Abstract
Objective
To prevent temporal depression after the pterional craniotomy, this study was designed to examine the safety and aesthetic efficacy of the brushite calcium phosphate cement (CPC) in the repair and augmentation of bone defects following the pterional craniotomy.
Methods
The brushite CPC was used for the repair of surgically induced cranial defects, with or without augmentation, in 17 cases of pterional approach between March, 2005 and December, 2006. The average follow-up month was 20 with range of 12-36 months. In the first 5 cases, bone defects were repaired with only brushite CPC following the contour of the original bone. In the next 12 cases, bone defects were augmented with the brushite CPC rather than original bone contour. For a stability monitoring of the implanted brushite CPC, post-implantation evaluations including serial X-ray, repeated physical examination for aesthetic efficacy, and three-dimensional computed tomography (3D-CT) were taken 1 year after the implantation.
Results
The brushite CPC paste provided precise and easy contouring in restoration of the bony defect site. No adverse effects such as infection or inflammation were noticed during the follow-up periods from all patients. 3D-CT was taken 1 year subsequent to implantation showed good preservation of the brushite CPC restoration material. In the cases of the augmentation group, aesthetic outcomes were superior compared to the simple repair group.
Conclusion
The results of this clinical study indicate that the brushite CPC is a biocompatible alloplastic material, which is useful for prevention of temporal depression after pterional craniotomy. Additional study is required to determine the long-term stability and effectiveness of the brushite calcium phosphate cement for the replacement of bone.
Keywords: Bony defect, Pterional craniotomy, Brushite calcium phosphate cement, Augmentation
INTRODUCTION
Pterional craniotomy is commonly used for anterior circulating artery aneurysms and anterior cranial base tumors. This approach involves a simple method that allows wide and safe exposure of the anterior and middle cranial fossas. To secure more comfortable exposure and minimize the brain retraction, it becomes necessary to remove the greater wing of the sphenoid bone as well as some parts of the frontal and temporal bone9). Postoperative temporal muscle atrophy and bone defects after the pterional craniotomy create a depression of the temporal fossa that sometimes turns to the main postoperative complaint of patients2,9).
With the current emphasis on minimally invasive surgery, increasing care is being taken to prevent this complication13). In practice, however, we often see patients with temporal fossa depression after surgery9).
Since the first discovery of the calcium phosphate cement (CPC) by Brown and Chow in the 1980's, applications of a broad spectrum of calcium phosphate cements (CPCs) have been increased as an alternative to alloplast or even bone grafts in craniofacial reconstruction. The CPCs are easily applied for contouring as a paste and hardens in minutes. Accompanying a negligible exothermic reaction, the CPCs provide structure integrity in hours. In addition, the minor exothermic nature of the CPC setting, together with the fact that no special fixation elements e.g., screws, miniplates or suture materials are needed to fix the CPC, makes this material ideal for craniofacial reconstruction3,4,7,11,17-19).
In this article, we report our clinical experience in repair and augmentation of bone defects to prevent postoperative temporal depression in several pterional craniotomy cases using a brushite CPC, PolyBone® (Kyungwon Medical Co. Ltd., Seoul, Korea).
MATERIALS AND METHODS
Seventeen consecutive patients with anterior circulation aneurysm were included in this retrospective study. The objective patients were operated by a surgeon at our institution between March 5, 2005 and June 25, 2006. The series included 5 men and 12 women, ages ranging from 45 to 73 years, at the time of surgery.
All patients had been followed by postoperative aesthetic outcomes for 1 year and the cosmesis was graded by the scale described in Table 114). The minimum follow-up lasted for 12 months. After a year following the completion of reconstruction, 3-dimensional skull computer tomography was taken for the documentation of the fate of the brushite CPC.
Table 1.
Grading system for cosmetic outcomes
Operative technique
After fixing the craniotomy bone flap with minifixation (Biomet), the powder component of calcium phosphate was mixed with liquid component, and left for 1-2 minutes, for dense viscosity of the paste. The paste of brushite CPC was applied to the surgically induced bone defect area such as pterion, sphenoid ridge, burr hole sites, or temporal bone defect area. Then the CPC paste was contoured with a spatula. Once the desired contour was formed, the cement was left untouched for 10 minutes.
