Abstract
Background
Perinatal mood disorders affect up to 20% of women in the United States. Little is known about how disorders in maternal mood may affect rates of breastfeeding.
Objective
To determine the impact of prenatal depressive symptoms and high pregnancy-related anxiety on (1) prenatal intention to breastfeed and (2) breastfeeding initiation.
Methods
We prospectively followed 1436 pregnant women enrolled in the cohort study Project Viva. The main outcome measures were (1) mother's second trimester self-report of intention to use all or mostly formula in the first week of life and (2) failure to initiate breastfeeding. We defined prenatal depressive symptoms as a second trimester Edinburgh Postpartum Depression Scale (EPDS) score of ≥13 and high pregnancy-related anxiety as a “very much” response to three or more questions on a first trimester pregnancy anxiety scale.
Results
Of the 1436 participants, 9% (n = 125) had prenatal depressive symptoms indicative of depression, and 10% (n = 141) reported high pregnancy-related anxiety; 11% (n = 159) intended to give mostly or only formula in the first week of life, and 86% (n = 1242) initiated breastfeeding. In multivariate analyses, women with prenatal depressive symptoms (OR 1.92, 95% CI 1.11, 3.33) and high pregnancy-related anxiety (OR 1.99, 95% CI 1.12, 3.54) were roughly two times more likely than women without these mood disorders to plan to formula feed. However, neither prenatal depressive symptoms (OR 1.06, 95% CI 0.61, 1.84) nor high pregnancy-related anxiety (OR 1.28, 95% CI 0.74, 2.20) was associated with failure to initiate breastfeeding.
Conclusions
In a healthcare setting highly supportive of breastfeeding, women with prenatal depressive symptoms and possibly those with high pregnancy-related anxiety were less likely to plan prenatally to breastfeed, although this tendency did not translate into lower breastfeeding initiation rates.
Introduction
Perinatal mood and anxiety disorders affect an estimated 20% of women during pregnancy1 or in the postpartum period2 and include prenatal depression3–6 (7%–11%), postpartum depression2,3,7 (11%–14%), postpartum psychosis8 (0.1%–0.2%), and anxiety disorders7,9,10 (6.6%–16.8%). Some experts also include high levels of pregnancy-related anxiety—maternal fears and anxiety related to the outcome of the pregnancy, the experience of labor, and the health and well-being of the fetus11,12—within the spectrum of perinatal mood and anxiety disorders.13–17 Perinatal mood and anxiety disorders are associated with many aspects of maternal health, including breastfeeding.18–23 However, the few studies that have examined the association between perinatal mood disorders and breastfeeding have been cross-sectional studies of postpartum depression rates among breastfeeding vs. nonbreastfeeding women, often reporting lower rates of depression or better mood among women who breastfeed.21–23 Studies have shown decreased breastfeeding duration in women with postpartum depression,18–20 although few of these studies attempted to distinguish new-onset postpartum depression from persistent perinatal depression beginning prior to delivery. Two separate studies have shown that prenatal depression was not associated with decreased prenatal intention to breastfeed after controlling for maternal socioeconomic factors or behavioral characteristics.24,25 No study has specifically examined the effect of prenatal depression on breastfeeding initiation, although a study by Chung et al.26 in Philadelphia showed that persistent perinatal (i.e., prenatal and postpartum) depression had no effect on continuation of breastfeeding for 1 month or less. To our knowledge, no studies have examined links between prenatal maternal anxiety disorders or pregnancy-related anxiety and breastfeeding, although one study showed a positive association between postnatal maternal anxiety and infant formula supplementation during the postpartum hospital stay.27
The studies of postpartum mood and breastfeeding do not take into account the process by which women choose to breastfeed. Breastfeeding behavior is a two-step process: (1) decision to breastfeed, which is shaped by sociodemographic, clinician, and psychosocial factors,20,28–31 and (2) initiation of breastfeeding, which is dependent on the same factors as well as hospital maternity practices, mode of delivery, and perinatal complications.32–38 Studies estimate that 82%–97% of women make their decision about breast or formula feeding prior to delivery.39–42 The vast majority (75%–97%) of women who indicate during pregnancy that they plan to breastfeed do initiate breastfeeding.39–41 Prenatal mood disorders may affect a woman's plans to breastfeed and may be early risk factors for failure to breastfeed.
The aims of this study were to examine the associations of prenatal depressive symptoms and of high pregnancy-related anxiety with (1) prenatal intention to breastfeed and (2) initiation of breastfeeding.
