Abstract
Background
Women diagnosed with gestational diabetes mellitus (GDM) are at substantially increased risk of developing type 2 diabetes and obesity, currently at epidemic rates in the United States. GDM, therefore, identifies a population of women at high risk of developing type 2 diabetes and provides an opportunity to intervene before the development of this disorder. It is well recognized that acute as well as chronic physical activity improves glucose tolerance in type 2 diabetes. To date, however, primary prevention trials have not been conducted to test whether an increase in physical activity reduces risk of developing GDM among women at high risk of this disorder.
Methods
The aims of this study are to investigate the effects of a motivationally targeted, individually tailored 12-week physical activity intervention on (1) development of GDM, (2) serum biomarkers associated with insulin resistance, and (3) the adoption and maintenance of exercise during pregnancy. Women at high risk of GDM are recruited in early pregnancy and randomized to either an individually tailored exercise intervention or a comparison health and wellness intervention.
Results
The overall goal of the exercise intervention is to encourage pregnant women to achieve the American College of Obstetricians and Gynecologists guidelines for physical activity during pregnancy through increasing walking and developing a more active lifestyle.
Conclusions
The intervention takes into account the specific social, cultural, economic, and physical environmental challenges faced by pregnant women of diverse socioeconomic and ethnic backgrounds.
Introduction
As the prevalence of diabetes continues to rise worldwide,1 it becomes increasingly important to identify high-risk populations and to implement strategies to delay or prevent diabetes onset.2,3 Women diagnosed with gestational diabetes mellitus (GDM) are at high risk for future diabetes, with approximately 50% of women developing type 2 diabetes within 5 years of delivery.4 It has been estimated that, in some populations, women with a history of GDM may account for up to one third of diabetes cases among parous women.5 Women diagnosed with GDM are also more likely to display features of the insulin resistance syndrome, which are linked to cardiovascular disease (CVD).6 Their children are at increased risk of perinatal morbidity7–9 and, in the long term, obesity and glucose intolerance.10–12 GDM, therefore, identifies a population of women at high risk of developing type 2 diabetes and thus provides an excellent opportunity to intervene years before the development of this disorder.
The value of regular physical activity for reducing the risk of CVD and type 2 diabetes in nonpregnant adults is well established.13 Similarly, recent epidemiological studies have suggested that women reporting higher levels of physical activity have reduced risk of GDM.14–20 Increased physical activity improves insulin sensitivity and glucose control in pregnant patients with GDM. To date, however, primary prevention studies have not intervened to test whether making a change in physical activity reduces the risk of developing GDM among women at high risk of this disorder.
Current American College of Obstetricians and Gynecologists (ACOG) guidelines recommend engaging in 30 minutes of moderate-intensity physical activity (e.g., brisk walking) during most days of the week for pregnant women without medical or obstetrical complications21 and are consistent with guidelines for nonpregnant women.22 The guidelines, which emphasize the accumulation of physical activity through bouts of at least 10 minutes, may be more acceptable to pregnant women than traditional exercise recommendations.23 However, the majority of pregnant women currently do not meet physical activity guidelines, with rates of compliance ranging from 13%–20%.24–27 Individually tailored, motivationally matched exercise interventions have been found to be an effective, low-cost approach for enhancing physical activity participation among nonpregnant women in the community.28–31 This study applies these programs to healthy pregnant women at high risk of GDM.
Conceptual model
Theory-driven interventions using the transtheoretical model32 and social cognitive theory33 have been shown to be effective for exercise adoption.13,34,35 The transtheoretical model was developed to help understand how and when indi-viduals make behavior change. According to this model, individuals adopting a new behavior move through a series of stages: precontemplation (not intending to make changes), contemplation (considering a change), preparation (making small changes), action (actively engaging in the behavior), and maintenance (sustaining the change over time). Also included in this model are processes of change that constitute strategies that individuals use to help make changes in their behavior. Such interventions, matched to the individual's stage of motivational readiness for physical activity adoption, have been found to be more effective than nontargeted interventions.36
Testable hypotheses
The primary goals of the study are to investigate the effects of a motivationally targeted, individually tailored 12-week physical activity intervention among women at high risk of GDM on (1) development of GDM, (2) serum biomarkers associated with insulin resistance, (3) and the adoption and maintenance of exercise during pregnancy. The study is designed to test the following primary hypotheses.
Compared with subjects who are randomized to an intervention on general issues related to health and wellness during pregnancy (the comparison health and wellness intervention), women who are randomized to an individually tailored exercise intervention will:
Have a lower incidence of GDM.
Have lower fasting concentrations of glucose, insulin, leptin, tumor necrosis factor-α (TNF-α), resistin, and C-reactive protein (CRP) and higher concentrations of adiponectin.
Participate in more physical activity in mid and late pregnancy.
Secondary goals are to investigate the impact of the intervention on gestational weight gain and selected birth outcomes (i.e., accelerated fetal growth, Apgar score).
