Abstract
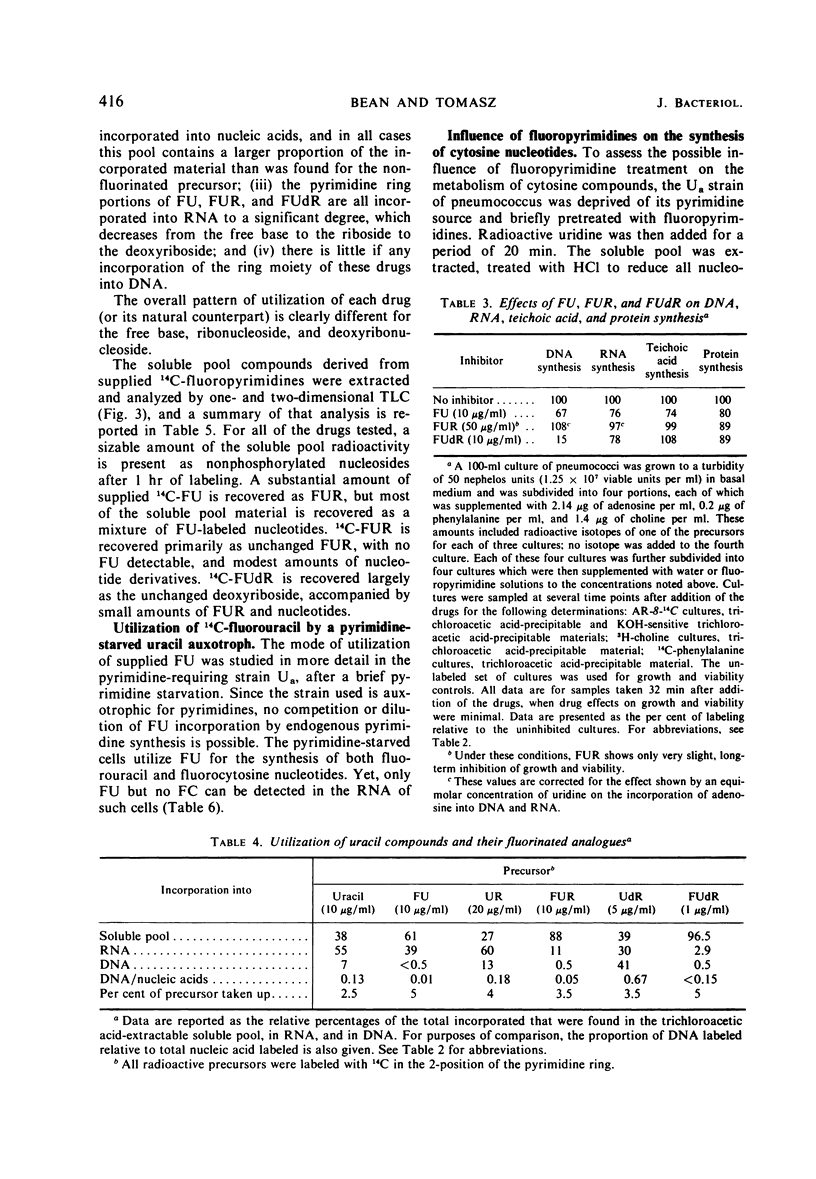
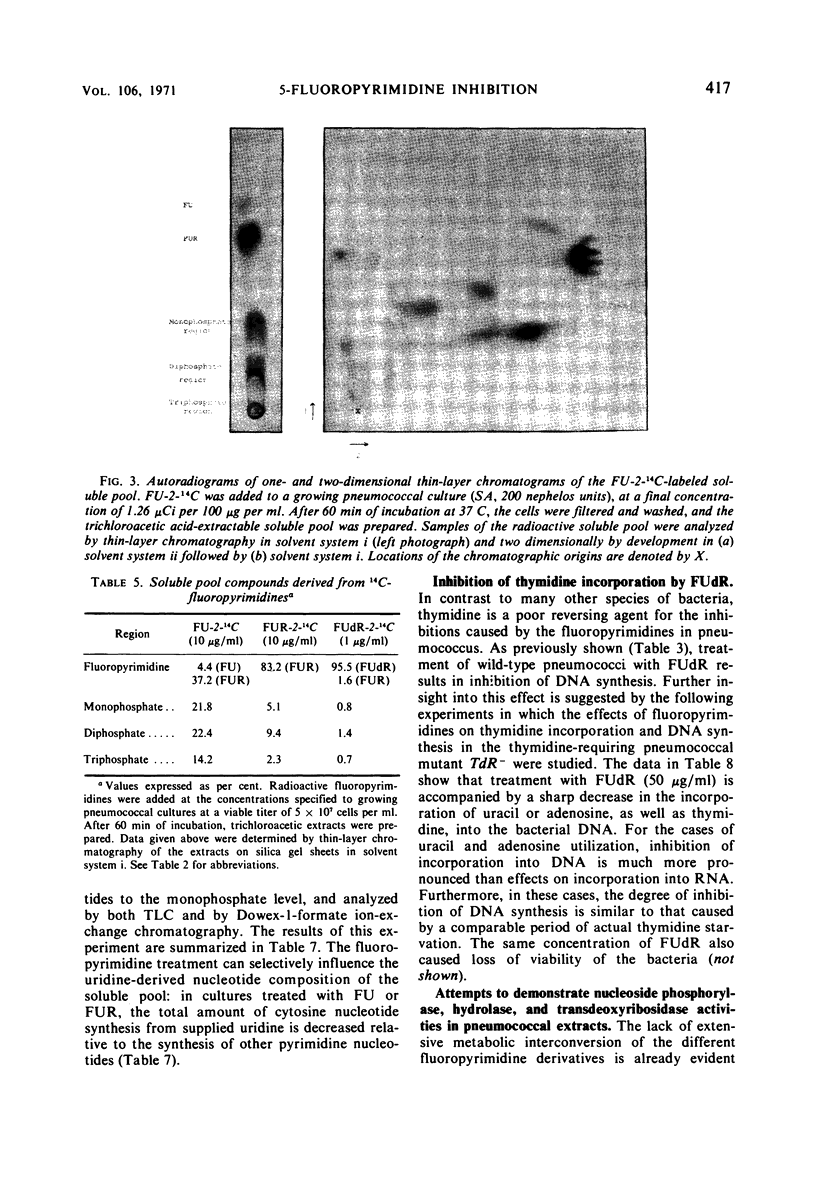
5-Fluorouracil (FU), 5-fluorocytosine, and the riboside and deoxyriboside derivatives of these fluoropyrimidines each exhibit a different spectrum of inhibitory effects in pneumococci. The biochemical basis of this finding seems to be the extremely low level of nucleoside phosphorylase (hydrolase) and N-trans-deoxyribosylase activity in pneumococcus and the consequent, relatively limited metabolic interconversion of the different fluoropyrimidines, which can therefore selectively affect one or the other of the several drug-sensitive biochemical reactions in this bacterium. Special attention was paid to the effect of fluoropyrimidines on the metabolism of cytosine and thymidine. In spite of the fact that FU is converted to both fluorouridine triphosphate and fluorocytidine triphosphate, only fluorouridylate but no fluorocytidylate can be detected in the ribonucleic acid Exogenous FU and fluorouridine also inhibit the synthesis of cytosine nucleotides from supplied uridine in a pyrimidine auxotroph. Thymidine was found to be a poor reversing agent for any of the fluoropyrimidine inhibitions. In both the wild type and in a thymidine-requiring (thymidylate-synthetase deficient) mutant, growing with supplied thymidine in the medium, fluorodeoxyuridine (FUdR) treatment caused cell death and inhibition of the incorporation of radioactive thymidine, adenosine, or uracil into deoxyribonucleic acid. It is suggested that FUdR (or a metabolic derivative) inhibits the transport of phosphorylation of thymidine in this microorganism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. P., BROCKMAN R. W. FEEDBACK INHIBITION OF URIDINE KINASE BY CYTIDINE TRIPHOSPHATE AND URIDINE TRIPHOSPHATE. Biochim Biophys Acta. 1964 Nov 15;91:380–386. doi: 10.1016/0926-6550(64)90067-2. [DOI] [PubMed] [Google Scholar]