Materials and Methods
We used data collected as part of Project Viva, a prospective cohort study of the determinants of pregnancy outcomes and offspring health.43 Pregnant members of eight selected obstetric offices of Harvard Vanguard Medical Associates, a large, multispecialty urban/suburban group practice in eastern Massachusetts, were enrolled in the study at their first prenatal visit and followed through delivery and postpartum. Human subjects committees of Harvard Pilgrim Health Care, Brigham and Women's Hospital, and Beth Israel Deaconess Medical Center approved the study protocols. At the first study visit, directly after the initial clinical prenatal visit, a trained research assistant approached the potential participant, described the study, obtained informed consent, administered a brief interview, and provided a take-home self-administered questionnaire. Research assistants were not associated with any of the clinical offices or hospitals involved in the participants' care; they informed participants at the initial screening visit that participation in the project would have no effect on routine care and that this information would not be communicated to their healthcare providers. Subjects were blinded to the specific hypotheses, and there were no specific hypotheses about lactation at the time of recruitment. Breastfeeding was not specifically mentioned during recruitment, and none of the recruiters were associated with breastfeeding promotion.
Exclusion criteria were multiple gestation, inability to answer questions in English, plans to move out of the area before delivery, and gestational age >22 completed weeks at initial prenatal clinical appointment. We enrolled 2670 pregnant women (64% of those eligible) between April 22, 1999, and July 31, 2002. Of the 2670 participants, 329 subsequently became ineligible because of multiple gestation (n = 19), transferring obstetric care to a nonstudy site (n = 115), or because they were no longer pregnant (n = 195). Of the 2341 remaining participants, 195 (8%) withdrew and 18 (<1%) were lost to follow-up, leaving 2128 who delivered a live infant. We excluded women from this analysis who did not complete all items on the anxiety questionnaire (n = 187) and who did not return (n = 366) or who did not complete one or more questions of the second trimester Edinburgh Postpartum Depression Scale (EPDS) (n = 44). We also excluded women who did not respond to the question regarding previous history of depression (n = 3), which we defined as self-reported depressive symptoms and either a (1) prepregnancy diagnosis of depression by a health professional or (2) prior use of prescription antidepressants. Finally, we excluded women whose postdelivery breastfeeding status could not be determined (n = 20) or who were uncertain what they would feed their baby in the first week of life (n = 72). Thus, we based these analyses on 1436 women.
Study representatives enrolled women immediately after the initial clinical prenatal appointment, usually during the first trimester of pregnancy. Study representatives then conducted a brief interview, and women were asked to complete a short questionnaire that included 7 items on pregnancy-related anxiety of the 10 previously used by Rini et al.44 The questionnaire included such items as concern about how the baby is growing and developing and concern about having a hard or difficult labor and delivery. For each item, the possible responses were very much, moderately, somewhat, and not at all.44–46 The mean gestational age at the time the woman completed the anxiety questionnaire was 10.4 weeks.
The second trimester study visit occurred in late second trimester, typically at 26–28 weeks. At that time, participants completed a questionnaire on history of depressive symptoms, previous diagnosis of depression by a medical professional, and previous use of antidepressant medication. Participants also completed the 10-item Edinburgh EPDS.47 We have described the study procedures in more detail elsewhere.48 The EPDS has been validated for use in prenatal patients and is 86% sensitive and 78% specific for diagnosis of prenatal depression.49
At the same second trimester visit, we determined each subject's intention to breastfeed with written questions asking if she planned to feed her infant breast milk only, mostly breast milk, some formula, mostly formula, some breast milk, or formula only during the first week of infant life or if she was uncertain about what she planned to feed her infant in the first week. Women who planned mostly or exclusively to bottle feed during the first week of life (n = 159) were categorized as “planned to formula feed.” Women who indicated that they would only or mostly breastfeed during the first week of life were categorized as “planned to breastfeed.”
Information on initiation of breastfeeding was collected at postdelivery interviews. Participants were asked: “Have you breastfed your baby? By breastfeeding, we mean that you have put your baby to your breast whether or not your baby actually received breast milk, or that you have fed your baby your breast milk?” to report whether or not they had initiated breastfeeding. We defined failure to initiate breastfeeding as a response of No to this question.