Materials and Methods
Overview
A projected total of 364 women at high risk of GDM are recruited in early pregnancy and randomized to either an individually tailored exercise intervention (n = 182) or a comparison health and wellness intervention (n = 182). The overall goal of the exercise intervention is to encourage pregnant women to achieve ACOG guidelines for physical activity during pregnancy (30 minutes or more of moderate-intensity activity on most days of the week) through increasing walking and developing a more active lifestyle in one daily bout or accumulated through 10-minute bouts.21 The intervention consists of a 12-week program ending at routine GDM screen (24–28 weeks gestation) with approximately 14 weeks of follow-up (ending at birth).
Study population
The Behaviors Affecting Baby and You (B.A.B.Y.) study is based in Baystate Medical Center, a large tertiary care facility in Western Massachusetts, which serves an ethnically and socioeconomically diverse population, with approximately 4500 deliveries per year. Eligible women are sedentary, with a diagnosis of GDM in a prior pregnancy defined according to American Diabetes Association (ADA) criteria.37 Exclusion criteria include age <18 or >40 years, history of diagnosis of diabetes outside of pregnancy, hypertension, heart disease or chronic renal disease, current medications that adversely influence glucose tolerance, >16 weeks gestation, contraindications to participating in moderate physical activity, inability to read English at a 6th grade level, self-reported participation in >30 minutes of moderate-intensity or vigorous-intensity exercise on >3 days/week, and nonsingleton pregnancy.
Formative development of the intervention
Irrespective of ethnicity and race, women encounter unique barriers to physical activity because of their social roles as primary caregivers, their household responsibilities, and consequent perception of lack of time.38–41 These barriers are often accompanied by lack of empowerment to devote time for personal benefit.42 We used well-established qualitative intervention development methods to adapt the intervention materials to the target Baystate study population.43,44 Specifically, modifications to the intervention materials were informed by the literature, the cultural expertise of the study team, and focus groups of participants recruited from the Baystate study population. The focus groups gathered data on contextual factors impinging on physical activity levels, facilitators of physical activity, and participant reactions to the intervention materials.45 Participants in the focus groups met the same entry criteria as those in the full randomized controlled trial.
Recruitment
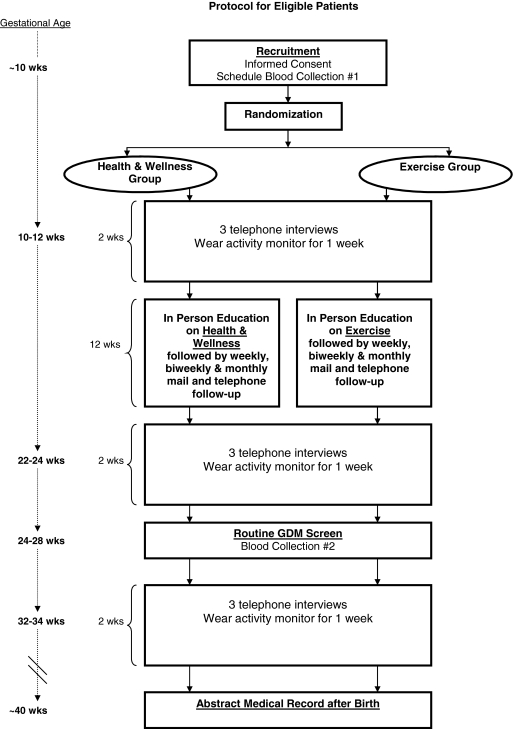
Women are recruited at the first prenatal care visit (Fig. 1) and are informed of the aims and procedures of the project, provide informed consent, and complete a brief screening form to determine eligibility. Eligible subjects are provided with a small gift. Women are then referred for a first morning fasting blood draw within the following 7 days. A gift certificate is provided for this visit, as well as for the completion of each subsequent study component. Immediately after enrollment, but prior to randomization, the study obstetrician reviews the medical records of eligible participants to exclude those with contraindications to physical activity in pregnancy.
FIG. 1.
Study design.
Randomization
Eligible subjects are randomized into either the individually tailored exercise intervention or the comparison health and wellness intervention. Randomization is stratified based on age (<30, >30 years), prepregnancy body mass index (BMI) (overweight ≥25 kg/m2 vs. normal weight BMI <25 kg/m2), and ethnicity (Hispanic vs. non-Hispanic). Within each stratum, a blocked randomization is used such that both treatment groups are assigned an equal number of times in each set of four sequentially enrolled subjects.
Intervention content
Both the exercise intervention and the health and wellness intervention consist of a 12-week program ending at routine GDM screen, with approximately 14 weeks of follow-up (until birth) (Table 1). A baseline face-to-face session with individualized counseling is followed by weekly and biweekly mailed, print-based materials as well as telephone booster calls to provide motivationally based individualized feedback. The face-to-face session takes place at the hospital or at the participant's home, according to the participant's preference. All intervention materials are written at a 6th grade reading level. Contact time between the health educators and the participants is consistent across the two conditions. In this way, we control for contact time while keeping the content of the two interventions distinct.