We divided patients into 2 groups : For group A (first 5 patients, repair group) (Fig. 1A), bony defect areas such as pterion, sphenoid ridge and temporal bone were only repaired to its original contour without augmentation. For group B (12 patients, augmentation group) (Fig. 1B), bony defect areas of pterion, sphenoid ridge and temporal bone were augmented rather than repairing original contour. The brushite CPC graft material was allowed for 5-8 minutes to set in situ until it turned into a harder state. No special fixing elements such as screws, miniplates, or suture materials were necessary to fix the brushite CPC graft material.
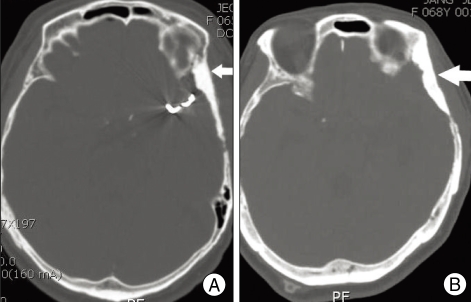
Fig. 1.
Postoperative computed tomography scans show the corresponding radiopaque brushite calcium phosphate cement which is filled homogeneously at bone defect area. A : Repair following the original contour of skull bone defect (white arrow). B : Augmentation of skull bone defect (white arrow).
RESULTS
Patients were followed-up for 12 to 27 months subsequent to surgery, with a mean period of 20 months. Postoperative recovery has been excellent thus far in all patients. None of the patients showed any evidence of foreign body reaction to the implant material or infection at the implant site. Depending on methods of cranioplasty, the results of aesthetic effect were variable. In group A (repair group), only 1 case was categorized into Grade A (Fig. 2), 3 cases were considered as Grade B (Fig. 3), and 1 case was classified as Grade C (Fig. 4). However, in group B (augmentation group), 7 cases were categorized into Grade A and 5 cases were considered as Grade B.
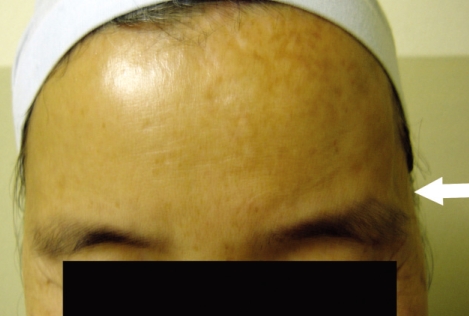
Fig. 2.
Photogragh of Grade A cosmetic outcome. No evidence of surgery with mild prominence of temporal fossa is seen on left side (white arrow). An excellent cosmetic result was obtained one year after surgery.
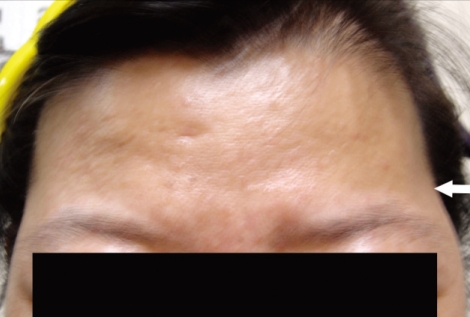
Fig. 3.
Photograph of Grade B cosmetic outcome. Slight depression of temporal fossa was seen on left side (white arrow).
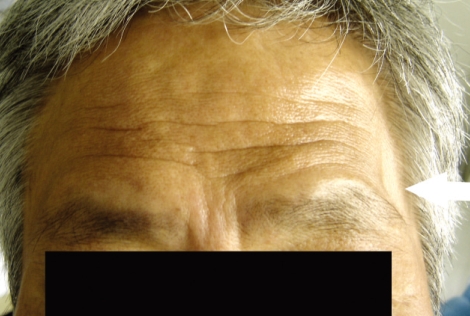
Fig. 4.
Photograph of Grade C cosmetic outcome. Prominent depression of temporal fossa is seen on left side (white arrow).
