Abstract
Posttraumatic syringomyelia may result from a variety of inherent conditions and traumatic events, or from some combination of these. Many hypotheses have arisen to explain this complex disorder, but no consensus has emerged. A 28-year-old man presented with progressive lower extremity weakness, spasticity, and decreased sensation below the T4 dermatome five years after an initial trauma. Magnetic resonance imaging (MRI) revealed a large, multi-septate syrinx cavity extending from C5 to L1, with a retropulsed bony fragment of L2. We performed an L2 corpectomy, L1-L3 interbody fusion using a mesh cage and screw fixation, and a wide decompression and release of the ventral portion of the spinal cord with an operating microscope. The patient showed complete resolution of his neurological symptoms, including the bilateral leg weakness and dysesthesia. Postoperative MRI confirmed the collapse of the syrinx and restoration of subarachnoid cerebrospinal fluid (CSF) flow. These findings indicate a good correlation between syrinx collapse and symptomatic improvement. This case showed that syringomyelia may develop through obstruction of the subarachnoid CSF space by a bony fracture and kyphotic deformity. Ventral decompression of the obstructed subarachnoid space, with restoration of spinal alignment, effectively treated the spinal canal encroachment and post-traumatic syringomyelia.
Keywords: Syringomyelia, Magnetic resonance imaging, Trauma, Subarachnoid space, Obstruction
INTRODUCTION
Posttraumatic syringomyelia is a complicated disorder that involves a cystic or tubular cavity formed in the spinal cord after spinal injury. Progressive pain, loss of sensorimotor function, and bladder dysfunction characteristically follow the initial trauma by several years. Despite controversy surrounding the pathogenesis, cyst formation is believed to involve many factors, including hematoma, necrosis, liquefaction of the cord, arachnoiditis, and myelomalacia16,18). Cephalocaudal extension of the cavity may result from the "slosh and suck" phenomenon17).
Posttraumatic syringomyelia has been treated by syringotomy or by syrinx diversion operations such as the syringoarachnoid shunt, syringo-peritoneal shunt, or syringopleural shunt3,5,9,15). However, shunt-related complications [e.g., obstruction, infection, adhesion, cerebrospinal fluid (CSF) over-drainage] may impede neurological improvement, requiring a shunt revision surgery3,7,11). Some authors report that decompression laminectomy, involving creation of a wide opening in the subarachnoid space, is more effective than other shunt diversion surgeries6,10,12).
Our report concerns a patient with a spinal canal obstruction, not corrected at the time of trauma, and syringomyelia, observed five years later. We performed extradural osseous decompression to treat the syringomyelia, and the patient showed neurological improvement with collapse of the syrinx.
CASE REPORT
A 28-year-old man presented with lower extremity weakness, spasticity, and numbness that had been progressing over the previous 12 months. He had experienced a five meter fall at work six years before he came to the hospital. He suffered severe back pain at the time of the fall, but felt no weakness. Magnetic resonance imaging (MRI) revealed no spinal cord injury except for a fractured L2 vertebral body. He underwent a posterior thoracolumbar fixation from T12 to L3 to treat the L2 burst fracture, with no aftereffects other than mild back pain. In the subsequent five years, however, he experienced progressive weakness in the lower extremities and decreased sensation below the T10 dermatome. He underwent a second surgery at another hospital to remove a posterior screw, after which a thoracolumbar MRI showed a large syrinx extending from T2 to L1. By that time, the weakness in both lower extremities had worsened. Although he could still walk without support, he had, in the eight months prior to entering the other hospital, experienced many stumbles. He was referred to our institution for further management.