Table 1.
Schedule of Intervention Contacts
| Intervention week | Mode of delivery | Intervention | Comparison |
|---|---|---|---|
| 1 | Face-to-face | Tailoring Questionnaire | Tailoring Questionnaire |
| Stage-matched manual | Healthy pregnancy brochures | ||
| Safe exercise brochure | Safe exercise brochure | ||
| Pedometer and activity log | |||
| Goal setting | |||
| 2 | Individually tailored report | Healthy pregnancy brochures | |
| Face-to-face/telephone | Booster contact | Booster contact | |
| 3 | Tip sheets | Healthy pregnancy brochures | |
| 4 | Tailoring Questionnaire | Tailoring Questionnaire | |
| Tips sheets | Healthy pregnancy brochures | ||
| 6 | Individually tailored report | Healthy pregnancy brochures | |
| Stage-matched manual | |||
| Face-to-face/telephone | Booster contact | Booster contact | |
| 8 | Tailoring Questionnaire | Tailoring Questionnaire | |
| Tips sheets | Healthy pregnancy brochures | ||
| 10 | Individually tailored report | Healthy pregnancy brochures | |
| Stage-matched manual | |||
| Face-to-face/telephone | Booster contact | Booster contact | |
| 12 | Tailoring Questionnaire | Tailoring Questionnaire | |
| Tips sheets | Healthy pregnancy brochures |
Exercise intervention
During the face-to-face visit for the exercise intervention, the health educator administers a 65-item Tailoring Questionnaire to assess current stage of motivational readiness for physical activity adoption, self-efficacy, decisional balance, use of cognitive and behavioral processes of change, and the time spent in physical activity. The Tailoring Questionnaire takes 10–15 minutes to complete. Based on responses to this questionnaire, the participant is given a stage-matched manual targeted at a specific stage of motivational readiness to adopt physical activity (precontemplation, contemplation, preparation, action, and maintenance). These manuals include motivationally targeted materials combined with stretching tips, tip sheets on goal setting, benefits of physical activity, building social support for new behavioral patterns, and strategies for overcoming barriers to physical activity that are specific to ethnically/racially diverse women and women with young children. Participants are also given an ACOG Pregnancy Fitness brochure, which reviews special considerations for physical activity during pregnancy.21
Individualized week-by-week physical activity goals are determined in conjunction with the participant and informed by recent literature on barriers to physical activity among minority women and among women with young children.38,41,46 The overall activity goal is to increase the time spent in moderate activity by 10% each week, with a 12-week goal of 30 minutes of moderate-intensity physical activity performed on 5 or more days per week. Women choose what form of activity to engage in, from dancing to walking in a shopping mall to yard work. The accumulation of short bouts (i.e., <10 minute episodes of walking) is encouraged.
Participants are provided with a digital pedometer to encourage self-monitoring. The pedometer can be kept in a shirt pocket or worn as a necklace, which may be particularly appealing for pregnant women. Women are also given a pedometer activity log to keep track of their daily total steps. Previous studies support the use of pedometers as a motivational tool and have observed a significant impact on walking behavior.47
Following the face-to-face visit, the participant receives, via mail, follow-up Tailoring Questionnaires (with a postage-paid envelope). Return of these questionnaires triggers the mailing of individually tailored reports generated by a computer expert system and corresponding stage-matched manuals. The individually tailored reports provide (1) an assessment of the individual's current stage of motivational readiness to adopt a physical activity regimen,48 (2) assessments of the individual's self-efficacy,49 benefits of and barriers to the adoption of physical activity, decisional balance,50 use of cognitive and behavioral processes associated with physical activity adoption,48 and normative feedback, and (3) feedback regarding progress the individual made on these constructs and minutes of physical activity participation since the prior assessment. The individually tailored reports and the stage-matched manuals have been field tested and shown to be effective in prior interventions by Marcus et al.36,51
Over the course of the intervention, weekly and biweekly booster telephone calls provide motivationally based individualized feedback as well as review of progress toward behavioral goals, including a review of the pedometer activity log. Those not achieving their weekly physical activity goals are given additional individualized physical activity counseling with a focus on overcoming barriers.
Health and wellness intervention
During the face-to-face visit for the comparison intervention, the health educator gives participants a book published by ACOG that covers every aspect of pregnancy from preconceptional and prenatal care, health insurance, and labor and delivery to breastfeeding and child care options. The health educator reviews with the participant general issues related to health and wellness during pregnancy. Following the face-to-face visit, participants receive weekly and biweekly mailings of ACOG informational brochures on such topics as alcohol and drug use during pregnancy, easing back pain, travel during pregnancy, and other topics. Participants are also given the ACOG Pregnancy Fitness brochure, which reviews special considerations for physical activity during pregnancy.21 These materials are selected to represent high-quality, standard, low-cost, self-help material currently available to the public. Weekly and biweekly booster telephone calls provide an opportunity for participants to ask questions about the materials they received.
