Abstract
Objective
The normal anatomic relationships characteristic of the pituitary stalk area were previously thought to involve only one location. The purpose of this study was to re-evaluate the anatomic location of the pituitary stalk and possible varying locations in relation to the tuberculum sellae and dorsum sellae using morphometric evaluation and anatomic dissection of human cadaveric specimens. The surgical implications of the variations are discussed.
Methods
The calvaria were removed via routine autopsy dissections, and the brains were removed from the skull while preserving the pituitary stalk. The diaphragma sellae, tuberculum sellae, and the location of the pituitary stalk were examined in 60 human cadaveric heads obtained from fresh adult cadavers. Empty sellae were excluded.
Results
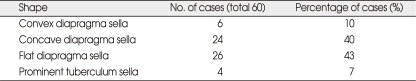
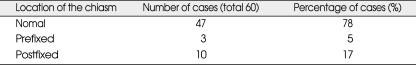
The openings of the diaphragma sellae averaged 6.62 ± 1.606 mm (range, 3-9 mm). The distance between the tuberculum sellae and the posterior part of the pituitary stalk was 1 to 8 mm. The upper face of the diaphragma sellae appeared flat in 26 (43%), concave in 24 (40%), and convex in 6 cases (10%), with a prominent tuberculum sellae in 4 cases (7%). The location of the chiasm was normal in 47 cases (78%), with a prefixed chiasm in 3 cases (5%) and a postfixed chiasm (17%) in the 10 cases. Four cadaver specimens had prominent tuberculum sellae and other parameters were not evaluated.
Conclusion
When opening the chiasmatic cistern, neurosurgeons should be aware about the relationship between the pituitary stalk and the surrounding structures to prevent inadvertent injury to the pituitary stalk.
Keywords: Chiasmatic cistern, Location, Pituitary stalk, Sellae
INTRODUCTION
Thorough knowledge of the anatomy of the sellar region, including the diaphragma sellae and the pituitary stalk, is important to neurosurgeons dealing with pathologies in this region. Recognizing variations is important in preventing damage during surgery.
The dura forming the roof of the oculomotor trigones extends medially across the sellae to form the diaphragma sellae, which covers the pituitary gland and contains an opening for the pituitary stalk. The diaphragma sellae is a small, circular, horizontal fold, which roofs in the sellae turcica and almost completely covers the hypophysis; a small central opening transmits the pituitary stalk4,10,13). When viewed from above, the upper face of the diaphragma sellae has 3 different shapes : convex (2% of cases), concave (56% of cases), and flat (42% of cases)13). The connecting pituitary stalk passes inferiorly through an opening in the dura, allowing delivery of hypothalamic peptides to the pituitary gland. The pituitary stalk contains the fibers of the neurohypophysis, which connect the posterior lobe to the supraoptic and paraventricular nuclei of the hypothalamus, and the portal venous system, transmitting hypothalamic peptides that control anterior lobe secretion via the median eminence5,7,8). The objective of the current study was to investigate the relationship between the pituitary stalk and its surrounding structures such as the diaphragma sellae, the tuberculum sellae, and the dorsum sellae and chiasmatic cistern in human cadaver specimens.
MATERIALS AND METHODS
We examined the relationship between the pituitary stalk and its surrounding structures in 60 fresh adult cadavers. The cadavers were in the supine position and the necks were extended 20 degrees. The calvarium was removed via routine autopsy dissections; the frontal lobe was elevated and pulled posteriorly. In addition, the optic nerves, chiasma, chiasmatic cistern, and the distal parts of the internal carotid artery were exposed. The midline of the chiasmatic cistern was then opened to demonstrate the pituitary stalk. The vascular structures and pituitary stalk were cut with an uppermost incision, and the whole brain was extracted from the calvarium. The sellar region was thoroughly examined from all directions. The cases with empty sellae were excluded from this study. The circumference, the dimensions of the opening, the shape, and the exit point of the pituitary stalk were inspected. Pictures were taken using a camera (2005 model 7.2 megapixels, 3× zoom) (Sony, made in China) and measurements were made using a regular compass and ruler.