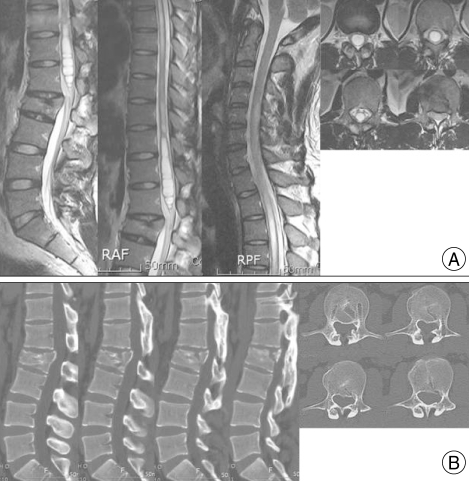
On examination, the patient had decreased feeling to light touches and pinpricks below the T4 dermatome area. The patient's gait also appeared to be ataxic and spastic. Strength testing of both lower extremities revealed weakness in the hip (4/5), knee (4/5), ankle (4/5), and big toe (4/5). Deep tendon reflexes were increased in the knee jerk and ankle jerk responses. Babinski's sign and ankle clonus were positive. Repeated MRI findings showed a large, multiseptate, non-enhancing syrinx from C5 to L1, with a bony fragment retropulsed from L2. The spinal cord was expanded, with compression estimated to be 56.4% at L2 (Fig. 1).
Fig. 1.
A 28-year-old man had progressive paraparesis five years after a fall. He complained of weakness in the lower extremities and decreased sensation below the T10 dermatome. A : Magnetic resonance images show a large multiseptate syrinx from C5 to L1, with a bony fragment retropulsed from L2. The spinal cord is expanded over the fractured L2 vertebra. B : Computed tomography shows the retropulsed bony fragment at the fractured L2 vertebra, and spinal curvature reveals the kyphotic deformity.
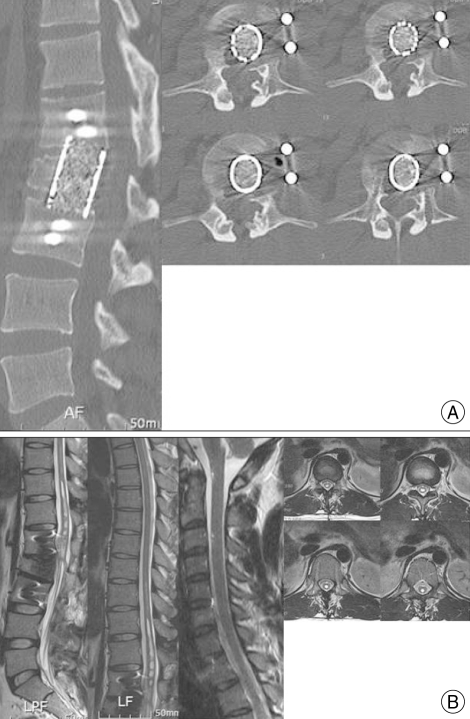
The clinical diagnosis was posttraumatic syringomyelia and a burst fracture of L2. We considered several surgical interventions, including syringotomy, shunt diversion, decompressive laminectomy and expansible duraplasty, and correction of the spinal deformity from the ventral side. Since the spinal canal stenosis had resulted from a bony intrusion, normal CSF circulation was hindered. We performed an L2 corpectomy to decompress the epidural space of the ventral spinal canal and a wide decompression of the ventral dural space. We also performed an L1-3 interbody fusion using a mesh cage and screw fixation (Fig. 2A).
Fig. 2.
A : Computed tomography after surgery shows a wide decompression in the ventral dural space of the spinal cord in the region of the L2 vertebral fracture, with improvement in the spinal stenosis. B : At three months post-surgery, magnetic resonance images show a marked reduction in the syrinx and improvement in cord signal changes from the rostral to caudal regions.