Outcome variables
Baystate Obstetrical Practices routinely screen all prenatal care patients for GDM. The screening test consists of a random 50-g glucose load and a plasma glucose determination 1 hour later (1-hr OGTT). If the plasma glucose value was >135mg/dL, a 3-hour GTT was performed. After the subject fasted overnight, a serum glucose value was obtained and a 100-g glucose load is given orally. Plasma samples were drawn at 1, 2, and 3 hours after glucose administration. Diagnosis of GDM was defined according to any one of the following three criteria: (1) two or more elevated values at fasting and 1, 2, and 3 hours, respectively, based on the ADA criteria of 95, 180, 155, and 140 mg/dL,37 (2) a 1-hr OGTT >200 mg/dL,52 and (3) elevated fasting (>105 mg/dL) or elevated 2-hr postprandial blood sugar (>120 mg/dL) in patients unwilling or unable to tolerate the OGTT.53 Diagnosis of GDM is confirmed by an obstetrician who reviewed the medical records of each suspected case.
Serum biomarkers associated with insulin resistance (fasting glucose, insulin, adiponectin, leptin, TNF-α, resistin, and CRP) are collected via a fasting serum sample at two time points: (1) within the 7-day period after recruitment and (2) within the 7-day period after routine second trimester screen for GDM (24–28 weeks gestation). Time since last meal and time of blood draw are included and incorporated in the analyses.
Pregravid weight is recorded in the medical record and is defined as weight at last menstrual period (LMP) as self-reported by the patient at the time of her first prenatal care visit. In those cases where prepregnancy weight is not recorded in the medical record, self-reported prepregnancy weight, obtained at the time of enrollment, is used. Weight during pregnancy is measured in all participants at each prenatal visit. Maternal weight gain is measured as change in weight from pregravid to delivery. Details of birth outcomes are abstracted from the electronic record after delivery and include birthweight, Apgar score, Cesarean delivery, accelerated fetal growth, and NICU admission. Accelerated fetal growth is defined according to ACOG guidelines54 as (1) macrosomia (>4000 g) and (2) large for gestational age, defined as newborn weight ≥ the 90th percentile for completed gestational weeks using cutoff points defined by Oken.55
Assessment measures
Baseline and follow-up assessments are conducted prior to randomization, at the end of the intervention, and in the third trimester to collect information on levels of and change in physical activity, diet, and other important GDM risk factors (Table 2). Each of the assessments includes the administration of the Pregnancy Physical Activity Questionnaire (PPAQ) as well as three 24-hour physical activity recalls. In addition, during each assessment period, an accelerometer is worn for 7 days. The interviewer is blinded to the treatment assignment throughout.
Table 2.
Variables to be Collected at Data Collection Times
| |
1st trimester |
2nd trimester |
3rd trimester |
|||
|---|---|---|---|---|---|---|
| Variable | Eligibility and blood sample (10 weeks) | Assessment 1 (10–12 weeks) | Assessment 2 (22–24 weeks) | GDM and blood sample (24–28 weeks) | Assessment 3 (32–34 weeks) | Birth (∼40 weeks) |
| Physical activity variables | ||||||
| 3 24-hour PA recalls | X | X | X | |||
| Accelerometer | X | X | X | |||
| PPAQ | X | X | X | |||
| Primary outcome variables | ||||||
| Gestational diabetes mellitus | X | |||||
| Glucose | X | X | ||||
| Insulin | X | X | ||||
| Adiponectin | X | X | ||||
| Leptin | X | X | ||||
| Resistin | X | X | ||||
| TNF-α | X | X | ||||
| CRP | X | X | ||||
| Secondary outcome variables | ||||||
| Maternal weight gaina | X | X | X | X | X | X |
| Birth weight | X | |||||
| Apgar score | X | |||||
| Exploratory outcome variables | ||||||
| Glycemic control/treatment | X | |||||
| Covariates | ||||||
| Dietary factors: 24-hour recall | X | X | X | |||
| Baseline and intrapartum medical historya | X | X | X | X | X | X |
| Sociodemographic factors | X | |||||
| Race/ethnicity | X | |||||
| Substance useb | X | X | X | X | X | |
Collected at every prenatal care visit.
Smoking assessed via serum cotinine and self-report.
The telephone interviewer uses interactive software to conduct a 24-hour physical activity recall, probing for detailed information on type and duration of each activity during the previous 24 hours. In addition, the PPAQ, a semiquantitative questionnaire that asks respondents to report usual physical activity during the past month,56 is administered. The PPAQ queries the time spent participating in 32 activities, including household/caregiving (13 activities), occupational (5 activities), sports/exercise (8 activities), transportation (3 activities), and inactivity (3 activities). For every individual for both measures, the number of minutes spent in each reported activity type will be multiplied by its MET intensity and summed to arrive at a measure of average weekly energy expenditure (MET-hrs/wk). MET intensity scores will be based on the Compendium of Physical Activities,57 with the exception of walking and light housework activities, for which field-based measures among pregnant women will be used.56 Average weekly energy expenditure will be further classified into categories based on activity intensity and type. Intensity categories are sedentary (<1.5 METs), light (1.5–2.9 METs), moderate (3.0–6.0 METs), and vigorous (>6.0 METs). Categories of activity type include household, occupational, sports and exercise, and sleep.