We attempted to minimize bias due to self-report of breastfeeding behaviors in many ways. First, Project Viva research assistants who conducted the delivery interview typically had not met with participants prior to the delivery interview, which included the breastfeeding behavior questions. None of the research assistants involved in Project Viva were involved in breastfeeding promotion or education, and none were clinical care providers in any of the hospital or clinic sites associated with Project Viva. Participants were reminded during each of the in-person interviews that their answers were confidential and that their answers would not affect the healthcare provided to them. Research assistants also underwent a lengthy training process on interviewing and were observed several times prior to being allowed to interview participants independently. The script used for the delivery interview was written and piloted to be value neutral to attempt to encourage accurate self-report. Specifically, the research assistant's delivery interview dialogue reads: “This next section is about feeding your baby. I'd like to begin by stressing that there are no right or wrong answers. Please just answer as best you can.”
We estimated odds ratios (ORs) of prenatal intention to formula feed and failure to initiate breastfeeding from logistic regression models fit using SAS version 8.02 software (SAS Institute, Cary, NC). We categorized prenatal depressive symptoms into two groups based on the second trimester EPDS score. We considered women with scores of <13 to have no complaint of prenatal depressive symptoms, and we designated these women as the reference group. We classified women with scores of ≥13 on a 0–30 scale as having prenatal depressive symptoms, in keeping with studies validating the EPDS as a measure of major and minor depression in English-speaking populations.47,49,50 We categorized pregnancy anxiety into two groups based on the number of times a woman chose the response very much to any of the anxiety questions. We have previously used this categorical measure of anxiety because it does not require an assumption of equidistance between response levels of somewhat, moderately, and very much. We classified women with three or more very much responses as the high anxiety group. We categorized all other women as having low-moderate levels of pregnancy-related anxiety.
We considered the following variables as possible confounders or effect modifiers: maternal age, race/ethnicity, maternal country of origin, gestational age, mode of delivery, partner status, household income, and maternal educational attainment.
Results
Table 1 shows the distribution of study characteristics and the percent of women reporting prenatal depressive symptoms and high pregnancy-related anxiety. The study population was largely white, well educated, and married. The average age of the participants was 32.4 years, and 51% had a previous live birth. Nonwhite women more frequently reported prenatal depressive symptoms and high pregnancy-related anxiety, as did women in the lowest category of household income. In a previous analysis, we showed that the higher prevalence of prenatal depressive symptoms in minority women was largely explained by lower socioeconomic position.48 High pregnancy anxiety was more prevalent among younger women (20% of women under 25) and among women with prenatal depressive symptoms (24% of women with depressive symptoms compared with 8% of women without prenatal depressive symptoms).
Table 1.
Maternal Characteristics and Associated Prevalence of Prenatal Depressive Symptoms and High Pregnancy-Related Anxiety: Data from 1436 Participants in Project Viva
| Maternal characteristic | n (%) of total | n (%) of category with prenatal depressive symptoms | n (%) of category with high pregnancy-related anxiety |
|---|---|---|---|
| Total | 1436 | 125 (9%) | 141 (10%) |
| Age, years | |||
| <25 | 86 (6%) | 17 (20%) | 17 (20%) |