- Andoh T., Chargaff E. Formation and fate of abnormal ribosomes of E. coli cells treated with 5-fluorouracil. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1181–1189. doi: 10.1073/pnas.54.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESNICK E. Feedback inhibition of aspartate transcarbamylase in liver and in hepatoma. Cancer Res. 1962 Nov;22:1246–1251. [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. Reversal of mutant phenotypes by 5-fluorouracil: an approach to nucleotide sequences in messenger-RNA. Proc Natl Acad Sci U S A. 1962 Apr 15;48:532–546. doi: 10.1073/pnas.48.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUDHURI N. K., MONTAG B. J., HEIDELBERGER C. Studies on fluorinated pyrimidines. III. The metabolism of 5-fluorouracil-2-C14 and 5-fluoroorotic-2-C14 acid in vivo. Cancer Res. 1958 Apr;18(3):318–328. [PubMed] [Google Scholar]
- COOPER P. D. THE MUTATION OF POLIOVIRUS BY 5-FLUOROURACIL. Virology. 1964 Feb;22:186–192. doi: 10.1016/0042-6822(64)90003-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK K., CLINE R. E., HENDERSON R. B., FINK R. M. Metabolism of thymine (methyl-C14 or -2-C14) by rat liver in vitro. J Biol Chem. 1956 Jul;221(1):425–433. [PubMed] [Google Scholar]
- Gray P. N., Rachmeller M. The effect of 5-fluorouracil and 6-thioguanine incorporation on the amino acid acceptor activity of Escherichia coli tRNA. Biochim Biophys Acta. 1967 Apr 18;138(2):432–435. doi: 10.1016/0005-2787(67)90505-9. [DOI] [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Analysis of the complex sulfonamide resistance locus of pneumococcus. Cold Spring Harb Symp Quant Biol. 1958;23:85–97. doi: 10.1101/sqb.1958.023.01.012. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Heidelberger C. Fluorinated pyrimidines. Prog Nucleic Acid Res Mol Biol. 1965;4:1–50. doi: 10.1016/s0079-6603(08)60783-7. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Kaiser I. I. Studies on 5-fluorouracil-containing ribonucleic acid. I. Separation and partial characterization of fluorouracil-containing transfer ribonucleic acids from Escherichia coli. Biochemistry. 1969 Jan;8(1):231–238. doi: 10.1021/bi00829a033. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J., PERKINS H. R. 5-Fluorouracil and mucopeptide biosynthesis by Staphylococcus aureus. Biochem J. 1960 Dec;77:448–459. doi: 10.1042/bj0770448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B., Rothman F., Weigert M. G. Miscoding caused by 5-fluorouracil. J Mol Biol. 1969 Sep 14;44(2):363–375. doi: 10.1016/0022-2836(69)90181-8. [DOI] [PubMed] [Google Scholar]
- Sabatini Z. J., Hotchkiss R. D. Preparation of an exonuclease from Lactobacillus which releases 5-mononucleotides from polynucleotides. Biochemistry. 1969 Dec;8(12):4831–4837. doi: 10.1021/bi00840a027. [DOI] [PubMed] [Google Scholar]
- Saunders P. P., Bass R. E., Saunders G. F. Properties of 5-fluorouracil-containing ribonucleic acid and ribosomes from Bacillus subtilis. J Bacteriol. 1968 Aug;96(2):525–532. doi: 10.1128/jb.96.2.525-532.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. A., Neuhaus F. C. On the initial stage in peptidoglycan synthesis. Effect of 5-fluorouracil substitution on phospho-N-acetylmuramyl-pentapeptide translocase (uridine 5'-phosphate). J Biol Chem. 1967 Mar 25;242(6):1331–1337. [PubMed] [Google Scholar]
- Tomasz A., Borek E. THE MECHANISM OF BACTERIAL FRAGILITY PRODUCED BY 5-FLUOROURACIL: THE ACCUMULATION OF CELL WALL PRECURSORS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):324–327. doi: 10.1073/pnas.46.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG T. P., SABLE H. Z., LAMPEN J. O. Enzymatic deamination of cytosine nucleosides. J Biol Chem. 1950 May;184(1):17–28. [PubMed] [Google Scholar]