The relative validity of 24-hour physical activity recalls has been described in detail, with correlations for women ranging from 0.54 for household activity, 0.74 for occupational activity, and 0.68 for leisure time physical activity when compared with a physical activity diary.58 Intraclass correlation coefficients used to measure reproducibility of the PPAQ were 0.78 for total activity, and 0.82 for moderate activity, and 0.81 for vigorous activity and ranged from 0.83 for sports/exercise to 0.93 for occupational activity. Spearman correlations between the PPAQ and three published cutoff points used to classify accelerometer data ranged from 0.08 to 0.43 for total activity, 0.25 to 0.34 for vigorous activity, and 0.20 to 0.49 for moderate activity. A series of three 24-hour physical activity recalls has been found to provide a reasonable measure of short-term physical activity energy expenditure.58
At enrollment, women are fitted with the ActiGraph accelerometer (Fort Walton Beach, FL) a uniaxial actigraph that detects vertical accelerations ranging in magnitude from 0.05 to 2.00 G, with frequency response from 0.25 to 2.50 Hz. These parameters detect normal human movement while filtering out high-frequency movements, such as vibrations. The filtered acceleration signal is digitized, and the magnitude is summed over a user-specified time interval (epoch). At the end of each epoch, the activity count is stored in memory and the accumulator is reset to zero.59 A 1-minute epoch will be used in the current study. The accelerometer is affixed with an adjustable elastic belt or a clip on the right hip and worn for the 7-day period starting the morning after its receipt. Women are asked to wear the accelerometer all day except while sleeping, bathing, and swimming. Estimates of the average number of minutes/week spent in activity of moderate intensity and greater will be calculated using the count cutoff point of >2000 recommended by Matthews.60 Significant correlations between the accelerometer and directly measured energy expenditure during treadmill walking and running have been observed using open-circuit spirometry (r = 0.88)61 as well as between the accelerometer and directly measured oxygen consumption during moderate-intensity activities in the field (r = 0.59 for all activities combined and 0.77 for outdoor walking).62 Women are provided with a padded, self-addressed envelope to return the accelerometer in the mail. For the follow-up assessments, the accelerometer is sent via the mail.
Covariates
Dietary factors are collected via three 24-hour dietary recalls conducted during the midpregnancy telephone assessment. The interviewer follows a script developed by the University of Minnesota's Nutrition Coordinating Center (NCC) in which participants are prompted to remember and report everything they have eaten in the past 24 hours. The interviewer probes for detailed information about specific quantities, brand names, and cooking methods for each food. Recent studies have found no appreciable change in mean food group intake between the first and second trimesters, suggesting that a midpregnancy dietary assessment may be sufficient to characterize dietary intake prior to the diagnosis of GDM.63
Baseline and intrapartum medical history, including previous infant with anomalies, stillbirth, or macrosomia, infertility, family history of diabetes, and parity, are abstracted from medical records. Clinical characteristics of the current pregnancy (e.g., pregnancy-induced hypertension or preeclampsia, infection, prescribed bed rest, and prenatal care) are also abstracted. Age, race/ethnicity, and prepregnancy BMI are assessed via self-report at recruitment, as randomization is stratified according to these variables. Participants also self-report morning sickness (nausea and vomiting), alcohol consumption, and cigarette smoking at recruitment. Serum concentration of cotinine is assessed via the two serum samples (collected at baseline and 24–28 weeks gestation).
Statistical approach and power
The impact of the exercise intervention compared with the health and wellness intervention on risk of developing GDM will be evaluated using chi-square tests and logistic regression, with an intent-to-treat approach. The equivalence of the treatment groups will be assessed by comparing the distribution of the potential confounders between each group. Important potential effect modifiers include pre-gravid BMI and maternal weight gain. We will model the relationship between physical activity scores and risk of GDM while adjusting for possible confounders using logistic regression models.
The second hypothesis will evaluate the effect of the intervention on serum biomarkers associated with insulin resistance. This analysis will be based on a mixed-effect model with random subject effects, including a common mean at baseline for the treatment groups, a period effect, and an intervention by period interaction.64 Finally, for the third hypothesis, mixed-effect models will be used to evaluate the effect of the intervention on the adoption and maintenance of physical activity. Similar methods will be used to evaluate the secondary hypotheses involving maternal weight gain and birth outcomes.