| 25–29 | 300 (21%) | 26 (9%) | 28 (9%) |
| 30–39 | 993 (69%) | 79 (8%) | 87 (9%) |
| 40+ | 57 (4%) | 3 (5%) | 9 (16%) |
| Parity | |||
| No previous live births | 706 (49%) | 58 (8%) | 96 (14%) |
| 1 or more previous live births | 730 (51%) | 67 (9%) | 45 (6%) |
| Race/ethnicity | |||
| White | 1051 (74%) | 75 (7%) | 86 (8%) |
| Black | 163 (11%) | 22 (14%) | 17 (10%) |
| Asian | 85 (6%) | 10 (12%) | 19 (22%) |
| Hispanic | 75 (5%) | 12 (10%) | 12 (16%) |
| Other/multiracial | 56 (4%) | 6 (11%) | 6 (11%) |
| Country of origin | |||
| United States | 1136 (79%) | 89 (8%) | 102 (9%) |
| Non-United States | 280 (20%) | 35 (13%) | 37 (13%) |
| Educational Attainment | |||
| Less than high school | 24 (2%) | 2 (9%) | 3 (13%) |
| High school diploma | 87 (6%) | 18 (21%) | 16 (18%) |
| Some college | 279 (20%) | 27 (10%) | 27 (10%) |
| 4-year college degree | 557 (39%) | 41 (7%) | 45 (8%) |
| Graduate degree | 483 (34%) | 37 (8%) | 49 (10%) |
| Partner status | |||
| Partnered | 1342 (94%) | 103 (8%) | 121 (9%) |
| Unpartnered | 87 (6%) | 22 (26%) | 18 (21%) |
| Household income | |||
| ≤$40,000 | 143 (10%) | 23 (17%) | 19 (13%) |
| $40,001–70,000 | 312 (23%) | 30 (10%) | 32 (10%) |
| >$70,000 | 889 (64%) | 55 (6%) | 76 (9%) |
| Prepregnancy history of depression | 164 (11%) | 40 (24%) | 27 (17%) |
| Gestational age delivered | |||
| <37 weeks | 92 (6%) | 7 (8%) | 12 (13%) |
| ≥37 weeks | 1344 (94%) | 118 (9%) | 129 (10%) |
| Body mass index (BMI) | |||
| <25 | 937 (65%) | 72 (8%) | 93 (10%) |
| 25–29 | 305 (21%) | 34 (11%) | 24 (8%) |
| ≥30 | 189 (13%) | 19 (10%) | 23 (12%) |
| Prenatal depressive symptoms + high pregnancy-related anxiety | 30 (2.1%) | – | – |
Table 2 shows breastfeeding intention and initiation in our study population. Overall, 89% of subjects planned prenatally to breastfeed; 81% of women with prenatal depressive symptoms and 86% of women with high pregnancy-related anxiety reported plans to breastfeed in the first week of life. With respect to breastfeeding initiation, 86% of subjects initiated breastfeeding, including 86% of women with prenatal depressive symptoms and 84% of women with high pregnancy-related anxiety.
Table 2.
Infant Feeding Plans Reported in Second Trimester and Actual Initiation of Breastfeeding by Prenatal Depressive Symptoms and High Pregnancy-Related Anxiety: Data from 1436 Women in Project Viva
| |
|
Prenatal infant feeding intention |
Initiation of breastfeeding |
||||
|---|---|---|---|---|---|---|---|
| Maternal mood | Total | Breastfeed only | Mostly breastfeed | Mostly formula | Formula feed only | Initiated | Did not initiate |
| No prenatal depressive symptoms | 1311 | 1032 (79%) | 144 (10%) | 25 (2%) | 110 (8%) | 1137 (87%) | 174 (13%) |
| Prenatal depressive symptoms | 125 | 79 (63%) | 22 (18%) | 7 (6%) | 17 (13%) | 105 (84%) | 20 (16%) |
| Low–moderate pregnancy-related anxiety | 1295 | 1007 (78%) | 149 (12%) | 23 (2%) | 116 (9%) | 1123 (87%) | 172 (13%) |
| High pregnancy-related anxiety | 141 | 104 (74%) | 17 (12%) | 9 (6%) | 11 (8%) | 119 (84%) | 22 (16%) |
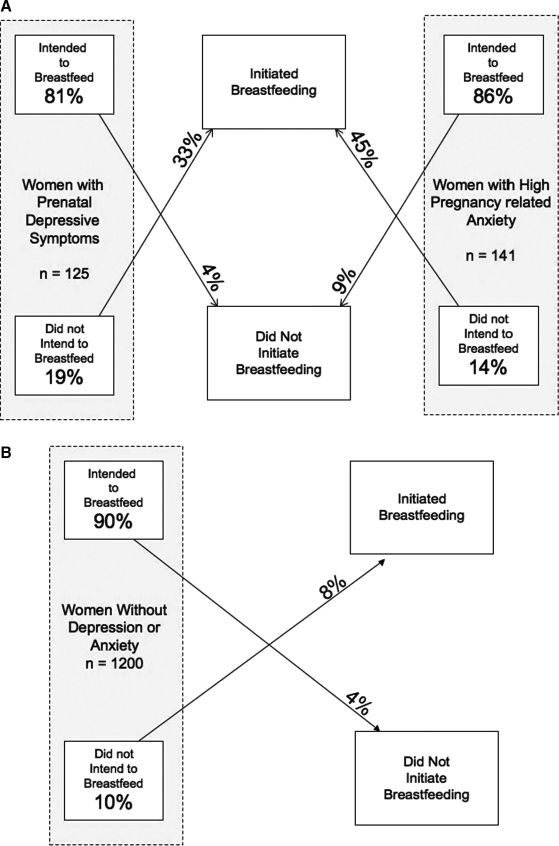
In Figure 1, we present the flow of participants from intention to initiation and the relationship to prenatal depressive symptoms and high pregnancy-related anxiety in our study population. Overall, prenatal intention to breastfeed predicted initiation; 91%–96% of women who intended to breastfeed initiated breastfeeding. Among women with prenatal depressive symptoms, 81% intended to breastfeed, and very few of those (4%) failed to initiate breastfeeding; of the 19% of depressed women who intended to formula feed, one third (33%) chose to initiate breastfeeding. Similar patterns were seen for women with high pregnancy-related anxiety. In contrast, whereas 90% of women without depressive symptoms or high pregnancy-related anxiety intended to breastfeed, a lower proportion (8%) of women who intended to formula-feed converted to initiate breastfeeding.