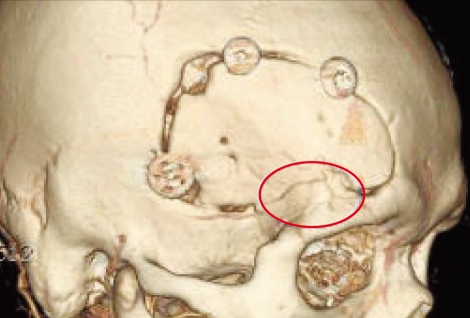
Three-dimensional computed tomography scans that were taken 1 year after reconstruction confirmed the slightly reduced volume of the CPC implants. However, the secondary temporal depression was not observed during the follow-up period (Fig. 5).
Fig. 5.
Postoperative 3-D-computed tomography scans, which was taken 1 year after operation, shows the well-preserved brushite calcium phosphate cement on the bone defect area (white circle).
DISCUSSION
Temporal depression after pterional craniotomy would be one of the complaints arising from patients. With the current emphasis on minimally invasive surgery, increasing care is being taken to prevent this complication13). In practice, however, we often see patients with temporal fossa depression after surgery9). Many alloplastic materials such as titanium mesh, polymethylmethacrylate (PMMA) bone cement, polymers and calcium phosphate cements have been used to repair the bone defects in craniofacial area15). To be an ideal cranioplasty material, the material should ideally feature the following characteristics : 1) good tissue compatibility, 2) the ability to be easily contoured, 3) stable through the patient's lifetime or if desorbed, replaced by structurally stabling bone without significant loss of volume or change in shape; 4) radiolucent to allow unimpeded computed tomography scanning of the intracranial contents, and 5) the ability to become ingrown by living tissue5,7,18).
Until present, one of the most commonly used materials to repair the cranial bone defect is PMMA bone cement. However, on the negative side of PMMA bone cement, PMMA may cause a marked inflammatory response and fibrous encapsulation of the implant, resulting in the possibility of infection and loosening and/or exposure of the implant4,19). In addition, the setting phase of PMMA is highly exothermic with temperatures as high as 140°F which may cause local cellular destruction11). In addition, it is hard to shape the contour of implant after polymerization has commenced. Furthermore, fixing the PMMA implant to the surrounding bone may be difficult to use with subtle defects without fixation systems3). Titanium mesh and polymer cannot be easily formed or shaped18); therefore, it is not easy to make satisfactory contouring in a complicated shape area, especially in the fronto-zygomatic-temporal area without special fixing elements. Calcium phosphate cement is highly biocompatible and is gradually replaced with autologous bone by osteoconduction7). Because of these characteristics, calcium phosphate cement has been used for the repair of craniofacial fractures in plastic and reconstructive surgeries3,4,7,11,17-19). Calcium phosphate cements are composed of an aqueous solution and of one or several calcium phosphates. These two components are mixed together during preparation; the calcium phosphates dissolve and precipitate into a less soluble calcium phosphate. Growth and entanglements of the calcium phosphate crystals provide mechanical rigidity to the cement10). Two types of cements are distinguished depending on the end product of the setting reaction : apatite and brushite (DCPD or dicalcium phosphate dihydrate) cements, of which the latter recently raises great interest16). Brushite cements are resorbed faster than apatite cements8-13,15-20), but concerning the mechanical strength, brushite calcium phosphate cement is slightly weaker than apatite calcium phosphate cement10). However, in the non-weight bearing conditions of the skull, the difference between two cements may be irrelevant10). The high biocompatibility of brushite calcium phosphate cement16) as well as no heat generation during the hardening process allows it to be directly placed onto the dura. In addition, its plasticity enables satisfactory contouring before hardening. After setting, undesirable irregularities could be removed using fine instruments such as a knife, and it achieves sufficient intraoperative strength for scalp closure without any problem. In these respects, calcium phosphate cements proves to be superior to acryl cement (PMMA). However, since the calcium phosphate cements have hydrophilic properties in general, it is recommended to be used as a dense paste and placed into a relatively dry operative field for a successful reconstruction5). The CPC is advised not to be applied at large craniofacial bone defect areas, due to its low compressive and tension strengths.