RESULTS
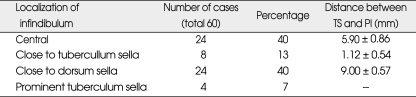
The openings of the diaphragma sellae averaged 6.62 ± 1.606 mm (range, 3-9 mm) in diameter. The distance between the tuberculum sellae and the posterior part of the pituitary stalk was 1 to 8 mm. The location of the pituitary stalk on the diaphragm sellae was not constant and could be central or adjacent to the dorsum sellae or adjacent to the tuberculum sellae (Fig. 1, 2, 3). After dissection of the chiasmatic cistern, the pituitary stalk was exposed in cases with a pituitary stalk that was adjacent to the tuberculum sellae (Fig. 3). The appearance of the upper face of the diaphragma sellae was flat in 26 (43%), concave in 24 (40%), and convex in 6 cases (10%), and there was a prominent tuberculum sellae in 4 cases (7%). The shape of the diaphragma sellae was round in 36 (65%) and elliptical in 20 cases (35%). Four cadaver specimens had prominent tuberculum sellae and their diaphragm openings and the shapes of the diaphragma sellae were therefore not evaluated (Fig. 4). The location of the chiasm was in normal position, which overlied the diaphragma sellae and the pituitary gland in 47 cases (78%), on the tuberculum sellae in 3 cases (5%) (prefixed chiasm) and overlying the dorsum sellae (postfixed chiasm) in the remaining 10 cases (17%). The results are presented in Tables 1, 2 and 3.
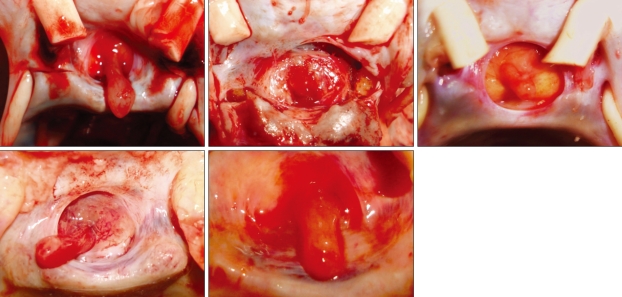
Fig. 1.
Superior view of the sellar region showing the pituitary stalk in the central position of the pituitary gland in different specimens.
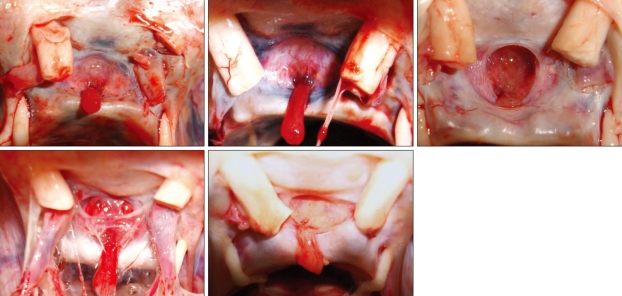
Fig. 2.
Superior view of the sellar region showing the pituitary stalk located just in front of the dorsum sellae in different specimens.
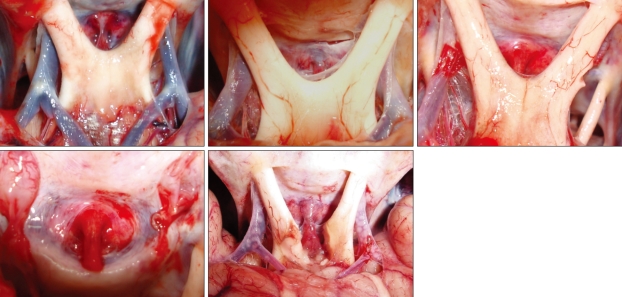
Fig. 3.
Superior view of the sellar region. The pituitary stalk is located just in front of the tuberculum sellae in different specimens.
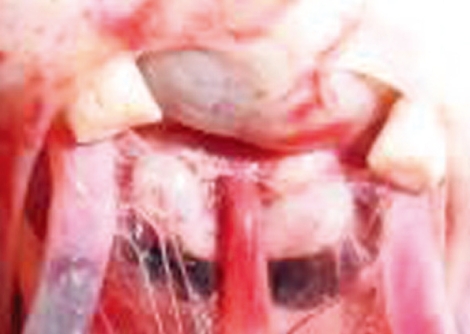
Fig. 4.
Superior view of the sellar region showing a prominent tuberculum sellae and the anatomic relation of the pituitary stalk.
Table 1.
Summary of the incidence of the variations in the shape of the upper face of the diapragma sellae
Table 2.
Anatomical localization of the infindibulum and the distance between the tuberculum sellae (TS) and the posterior part of the infindibulum (PI)
Table 3.