FIG. 1.
Maternal prenatal breastfeeding intention and relationship to initiation of breastfeeding in women with (A) and without (B) prenatal depressive symptoms and high pregnancy-related anxiety. Data from 1436 participants in Project Viva.
Prenatal intention
In Tables 3 and 4, we present the results of logistic regression modeling of the associations of prenatal depressive symptoms and high pregnancy-related anxiety on prenatal plans to formula feed. Compared with women with a EPDS score of <13, women whose scored ≥13 were more likely to have prenatal plans to formula feed, with an OR of 2.08 (95% CI 1.28, 3.38). Once adjusted for parity, country of origin, household income, maternal educational attainment, and prepregnancy body mass index (BMI, kg/m2), the OR fell to 1.92 (95% CI 1.11, 3.33). As shown in Table 4, the OR fell again to 1.81 (95% CI 1.04, 3.34) after adjusting further for high pregnancy-related anxiety. In unadjusted analyses, women with high pregnancy-related anxiety were somewhat more likely to plan to formula feed prenatally (OR 1.40, 95% CI 0.84, 2.33), although this association was not statistically significant. Adjustment for education, household income, and prepregnancy BMI increased the effect estimate (OR 1.99, 95% CI 1.12, 3.54). In Table 4, however, we show that further adjustment for prenatal depressive symptoms lowered this effect estimate slightly, to 1.87 (95% CI 1.04, 3.34).
Table 3.
Odds Ratios (95% Confidence Intervals) for Associations of (1) Prenatal Depressive Symptoms and (2) High Pregnancy-Related Anxiety with Prenatal Intention to Formula-Feed
| Models | Prenatal depressive symptoms | High pregnancy-related anxiety |
|---|---|---|
| Age-adjusted | 2.08 (1.28, 3.38) | 1.40 (0.84, 2.33) |
| Multivariate, adjusteda | 1.92 (1.11, 3.33) | 1.99 (1.12, 3.54) |
Adjusted for parity, country of origin, household income, maternal educational attainment, and prepregnancy BMI.
Table 4.
Odds Ratios (95% Confidence Intervals) for Multivariate Models of Associations of Prenatal Depressive Symptoms and High Pregnancy-Related Anxiety with Prenatal Intention to Formula Feed and Failure to Initiate Breastfeeding
| Maternal characteristic | Prenatal intention to formula feed | Failure to initiate breastfeeding |
|---|---|---|
| Prenatal depressive symptoms | ||
| EDPS < 13 | 1.0 | 1.0 |
| EDPS ≥ 13 | 1.81 (1.04, 3.34) | 1.03 (0.59, 1.80) |
| High pregnancy-related anxiety | ||
| Low–moderate | 1.0 | 1.0 |
| High | 1.87 (1.04, 3.34) | 1.27 (0.74, 2.20) |
| Age, years | ||
| <25 | 0.29 (0.12, 0.70) | 0.89 (0.45, 1.77) |
| 25–29 | 1.36 (0.86, 2.14) | 0.98 (0.64, 1.50) |
| 30–39 | 1.0 | 1.0 |
| 40+ | 0.61 (0.20, 1.88) | 0.64 (0.24, 1.70) |
| Parity | ||
| Nulliparous | 1.0 | 1.0 |
| Parous | 3.32 (2.14, 5.13) | 1.99 (1.38, 2.86) |
| Origin | ||
| United States | 1.0 | 1.0 |
| Non-United States | 0.27 (0.14, 0.50) | 0.29 (0.16, 0.50) |
| Education | ||
| Less than high school | 15.02 (4.08, 55.31) | 6.58 (2.17, 19.92) |
| High school graduate | 7.85 (3.50, 17.58) | 5.17 (2.58, 10.34) |
| Some college | 7.35 (3.82, 14.14) | 4.50 (2.65, 7.63) |
| College degree | 3.29 (1.75, 6.19) | 2.15 (1.31, 3.53) |
| Graduate degree | 1.0 | 1.0 |
| Household income | ||
| ≤$40,000 | 2.79 (1.57, 4.96) | 1.74 (1.01, 3.07) |
| $40,001–$70,000 | 1.68 (1.07, 2.63) | 1.50 (1.00, 2.24) |
| $70,000+ | 1.0 | 1.0 |
| Prepregnancy BMI (kg/m2) | ||
| <25 | 1.0 | 1.0 |
| 25–29 | 1.43 (0.92, 2.22) | 1.33 (0.89, 1.99) |
| 30+ | 1.15 (0.71, 1.86) | 1.57 (1.02, 2.40) |
| Gestational age | ||
| <37 weeks | – | 3.33 (1.92, 5.76) |
| 37 + weeks | – | 1.0 |
Initiation