Sample size calculations were based on the power to detect recurrent GDM for a range of relative risks given a 1:1 ratio of intervention to comparison groups and alpha = 0.05. Given a sample size of 364 and a recurrence rate of 50%, we will have power to detect relative risks of ≥0.70, a clinically significant 30% reduction in risk.65
Data safety and monitoring plan
In light of the minimal risks of the intervention, but recognizing the vulnerable nature of the pregnant population, this trial is monitored in compliance with an independent Data and Safety Monitoring Board (DSMB). The DSMB (1) reviewed the research protocol and plans for data safety and monitoring, (2) evaluate the progress of the trial with biannual assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk vs. benefit, and reports from related studies, and (3) makes recommendations to the IRB and investigators concerning continuation or conclusion of the trial.
Discussion
The B.A.B.Y. study will test the ability of a physical activity intervention to prevent GDM in an ethnically diverse high-risk population at a life period conducive to behavior change. The intervention draws from the transtheoretical model and social cognitive theory constructs for physical activity behavior and takes into account the specific social, cultural, economic, and physical environmental challenges faced by pregnant women of diverse socioeconomic and ethnic backgrounds. It addresses the rapidly changing context of pregnancy, which brings opportunities for adoption and maintenance of new behaviors.
Strengths of the B.A.B.Y. study include the formative development component conducted within the target population, the use of multidimensional (subjective and objective) measures of physical activity, and multiple and fasting measures of serum biomarkers associated with insulin resistance. The study is also novel in developing a culturally tailored exercise intervention for a multiethnic population.
We considered but chose not to add a dietary component to the intervention, as the literature on diet and risk of GDM is somewhat sparse and conflicting. High-fat diets have been associated with the development of glucose abnormalities in pregnancy66 and with the recurrence of GDM in future pregnancies.67 However, a more recent prospective cohort study among 1733 pregnant women found that dietary fats, carbohydrate, and glycemic load were not associated with GDM or risk of impaired glucose tolerance.68 Thus, we thought that a dietary intervention would be premature at this time. Counseling is routinely provided to all prenatal care patients at Baystate regarding the Institute of Medicine Guidelines for appropriate nutrition and weight gain in pregnancy.69 In addition, we collect comprehensive information on diet through 24-hour recalls and will statistically adjust for diet in analyses.
Because participants are recruited at their first prenatal care visit, we are excluding, by definition, high-risk women who do not attend prenatal care. However, our study population includes a sizeable proportion of women who are at high risk based on socioeconomic factors and ethnicity. For example, statewide data for Latina births in Massachusetts indicate that 64.4% of Latinas in Massachusetts begin prenatal care in the first trimester and have a total of nine or more visits.70 In the current study, women were eligible to participate up to and including 16 weeks gestation.
Conclusions
The B.A.B.Y. study is innovative in testing a physical activity intervention designed to prevent GDM among high-risk women. It is also novel in developing a culturally tailored exercise intervention for a multiethnic population. The intervention protocol can readily be translated into clinical practice in underserved and minority populations. Adoption of such a lifestyle-based intervention by pregnant women is facilitated by custom fitting the physical activity into a daily routine appropriate to individual lifestyles.
Acknowledgments
This work was supported by NIH NIDDK 1R01DK074876.
Disclosure Statement
No competing financial interests exist.
References
- 1.King H. Aubert RE. Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Reichard P. Nilsson BY. Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 3.U.K. Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Kjos SL. Postpartum care of the woman with diabetes. Clin Obstet Gynecol. 2000;43:75–86. doi: 10.1097/00003081-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kim C. Newton KM. Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan TA. Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt MI. Duncan BB. Reichelt AJ, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151–1155. doi: 10.2337/diacare.24.7.1151. [DOI] [PubMed] [Google Scholar]
- 8.Naylor CD. Sermer M. Chen E. Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: Pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA. 1996;275:1165–1170. [PubMed] [Google Scholar]
- 9.Magee MS. Walden CE. Benedetti TJ. Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA. 1993;269:609–615. [PubMed] [Google Scholar]
- 10.Pettitt DJ. Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 1998;21(Suppl 2):B138–141. [PubMed] [Google Scholar]
- 11.Silverman BL. Rizzo TA. Cho NH. Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl 2):B142–149. [PubMed] [Google Scholar]