The location of the chiasm on the sellae (normal, prefixed, postfixed)
The nomal : chiasm places over the diapragma sellae and pituitary gland, The prefixed : chiasm places over the tuberculum sellae, The postfixed : chiasm places over the dorsum sellae
DISCUSSION
The importance of understanding the anatomic variations in the sellar region and the influence these variations have on the surgical approach have been described by Rhoton and Campero. They postulated that the variability in the diameter of the opening of the diaphragma sellae may affect the growth of the pituitary tumor toward the suprasellar region or cavernous sinus3,13). Some authors also speculated that there may be an eccentric insertion of the pituitary stalk as regards the magnetic resonance imaging findings1,2,6,9). Rhoton described three different relationships between the optic chiasm and its surrounding structures including the tuberculum sellae, diaphragma sellae, dorsum sellae and the pituitary gland. The first one, the prefix optic chiasm, in which the optic chiasm is located on top of the tuberculum sellae, occurs in 15% of the patients. The second one, the normal chiasm, in which the optic chiasm is located on top of the diaphragma sellae, occurs in 70% of the patients. The third one, the postfix chiasm, in which the optic chiasm is located on top of the dorsum sellae, occurs in 15% of the patients. Another important variation in this region is a prominent tuberculum sellae, which could lead to an exposure problem during transcranial surgery in all three types of optic chiasm13). In our series, we obtained results that were similar to Rhoton's series, but our prefix chiasm incidence was lower. Prefixed chiasma and prominent tuberculum sellae could obviate the access to the some pathology in this region including craniopharyngioma and pituitary tumors as regards the transcranial approach. Finally, the upper face of the diaphragma sellae may be convex in 4%, concave in 54%, and flat in 42% of patients, and the shape may be round in 56% and elliptical in 44% of patients, according to the Rhoton13).
The descriptions above have been very useful to neurosurgeons that regularly operate in this area. However, to the best of our knowledge, no one has described the relationship between the pituitary stalk and its surrounding structures. The common anatomic description of the pituitary stalk is that it is located at the center of the diaphragma sellae. The dura covering the roof of the oculomotor trigone extends medially across the sellae to form the diaphragma sellae, which covers the pituitary gland and contains an opening for the pituitary stalk. The opening of the center of the diaphragma sellae is wider than the thickness of the pituitary stalk; this opening was bigger than 5 mm in 56% of the specimens in Rhoton's study12,13). Recently, Campero et al.3) described the diaphragma sellae anatomy and its role in directing the pattern of the growth of pituitary adenoma. They speculated that pituitary adenomas could grow towards the cavernous sinus or the suprasellar region depending on the variability in the diameter of the opening of the diaphragma sellae, but they did not mention the variability of the localization of the pituitary stalk. Our investigation demonstrated differences in the location of the pituitary stalk, but we did not find any relationship between the shape of the diaphragma sellae and the location of the pituitary stalk. With a prominent tuberculum sellae, the pituitary stalk is located very closely to the dorsum sellae and tuberculum sellae.
Finally, we propose that there are 4 different locations for the pituitary stalk. In the first location, the pituitary stalk is positioned at the center of the diaphragma sellae (Fig. 1) In the second location, the pituitary stalk is positioned more anteriorly on the diaphragma sellae (toward the tuberculum sellae) (Fig. 3). In the third location, the pituitary stalk is more posterior (toward the dorsum sellae) (Fig. 2). In the fourth location, the pituitary stalk is located very close to the tuberculum sellae and to the dorsum sellae, because prominent tuberculum sellae that is anatomically expanded toward the dorsum sellae. In these cases the distance between the pituitary stalk and dorsum sellae and between the pituitary stalk and tuberculum sellae is very small as shown in Fig. 4. During surgery for sellar zone pathology, awareness of the chiasmal location, a prominent tuberculum sellae, and cisternal anatomy is vitally important for preventing inadvertent damage to structures such as the optic nerve, the chiasma, the third and fourth cranial nerves, vascular structures, and the pituitary stalk. If the pituitary stalk is located just behind the tuberculum sellae, pituitary stalk injury may ensue due to the location of the pituitary stalk during opening of the chiasmal cistern (Fig. 3). If the pituitary stalk is inadvertently damaged during a surgical intervention but not completely sectioned, the remnant of pituitary stalk tissue which connects to the median eminence of the pituitary gland may serve as a matrix on which the pituitary portal system may reform4,5,10). It is therefore important to know the various positions of the pituitary stalk during surgery to prevent inadvertent injury and protect the pituitary stalk as much as possible. Various studies have reported different rates of panhypopituitarism. Honegger et al.11) reported that the rate of panhypopituitarism increased from 10.9% before surgery to 34.8% after transcranial surgery for craniopharyngioma. Some series have shown diabetes insipidus to be the most common complication after complete evacuation of the craniopharyngioma. Yasargil et al.14) reported a total resection rate of 90% in their series; the incidence of permanent diabetes insipidus after surgery was 90%. Zhou et al.15) reported that the incidence of diabetes insipidus before and after surgery was 52% and 92%, respectively. Devile et al.8) reported that the incidence of diabetes insipidus depended on the extent of tumor removal, and that the incidence of diabetes insipidus after limited surgery was 6%, but it was as high as 70% to 90% after aggressive surgery. At present, sellar region pathologies may be completely removed with the help of modern neuroimaging and microneurosurgical techniques, but how to guard patients from endocrine abnormalities after this removal remains a challenge to neurosurgeons worldwide. We think that our study showed that the variant location of the pituitary stalk affects the surgery regarding preservation of the pituitary stalk. If the pituitary stalk is localized adjacent to the tuberculum sellae, it may be damaged inadvertently during chiasmatic cistern opening or during pituitary tumor removal in transcranial surgery as shown (Fig. 3). In addition, tumoral invasion would distort the structure and localization of the pituitary stalk, and pituitary stalk injury would be inevitable unless one takes these anatomic variations into account. Knowing the shape of the diaphragma sellae during transcranial surgery of the sellar region may not be helpful as the invasion itself could alter the shape and appearance of the diaphragma sellae; however, knowing the variant location of the pituitary stalk during surgery might be helpful during exploration of this area and increase the chance of preserving the pituitary stalk. In our opinion, awareness of the various locations of the pituitary stalk is as important as knowing other anatomic variations. If we preserve the pituitary stalk even partly, we may prevent the patient' becoming dependent on lifelong external supplemental hormonal therapy.