The associations of prenatal depressive symptoms and high pregnancy-related anxiety with initiation of breastfeeding are shown in Tables 4 and 5. We saw little evidence of an association between prenatal depressive symptoms and failure to initiate breastfeeding (age-adjusted OR 1.16, 95% CI 0.69, 1.93). In Table 4, we show that adjustment for parity, origin, household income, maternal educational attainment, prepregnancy BMI, gestational length, and high pregnancy-related anxiety decreased the effect estimate slightly (OR 1.03, 95% CI 0.59, 1.80). After adjustment for multiple covariates (Table 4), women with high pregnancy-related anxiety did not appear appreciably more likely to initiate breastfeeding (OR 1.27, 95% CI 0.74, 2.20) than did women with low to moderate pregnancy-related anxiety.
Table 5.
Odds Ratios (95% Confidence Intervals) for Associations of (1) Prenatal Depressive Symptoms and (2) High Pregnancy-Related Anxiety with Failure to Initiate Breastfeeding
| Models | Prenatal depressive symptoms | High pregnancy-related anxiety |
|---|---|---|
| Age-adjusted | 1.16 (0.69, 1.93) | 1.15 (0.71, 1.88) |
| Multivariate, adjusteda | 1.06 (0.61, 1.84) | 1.28 (0.74, 2.20) |
Adjusted for parity, country of origin, household income, maternal educational attainment, prepregnancy BMI, and gestational age.
Women with pregnancy-related anxiety or prenatal depressive symptoms may have been prescribed psychiatric medication prior to or during pregnancy, and this may have potentially influenced their prenatal intention to breastfeed. We examined this by conducting stratified analyses to determine if the effects of depression on breastfeeding behaviors were significantly different in women who had or had not been prescribed medicine for depression prior to or during pregnancy. Our stratified analyses showed consistency between the two subgroups. No significant interactions were detected between preconception use of medication for depression and prenatal depressive symptoms in predicting either intention (p = 0.77 for prepregnancy and p = 0.96 for prenatal prescription) or initiation (p = 0.66 for prepregnancy and p = 0.43 for prenatal prescription). We also detected no significant interactions between high pregnancy-related anxiety and use of psychiatric medications during pregnancy (intention, p = 0.30; initiation, p = 0.36).
Discussion
These results indicate that women reporting prenatal depressive symptoms were nearly twice as likely to plan to formula feed prenatally. Neither prenatal symptoms of depression nor high pregnancy-related anxiety, however, was a strong predictor of actual initiation of breastfeeding in the hospital. This seeming contradiction appeared to be due to changes in breastfeeding intention in depressed and anxious women and did not appear to be influenced by the use of psychiatric medications either prior to or during pregnancy, neither of which predicted intention to formula feed or failure to initiate breastfeeding. Whereas intention to breastfeed predicted breastfeeding initiation in nondepressed and nonanxious women, women with prenatal depressive symptoms and high pregnancy-related anxiety more often chose to initiate breastfeeding despite their prenatal intention to formula feed. (The fact that prenatal depression did not affect initiation but did affect intent, a known predictor of initiation in this population and others, suggests that clinical care or other factors during pregnancy and at the delivery hospital may have either changed intention in late pregnancy or convinced women who had not intended to breastfeed to try breastfeeding, especially women with pregnancy-related anxiety and prenatal depressive symptoms). It may be that breastfeeding promotion services in the third trimester or prenatal care and at the delivery hospital were able to assuage some of the fears or lack of breastfeeding confidence that might have been carried by mothers with anxiety or depressive symptoms. The unusually high breastfeeding initiation rate in this population (86%) suggests that these services were particularly effective. Alternatively, the high pregnancy-related anxiety and depressive symptoms measured in early and midpregnancy, respectively, may have been resolved by the time of delivery.
