder. Journal of Anxiety Disorders. 2004;18(1):69–87. doi: 10.1016/j.janxdis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Pollack MH, et al. A novel cognitive-behavioral approach for treatment-resistant drug dependence. Journal of Substance Abuse Treatment. 2002;23(4):335–342. doi: 10.1016/s0740-5472(02)00298-2. [DOI] [PubMed] [Google Scholar]
- Powell J, Gray J, Bradley BP. Subjective craving for opiates: Evaluation of a cue exposure protocol for use with detoxified opiate addicts. British Journal of Clinical Psychology. 1993;32(Pt. 1):39–53. doi: 10.1111/j.2044-8260.1993.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, et al. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug and Alcohol Dependence. 2000;59(1):33–42. doi: 10.1016/s0376-8716(99)00103-9. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, et al. Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol. 1994;55(4):487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Sherman JE, et al. Subjective dimensions of heroin urges: Influence of heroin-related and affectively negative stimuli. Addictive Behaviors. 1989;14(6):611–623. doi: 10.1016/0306-4603(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Siegel S. Classical conditioning, drug tolerance, and drug dependence. In: Israel Y, Glaser FB, Kalant H, Popham RE, Schmidt W, Smart RG, editors. Research Advances in Alcohol and Drug Problems. Vol. 7. New York: Plenum; 1983. pp. 207–246. [Google Scholar]
- Spiegel DA, et al. Does cognitive behavior therapy assist slow-taper alprazolam discontinuation in panic disorder? American Journal of Psychiatry. 1994;151(6):876–881. doi: 10.1176/ajp.151.6.876. [DOI] [PubMed] [Google Scholar]
- Wikler A. Conditioning factors in opiate addiction and relapse. In: Wilner DI, Kassenbaum GG, editors. Narcotics. New York: McGraw-Hill; 1965. pp. 85–100. [Google Scholar]