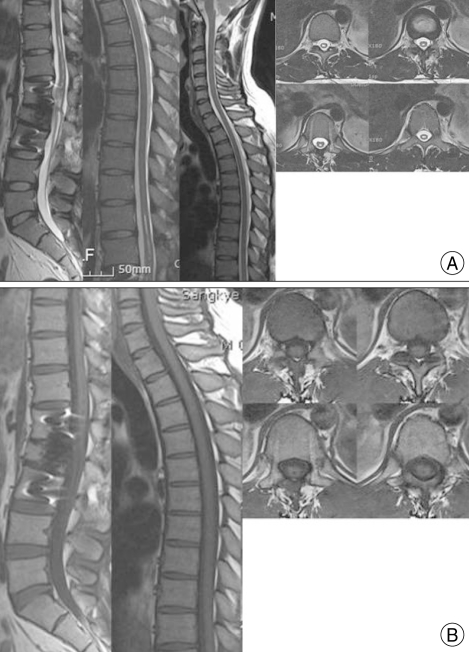
The patient showed gradual neurological improvement, notably in bilateral leg weakness and dysesthesia, at follow-ups three, six, and 12 months after surgery. Three months after the surgery, MRI showed a marked decrease in syrinx size, with re-establishment of normal subarachnoid CSF flow (Fig. 2B). At two years post-surgery, the patient had recovered full motor ability in both lower extremities, except for residual hypesthesia of the L2 dermatome. The last follow-up MRI confirmed that the syrinx had collapsed (Fig. 3). These findings indicate a good correlation between syrinx collapse and symptomatic improvement.
Fig. 3.
A : Magnetic resonance (MR) images at 12 months after decompressive surgery show some improvement in cord diameter and signal intensity. B : T1-weighted MR images show almost complete disappearance of the syrinx two years after surgery.
DISCUSSION
Syringomyelia was first described by Bastian in 18672). Post-traumatic syringomyelia has been increasingly recognized since the first report by Holmes in 19151). The time of onset may range from two months to 36 years (five years, in our patient) after the initial trauma1,14). Clinical symptoms include sensory loss, radical pain, spasticity, muscle weakness, urinary bladder dysfunction, and autonomic disorders such as hyperhidrosis. Among patients with spinal cord injuries, only 0.3-3.2% typically develop syringomyelia13,14).
The pathogenesis of syringomyelia is unclear. Cho et al.4) assert that the syrinx can develop at the injured site through scarring of the arachnoid, and they demonstrated in an experimental model that obstruction of the CSF pathway and subarachnoid obstruction by adhesive arachnoiditis play a prominent role in posttraumatic syringomyelia8). These studies support the hypothesis that disturbances of cerebrospinal fluid flow within the subarachnoid space may generate posttraumatic syringomyelia. As posttraumatic syringomyelia can develop from an obstructed subarachnoid at the area of injury, we think that treatment should focus on relieving the blocked space. In our case, the subarachnoid space was obstructed by bony particles, and the reflection of CSF pressure was produced in proximity to the obstruction. This consequently distended the spinal cord above the obstructed canal. We performed an L2 corpectomy, without syrinx drainage, to release the subarachnoid obstruction, and the collapse of the syrinx spontaneously followed.
We propose that a syrinx may develop if, as in our patient, cord compression persists for several years after the initial injury. Although syringomyelia is not easily prevented, correction of CSF blockage and decompression of the spinal cord in the initial post-injury surgery may help to avoid the tethering of the spinal cord and the formation of a syrinx. If the obstructed subarachnoid at the injury site is not corrected, follow-up MRIs should be performed to monitor the condition of the spinal cord.
When the anatomical distortion or obstruction of the subarachnoid space has been remedied, as described herein, CSF flow dynamics may normalize and the syrinx may decrease in size or disappear altogether, providing symptomatic relief for the patient. We believe that restoration of normal CSF circulation and cord traction may obviate the need for a shunt.
CONCLUSION
We propose that posttraumatic syringomyelia may arise from the disturbance of normal cerebrospinal fluid flow within the subarachnoid space caused by various factors after trauma. In the patient described here, we released the blockage of the subarachnoid space by removal of the fractured bone. Surgical realignment without syrinx drainage reinstated normal CSF circulation, and the syrinx completely vanished. This case shows the importance of decompression and reconstruction of the spinal subarachnoid space in patients with posttraumatic syringomyelia.