In this study, repairs of the surgically induced bone defects such as pterion, sphenoid ridge, squamosal temporal bone without (group 1) or with augmentation (group 2) are easily undertaken using the brushite calcium phosphate cement. The bone bonding effect of CPC makes it possible to have a great advantage of mold and adaptation to the individual defect with no need of special fixation elements. One of the first calcium phosphate cements, Bone Source has shown positive results when it is used in craniofacial reconstruction4,17,18). Bone Source shows excellent retention of implant volume, with no interference of craniofacial growth over a 3-year study period for the cases of pediatric patients6).
Nearly half of the reported complications have been related to infection, with an overall complication rate ranging from 0% to 11%12).
In this study, none of our patients showed any evidence of foreign body reaction to the implant material or infection at the implant site during the follow-up period. In the 3-D computed tomography scans which were taken 1 year after reconstruction, showed the slightly reduced volume of the CPC implants. However, the secondary temporal depression was not observed during the follow-up period. In respect of aesthetic efficacy, much better results were seen in the augmentation group. We think this finding was due to the compensation of the delayed temporal muscle atrophy. For better long term cosmetic results, we currently augment both pterion and temporal areas with somewhat larger volume of the CPC than the bone defect cases. Since the brushite calcium phosphate cement remains malleable for only a few minutes, it is prudent to complete all surgical preparation of the graft site before mixing the product. It has been well known that the CPC is gradually replaced with autogenous bone by osteoconduction. Even though the CPC shows almost equivalent CT density to bone, biopsy of the reconstructed site is required to assure bone ingrowth into cement restoration. Although no biopsy study is carried out during our follow-up period, numbers of orthopedic studies on long bones encourage the results with brushite cements1,10). From a practical view point, because the craniofacial skeleton is a non stress-bearing site, the fact that the CPC does not totally convert to bone is not vital. As long as the cement integrates into the host bone and maintains its volume over the period, it would appear to be satisfactory for craniofacial skeleton augmentation. In this study, we demonstrated an excellent tissue compatibility with anatomic tissue as well as superior aesthetic effects of cranioplasty using calcium phosphate cement during the follow-up period after the pterional craniotomy, based on the clinical evaluation and 3-D computed tomography.
CONCLUSION
The results from this clinical study show that brushite CPC is a biocompatible and alloplastic material, which is useful for the prevention of temporal depression after pterional craniotomy. In the cases of augmentation group, aesthetic outcome is much better than the cases of simple repair groups. Additional study is required to determine the long-term stability and effectiveness of brushite calcium phosphate cement replacement by bone.
References
- 1.Apelt D, Theiss F, El-warrak AO, Zlinszky K, Bettschart-Wolfisberger R, Bohner M, et al. In vivo behaviour of three different injectable hydraulic calcium phosphate cements. Biomaterials. 2004;25:1439–1451. doi: 10.1016/j.biomaterials.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 2.Badie B. Cosmetic reconstruction of temporal defect following pterional [correceted] craniotomy. Surg Neurol. 1996;45:383–384. doi: 10.1016/0090-3019(95)00452-1. [DOI] [PubMed] [Google Scholar]
- 3.Baker SB, Weinzweig J, Kirschner RE, Bartlett SP. Applications of new carbonated calcium phosphate bone cement : early experience in pediatric and adult craniofacial reconstrution. Plastic Reconstr Surg. 2002;109:1789–1796. doi: 10.1097/00006534-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chen TM, Wang HJ, Chen SL, Lin FH. Reconstruction of posttraumatic frontal-bone depression using hydroxyapatite cement. Ann Plast Surg. 2004;52:303–308. doi: 10.1097/01.sap.0000105522.32658.2f. discussion 309. [DOI] [PubMed] [Google Scholar]