This is the first study to examine the association of pregnancy-related anxiety with infant feeding intention. Our finding that highly anxious women more often planned to formula feed suggests that anxious patients might benefit from breastfeeding counseling. Two previous studies reported that a complaint of prenatal depressive symptoms was unassociated with infant feeding plans,26,51 but patients with symptoms of prenatal depression in Project Viva were more likely to plan to formula feed. To our knowledge, our study is also the first to examine whether prenatal depressive symptoms and pregnancy-related anxiety predict breastfeeding initiation. The lack of association between these prenatal mood disorders and breastfeeding initiation was surprising, as both prenatal depressive symptoms and anxiety were associated with prenatal infant feeding intentions. Given the impact of breastfeeding on child health and the potentially modifiable impact of mood disorders on breastfeeding intentions and behavior, it is important to replicate these findings in other populations.
We were able to examine how prenatal mood disorders affect two stages in the process of establishing breastfeeding: prenatal intention to breastfeed and initiation of breastfeeding. Other studies, which have been cross-sectional or retrospective or have not measured maternal feeding behaviors as precisely, are unable to document the impact of maternal mood on these critical early steps in the breastfeeding process.18–23,51–55
The Project Viva population is largely Caucasian and well educated and received care within a prenatal outpatient system with strong breastfeeding support practices. There was an unusually high prevalence of breastfeeding initiation within our cohort.56 It is difficult to determine if prenatal depressive symptoms or pregnancy-related anxiety would have the same effect in a population with low breastfeeding rates and less clinician support for breastfeeding.51
We recognize several limitations in these analyses. First, our study had a follow-up rate of 67% from the first prenatal visit to 6 months postpartum, mostly because women became ineligible for the study as a result of change in pregnancy or care status or withdrawal from the study. This study relied on cross-sectional data to examine associations of maternal mood with prenatal intention to breastfeed in the second trimester of pregnancy. In doing so, we were unable to assess if a participant's intention to breastfeed changed throughout her pregnancy, which seems the most likely explanation for the lack of association between mood disorder and actual initiation of breastfeeding. Finally, the outcome measure for initiation of breastfeeding does not necessarily provide verifiable proof of actual transfer of milk from mother to infant. However, maternal behaviors related to breastfeeding, not the success of such behaviors, were the objective measures in which we were interested, given the potential influence of maternal prenatal mood on behavior and motivation.
We acknowledge potential bias due to self-report in these analyses. However, we took care to minimize potential bias: trained interviewers who were associated neither with breastfeeding promotion nor with the subjects' healthcare providers conducted the interviews, and questions related to breastfeeding were phrased in a neutral manner to promote accurate self-report. Despite these provisions, it is still possible that some mothers misrepresented their breastfeeding status either intentionally or unintentionally. The proportion of mothers who self-reported breastfeeding at delivery in our study (86%) agrees with the proportion who self-reported having ever breastfed at our 6-month follow-up visit (88%), however, giving some measure of internal reliability. Additionally, of those who self-reported initiation of breastfeeding at delivery, only 0.6% denied that they had ever breastfed at the 6-month follow-up visit.
Our findings suggest a role for more fine-honed studies designed to examine mood and intention as they change throughout the entire prenatal and perinatal period. The most logical solution to our paradoxical findings seems to be that either mood or intention changed during pregnancy; if we had measured mood in the first weeks after delivery, we might have found that mood disorders at that time were associated with initiation. The support groups, lactation consultant services, and prenatal teaching on lactation offered by clinics and hospitals serving our study participants were particularly strong and may have contributed to a conversion between intent and initiation among women with mood disorders. This interpretation is supported by the data given in Figure 1, which shows that women with high prenatal anxiety or prenatal depressive symptoms whose prenatal intention was not to breastfeed more frequently changed their intention and initiated breastfeeding.