References
- 1.Barnett HJ, Botterell EH, Jousse AT, Wynn-Jones M. Progressive myelopathy as a sequel to traumatic paraplegia. Brain. 1966;89:159–174. doi: 10.1093/brain/89.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Bastian HC. On a case of concussion lesion with extensive secondary degeneration of the spinal cord. Proc Royal Med Chirurg Soc Lond. 1867;50:499. doi: 10.1177/095952876705000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batzdorf U, Klekamp J, Johnson JP. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382–388. doi: 10.3171/jns.1998.89.3.0382. [DOI] [PubMed] [Google Scholar]
- 4.Cho KH, Iwasaki Y, Imamura H, Hida K, Abe H. Experimental model of posttraumatic syringomyelia : the role of adhesive arachnoiditis in syrinx formation. J Neurosurg. 1994;80:133–139. doi: 10.3171/jns.1994.80.1.0133. [DOI] [PubMed] [Google Scholar]
- 5.Hida K, Iwasaki Y, Imamura H, Abe H. Posttraumatic syringomyelia : its characteristic magnetic resonance imaging findings and surgical management. Neurosurgery. 1994;35:886–891. doi: 10.1227/00006123-199411000-00012. discussion 891. [DOI] [PubMed] [Google Scholar]
- 6.Hong SH, Jeong SK, Chung CK, Kim HJ. Treatment of syringomyelia with consideration on its pathophysiology. J Korean Neurosurg Soc. 2002;31:388–391. [Google Scholar]
- 7.Klekamp J. The pathophysiology of syringomyelia - historical overview and current concept. Acta Neurochir (Wien) 2002;144:649–664. doi: 10.1007/s00701-002-0944-3. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi I, Iwasaki Y, Hida K, Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005;63:350–355. doi: 10.1016/j.surneu.2004.05.038. discussion 355-356. [DOI] [PubMed] [Google Scholar]
- 9.Lee CW, Kim YS, Lee JS, Park MS, Ha HG, Kim JS. Syringopleural shunt for failed syringosubarachnoid shunt in posttraumatic Syringomyelia. J Korean Neurosurg Soc. 2001;30:633–637. [Google Scholar]
- 10.Lee JH, Chung CK, Kim HJ. Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation. Spinal Cord. 2002;40:501–506. doi: 10.1038/sj.sc.3101322. [DOI] [PubMed] [Google Scholar]
- 11.Lee TT, Alameda GJ, Camilo E, Green BA. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine. 2001;26:S119–S127. doi: 10.1097/00007632-200112151-00020. [DOI] [PubMed] [Google Scholar]
- 12.Levi AD, Sonntag VK. Management of posttraumatic syringomyelia using an expansile duraplasty. A case report. Spine (Phila Pa 1976) 1998;23:128–132. doi: 10.1097/00007632-199801010-00026. [DOI] [PubMed] [Google Scholar]
- 13.Rossier AB, Foo D, Shillito J, Dyro FM. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108:439–461. doi: 10.1093/brain/108.2.439. [DOI] [PubMed] [Google Scholar]
- 14.Umbach I, Heilporn A. Review article : post-spinal cord injury syringomyelia. Paraplegia. 1991;29:219–221. doi: 10.1038/sc.1991.32. [DOI] [PubMed] [Google Scholar]
- 15.Wiart L, Dautheribes M, Pointillart V, Gaujard E, Petit H, Barat M. Mean term follow-up of a series of post-traumatic syringomyelia patients after syringo-peritoneal shunting. Paraplegia. 1995;33:241–245. doi: 10.1038/sc.1995.55. [DOI] [PubMed] [Google Scholar]
- 16.Williams B. Pathogenesis of post-traumatic syringomyelia. Br J Neurosurg. 1992;6:517–520. doi: 10.3109/02688699209002367. [DOI] [PubMed] [Google Scholar]
- 17.Williams B. Post-traumatic syringomyelia, an update. Paraplegia. 1990;28:296–313. doi: 10.1038/sc.1990.39. [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Terry AF, Jones F, McSweeney T. Syringomyelia as a sequel to traumatic paraplegia. Paraplegia. 1981;19:67–80. doi: 10.1038/sc.1981.18. [DOI] [PubMed] [Google Scholar]