- 12.Vohr BR. McGarvey ST. Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care. 1999;22:1284–1291. doi: 10.2337/diacare.22.8.1284. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services. Physical activity and health: A report of the Surgeon General. US Dept of Health and Human Services, Office of Surgeon General; 1996. [Google Scholar]
- 14.Dempsey JC. Butler CL. Sorensen TK, et al. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2004;66:203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey JC. Sorensen TK. Williams MA, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004;159:663–670. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- 16.Oken E. Ning Y. Rifas-Shiman SL. Radesky JS. Rich-Edwards JW. Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108:1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C. Solomon CG. Manson JE. Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med. 2006;166:543–548. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal R. Rafique G. Badruddin S. Qureshi R. Cue R. Gray-Donald K. Increased body fat percentage and physical inactivity are independent predictors of gestational diabetes mellitus in South Asian women. Eur J Clin Nutr. 2007;61:736–742. doi: 10.1038/sj.ejcn.1602574. (Epub 2006, Dec 20.) [DOI] [PubMed] [Google Scholar]
- 19.Dyck R. Klomp H. Tan LK. Turnell RW. Boctor MA. A comparison of rates, risk factors, and outcomes of gestational diabetes between aboriginal and non-aboriginal women in the Saskatoon health district. Diabetes Care. 2002;25:487–493. doi: 10.2337/diacare.25.3.487. [DOI] [PubMed] [Google Scholar]
- 20.Dye TD. Knox KL. Artal R. Aubry RH. Wojtowycz MA. Physical activity, obesity, and diabetes in pregnancy. Am J Epidemiol. 1997;146:961–965. doi: 10.1093/oxfordjournals.aje.a009223. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: Exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 22.Pate RR. Pratt M. Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL. Lee IM. Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 24.Gollenberg A. Pekow P. Markenson G. Tucker KL. Chasan-Taber L. Dietary behaviors, physical activity, and cigarette smoking among pregnant Puerto Rican women. Am J Clin Nutr. 2008;87:1844–1851. doi: 10.1093/ajcn/87.6.1844. [DOI] [PubMed] [Google Scholar]
- 25.Haas JS. Fuentes-Afflick E. Stewart AL, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159:58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Evenson KR. Savitz DA. Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J. Savitz DA. Exercise during pregnancy among US women. Ann Epidemiol. 1996;6:53–59. doi: 10.1016/1047-2797(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 28.Marcus BH. Lewis BA. Williams DM, et al. A comparison of Internet and print-based physical activity interventions. Arch Intern Med. 2007;167:944–949. doi: 10.1001/archinte.167.9.944. [DOI] [PubMed] [Google Scholar]
- 29.Marcus BH. Lewis BA. Williams DM, et al. Step into Motion: A randomized trial examining the relative efficacy of Internet vs. print-based physical activity interventions. Contemp Clin Trials. 2007;28:737–747. doi: 10.1016/j.cct.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Marcus BH. Napolitano MA. King AC, et al. Telephone versus print delivery of an individualized motivationally tailored physical activity intervention: Project STRIDE. Health Psychol. 2007;26:401–409. doi: 10.1037/0278-6133.26.4.401. [DOI] [PubMed] [Google Scholar]
- 31.Marcus BH. Napolitano MA. King AC, et al. Examination of print and telephone channels for physical activity promotion: Rationale, design, and baseline data from Project STRIDE. Contemp Clin Trials. 2007;28:90–104. doi: 10.1016/j.cct.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochaska JO. DiClemente CC. Velicer WF. Rossi JS. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol. 1993;12:399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- 33.Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 34.Blair SN. Kohl HW., 3rd Paffenbarger RS., Jr Clark DG. Cooper KH. Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 35.King AC. Blair SN. Bild DE, et al. Determinants of physical activity and interventions in adults. Med Sci Sports Exerc. 1992;24(Suppl 6):S221–236. [PubMed] [Google Scholar]
- 36.Marcus BH. Bock BC. Pinto BM. Forsyth LH. Roberts MB. Traficante RM. Efficacy of an individualized, motivationally tailored physical activity intervention. Ann Behav Med. 1998;20:174–180. doi: 10.1007/BF02884958. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 38.Eyler AA. Matson-Koffman D. Vest JR, et al. Environmental, policy, and cultural factors related to physical activity in a diverse sample of women: The Women's Cardiovascular Health Network Project—Summary and discussion. Women Health. 2002;36:123–134. [PubMed] [Google Scholar]
- 39.Eyler AA. Wilcox S. Matson-Koffman D, et al. Correlates of physical activity among women from diverse racial/ethnic groups. J Womens Health Gend Based Med. 2002;11:239–253. doi: 10.1089/152460902753668448. [DOI] [PubMed] [Google Scholar]
- 40.King AC. Castro C. Wilcox S. Eyler AA. Sallis JF. Brownson RC. Personal and environmental factors associated with physical inactivity among different racial-ethnic groups of U.S. middle-aged and older-aged women. Health Psychol. 2000;19:354–364. doi: 10.1037//0278-6133.19.4.354. [DOI] [PubMed] [Google Scholar]