CONCLUSION
When accessing a sellar pathology during transcranial surgery, knowing the various variations of the pituitary stalk location is of vital importance in preventing inadvertent injury. When the pituitary stalk is unidentified, the anatomic relationship between the pituitary stalk and tuberculum sellae or dorsum sellae should be taken into consideration. As tumoral invasion progresses, unexpected displacement of the pituitary stalk could ensue. In addition, the pituitary stalk may be just next to the tuberculum sellae, so the chiasmatic cistern should be opened cautiously during any surgical exploration in this region.
References
- 1.Ahmadi H, Larsson EM, Jinkins JR. Normal pituitary gland : coronal MR imaging of infundibular tilt. Radiology. 1990;177:389–392. doi: 10.1148/radiology.177.2.2217774. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11–23. doi: 10.1016/s1042-3680(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 3.Campero A, Martins C, Yasuda A, Rhoton AL., Jr Microsurgical anatomy of the diaphragma sellae and its role in directing the pattern of growth of pituitary adenomas. Neurosurgery. 2008;62:717–723. doi: 10.1227/01.neu.0000317321.79106.37. discussion 717-723. [DOI] [PubMed] [Google Scholar]
- 4.Carmel PW. Surgical syndromes of the hypothalamus. Clin Neurosurg. 1980;27:133–159. doi: 10.1093/neurosurgery/27.cn_suppl_1.133. [DOI] [PubMed] [Google Scholar]
- 5.Carmel PW, Antunes JL, Ferin M. Collection of blood from the pituitary stalk and portal veins in monkeys, and from the pituitary sinusoidal system of monkey and man. J Neurosurg. 1979;50:75–80. doi: 10.3171/jns.1979.50.1.0075. [DOI] [PubMed] [Google Scholar]
- 6.Castillo M. Pituitary gland : development, normal appearances, and magnetic resonance imaging protocols. Top Magn Reson Imaging. 2005;16:259–268. doi: 10.1097/01.rmr.0000224682.91253.15. [DOI] [PubMed] [Google Scholar]
- 7.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery : results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. discussion 236; 236-237. [DOI] [PubMed] [Google Scholar]
- 8.DeVile CJ, Grant DB, Hayward RD, Stanhope R. Growth and endocrine sequelae of craniopharyngioma. Arch Dis Child. 1996;75:108–114. doi: 10.1136/adc.75.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elster AD. Modern imaging of the pituitary. Radiology. 1993;187:1–14. doi: 10.1148/radiology.187.1.8451394. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence H. Gray's Anatomy. ed 38. New York: ELBS & Churchill Livingstone; 1995. pp. 1882–1883. [Google Scholar]
- 11.Honegger J, Buchfelder M, Fahlbusch R. Surgical treatment of craniopharyngiomas : endocrinological results. J Neurosurg. 1999;90:251–257. doi: 10.3171/jns.1999.90.2.0251. [DOI] [PubMed] [Google Scholar]
- 12.Rhoton AL., Jr Tentorial incisura. Neurosurgery. 2000;47:S131–S153. doi: 10.1097/00006123-200009001-00015. [DOI] [PubMed] [Google Scholar]
- 13.Rhoton AL., Jr The sellar region. Neurosurgery. 2002;51:S335–S374. [PubMed] [Google Scholar]
- 14.Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73:3–11. doi: 10.3171/jns.1990.73.1.0003. [DOI] [PubMed] [Google Scholar]
- 15.Zhou ZQ, Shi XE. Changes of hypothalamus-pituitary hormones in patients after total removal of craniopharyngiomas. Chin Med J (Engl) 2004;117:357–360. [PubMed] [Google Scholar]