- 5.Cosrantino PD, Friedman CD, Jones K, Chow LC, Sisson GA. Experimental hydroxyapatite cement cranioplasty. Plastic Reconstr Surg. 1992;90:174–185. discussion 186-191. [PubMed] [Google Scholar]
- 6.David L, Argenta L, Fisher D. Hydroxyapatite cement in pediatric craniofacial reconstruction. J Craniofac Surg. 2005;16:129–133. doi: 10.1097/00001665-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Gómez E, Martín M, Arias J, Carceller F. Clinical application of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children : our experience at Hospital La Paz since 2001. J Oral Maxillofac Surg. 2005;63:8–14. doi: 10.1016/j.joms.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Klein CP, de Groot K, Driessen AA, van der Lubbe HB. Interaction of biodegradable beta-whitelockite ceramics with bone tissue: an in vivo study. Biomaterials. 1985;6:189–192. doi: 10.1016/0142-9612(85)90008-0. [DOI] [PubMed] [Google Scholar]
- 9.Kubo S, Takimoto H, Kato A, Yoshimine T. Endoscopic cranioplasty with calcium phosphate cement for pterional bone defect after frontotemporal craniotomy : technical note. Neurosurgery. 2002;51:1094–1096. doi: 10.1097/00006123-200210000-00046. discussion 1096. [DOI] [PubMed] [Google Scholar]
- 10.Kuemmerle JM, Oberle A, Oechslin C, Bohner M, Frei C, Boecken I, et al. Assessment of suitability of a new brushite calcium phosphate cement for cranioplasty - an experimental study in sheep. J Craniomaxillofac Surg. 2005;33:37–44. doi: 10.1016/j.jcms.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Mahr MA, Bartley GB, Bite U, Clay RP, Kasperbauer JL, Holmes JM. Norian craniofacial repair system bone cement for the repair of craniofacial skeletal defects. Ophthal Plast Reconstr Surg. 2000;16:393–398. doi: 10.1097/00002341-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Matic D, Phillips J. A contraindication for the use of hydroxyapatite cement in the pediatric population. Plast Reconstr Surg. 2002;110:1–5. doi: 10.1097/00006534-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Akagi K, Abekura M, Ohkawa M, Tasaki O, Tomishima T. Cosmetic and fuctional reconstruction achieved using a split myofascial bone flap for pterional craniotomy. Technical note. J Neurosurg. 2001;94:667–670. doi: 10.3171/jns.2001.94.4.0667. [DOI] [PubMed] [Google Scholar]
- 14.Raza SM, Thai QA, Pradilla G, Tamargo RJ. Frontozygomatic titanium cranioplasty in frontosphenotemporal ("pterional") craniotomy. Neurosurgery. 2008;62:262–264. doi: 10.1227/01.neu.0000317402.46583.c7. discussion 264-265. [DOI] [PubMed] [Google Scholar]
- 15.Tadros M, Costantino PD. Advances in cranioplasty : a simplified algorithm to guide cranial reconstruction of acquired defects. Facial Plast Surg. 2008;24:135–145. doi: 10.1055/s-2008-1037455. [DOI] [PubMed] [Google Scholar]
- 16.Theiss F, Apelt D, Brand B, Kutter A, Zlinszky K, Bohner M, et al. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26:4383–4394. doi: 10.1016/j.biomaterials.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Turk JB, Parhiscar A. Bone Source for craniomaxillofacial reconstruction. Facial Plast Surg. 2000;16:7–14. doi: 10.1055/s-2000-7321. [DOI] [PubMed] [Google Scholar]
- 18.Verheggen R, Merten HA. Correction of skull defects using hydroxyapatite cement (HAC)--evidence derived from animal experiments and clinical experience. Acta Neurochir (Wien) 2001;143:919–926. doi: 10.1007/s007010170022. [DOI] [PubMed] [Google Scholar]
- 19.Wolff KD, Swaid S, Nolte D, Böckmann RA, Hölzle F, Müller-Mai C. Degradable injectable bone cement in maxillofacial surgery : indications and clinical experience in 27 patients. J Craniomaxillofac Surg. 2004;32:71–79. doi: 10.1016/j.jcms.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Xia Z, Grover LM, Huang Y, Adamopoulos IE, Gbureck U, Triffit JT, et al. In vitro biodegradation of three brushite calcium phosphate cements by a macrophage cell-line. Biomaterials. 2006;27:4557–4565. doi: 10.1016/j.biomaterials.2006.04.030. [DOI] [PubMed] [Google Scholar]