- 41.Evenson KR. Sarmiento OL. Macon ML. Tawney KW. Ammerman AS. Environmental, policy, and cultural factors related to physical activity among Latina immigrants. Women Health. 2002;36:43–57. doi: 10.1300/J013v36n02_04. [DOI] [PubMed] [Google Scholar]
- 42.Henderson KA. Hodges S. Kivel BD. Context and dialogue in research on women and leisure. J Leisure Res. 2002;34:253–271. [Google Scholar]
- 43.Ayala GX. Elder JP. Campbell NR, et al. Nutrition communication for a Latino community: Formative research foundations. Fam Community Health. 2001;24:72–87. doi: 10.1097/00003727-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Rounsaville BJ. Carroll KM. Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clin Psychol Sci Pract. 2001;8:133–142. [Google Scholar]
- 45.Krueger RA. Casey MA. Focus groups: A practical guide for applied research. 3rd. Thousand Oaks, CA: Sage Publications; 2000. [Google Scholar]
- 46.Amesty SC. Barriers to physical activity in the Hispanic community. J Public Health Policy. 2003;24:41–58. [PubMed] [Google Scholar]
- 47.Tudor-Locke C. Bell RC. Myers AM, et al. Controlled outcome evaluation of the First Step Program: A daily physical activity intervention for individuals with type 2 diabetes. Int J Obes Rel Metab Disord. 2004;28:113–119. doi: 10.1038/sj.ijo.0802485. [DOI] [PubMed] [Google Scholar]
- 48.Marcus BH. Rossi JS. Selby VC. Niaura RS. Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychol. 1992;11:386–395. doi: 10.1037//0278-6133.11.6.386. [DOI] [PubMed] [Google Scholar]
- 49.Marcus BH. Selby VC. Niaura RS. Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 50.Marcus BH. Rakowski W. Rossi JS. Assessing motivational readiness and decision making for exercise. Health Psychol. 1992;11:257–261. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- 51.Marcus BH. Emmons KM. Simkin-Silverman LR, et al. Evaluation of motivationally tailored vs. standard self-help physical activity interventions at the workplace. Am J Health Promot. 1998;12:246–253. doi: 10.4278/0890-1171-12.4.246. [DOI] [PubMed] [Google Scholar]
- 52.Landy HJ. Gomez-Marin O. O'Sullivan MJ. Diagnosing gestational diabetes mellitus: Use of a glucose screen without administering the glucose tolerance test. Obstet Gynecol. 1996;87:395–400. doi: 10.1016/0029-7844(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 53.Atilano LC. Lee-Parritz A. Lieberman E. Cohen AP. Barbieri RL. Alternative methods of diagnosing gestational diabetes mellitus. Am J Obstet Gynecol. 1999;181:1158–1161. doi: 10.1016/s0002-9378(99)70100-6. [DOI] [PubMed] [Google Scholar]
- 54.ACOG practice bulletin Clinical management guidelines for obstetrician-gynecologist. Fetal Macrosomia. 2000;22:1–11. [Google Scholar]
- 55.Oken E. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chasan-Taber L. Schmidt MD. Roberts DE. Hosmer D. Markenson G. Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36:1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 57.Ainsworth BE. Haskell WL. Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl 9):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 58.Matthews CE. Freedson PS. Hebert JR. Stanek EJ., 3rd Merriam PA. Ockene IS. Comparing physical activity assessment methods in the Seasonal Variation of Blood Cholesterol Study. Med Sci Sports Exerc. 2000;32:976–984. doi: 10.1097/00005768-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Computer Science. Applications, Inc. Activity monitor operators manual, Model 7164. Release 1.2.1. Computer Science and Applications, Inc.; Shalimar, FL: [Google Scholar]
- 60.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(Suppl 11):S512–522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 61.Melanson EL., Jr Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27:934–940. [PubMed] [Google Scholar]
- 62.Hendelman D. Miller K. Baggett C. Debold E. Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exerc. 2000;32(Suppl 9):S442–449. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 63.Rifas-Shiman SL. Rich-Edwards JW. Willett WC. Kleinman KP. Oken E. Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20:35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanek EJ. Choosing a pretest-posttest analysis. Am Statistician. 1988;42:178–183. [Google Scholar]
- 65.Chapman DG. Asymptotic power of chi square tests for linear trends in proportions. Biometrics. 1968;24:315–327. [PubMed] [Google Scholar]
- 66.Saldana TM. Siega-Riz AM. Adair LS. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am J Clin Nutr. 2004;79:479–486. doi: 10.1093/ajcn/79.3.479. [DOI] [PubMed] [Google Scholar]
- 67.Moses RG. Shand JL. Tapsell LC. The recurrence of gestational diabetes: Could dietary differences in fat intake be an explanation? Diabetes Care. 1997;20:1647–1650. doi: 10.2337/diacare.20.11.1647. [DOI] [PubMed] [Google Scholar]
- 68.Radesky JS. Oken E. Rifas-Shiman SL. Kleinman KP. Rich-Edwards JW. Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol. 2008;22:47–59. doi: 10.1111/j.1365-3016.2007.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Institute of Medicine, Subcommittee on Nutritional on Status and Weight Gain during Pregnancy. National Academy Press; Washington, DC: 1990. [Google Scholar]
- 70.Averbach AR. Judge C. Orejuela M, et al. Hispanic births in Massachusetts 1996–1999. Commonwealth of Massachusetts, Dept of Public Health, Bureau of Health Statistics, Research, and Evaluation; 2001. November. [Google Scholar]