The success of the prenatal and perinatal breastfeeding promotion programs is indicated by the high proportion of women who breastfed in our cohort. Thus, we would suggest that mothers with prenatal mood disturbances may benefit from enhanced prenatal education and perinatal support for breastfeeding, coupled with mental health interventions if necessary. Given the relatively few infant contraindications for breastfeeding (there are few psychiatric medications contraindicated for use in lactating mothers, typically antipsychotics, which are used by only a small fraction of the adult mental health population) and the potential health benefits to mother and child, we think that this enhanced support and education may help women with prenatal mood disturbances to learn and become facile with maternal behaviors and skills. This enhanced support may be particularly helpful for women with prenatal mood disturbances whose sociodemographic characteristics (e.g., high BMI, low socioeconomic status) have already increased their risk to fail to initiate breastfeeding.
It would be useful to examine whether state or trait anxiety is associated with breastfeeding intention and behavior, as well as the more specific pregnancy-related anxiety that we examined in this study. Previous studies have determined that the prenatal period is a critical window during which breastfeeding intention is set.39–41 The association of prenatal depressive symptoms and high pregnancy-related anxiety with breastfeeding intention further reinforces the importance of the prenatal period to breastfeeding success. Although our data do not prove that women with prenatal depressive symptoms or high pregnancy-related anxiety can change their prenatal feeding intention, our results may suggest that these are potential modifiable risks for failure to initiate breastfeeding. Recognizing these exposures may be helpful in designing public health interventions to target women at high risk of failing to breastfeed and its associated negative effects on maternal-child health.
Acknowledgments
This project was supported by grants from the National Institutes of Health (HD34568, HL68041, HD44807, ES00002, POIES012874, MH068596, Harvard Medical School, and the Harvard Pilgrim Health Care Foundation.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Bennett H. Einarson A. Taddio A, et al. Prevalence of depression during pregnancy: Systematic review. Obstet Gynecol. 2004;103:698–703. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 2.Miller LJ. Postpartum depression. JAMA. 2002;287:762–765. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- 3.Josefsson A. Berg G. Nordin C. Sydsjo G. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet Gynecol Scand. 2001;80:251–255. doi: 10.1034/j.1600-0412.2001.080003251.x. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes B. Gavin N. Meltzer-Brody S, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llewellyn A. Stowe ZN. Nemeroff CB. Depression during pregnancy and the puerperium. J Clin Psychiatry. 1997;58 (Suppl 15):26–32. [PubMed] [Google Scholar]
- 6.Anderson L. Sundström-Poromaa I. Bixo M, et al. Point prevalence of psychiatric disorders during the second trimester of pregnancy: A population-based study. Am J Obstet Gynecol. 2003;189:149–154. doi: 10.1067/mob.2003.336. [DOI] [PubMed] [Google Scholar]
- 7.O'Hara M. Gorman L. Postpartum anxiety and depression: Onset and comorbidity in a community sample. J Nerv Ment Dis. 1998;186:420–424. doi: 10.1097/00005053-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Pfuhlmann B. Stoeber G. Beckmann H. Postpartum psychoses. Curr Psychiatry Rep. 2002;4:185–190. doi: 10.1007/s11920-002-0025-6. [DOI] [PubMed] [Google Scholar]
- 9.Levine RE. Oandasan A. Primeau LA. Berenson AB. Anxiety disorders during pregnancy and postpartum. Am J Perinatol. 2003;20:239–248. doi: 10.1055/s-2003-42342. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz JS. Schwartz S. Moore KM. Luenzmann KR. Obsessive-compulsive symptoms in pregnancy and the puerperium: A review of the literature. J Anxiety Disord. 2003;17:461–478. doi: 10.1016/s0887-6185(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 11.Huizink AC. Mulder E. Robles de Medina PG. Visser GH. Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Neuhaus W. Scharlaus S. Hamm W. Bolte A. Prenatal expectations and fears in pregnant women. J Perinat Med. 1994;22:409–414. doi: 10.1515/jpme.1994.22.5.409. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira JM. Fisk N. Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: Cohort based study. BMJ. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjögren B. Thomassen P. Obstetric outcome in 100 women with severe anxiety over childbirth. Acta Obstet Gynecol Scand. 1997;76:948–952. doi: 10.3109/00016349709034907. [DOI] [PubMed] [Google Scholar]
- 15.Ryding EL. Wijma B. Wijma K. Rydhstrom H. Fear of childbirth during pregnancy may increase the risk of emergency cesarean section. Acta Obstet Gynecol Scand. 1998;77:542–547. [PubMed] [Google Scholar]
- 16.Bhagwanani SG. Seagreaves K. Dierker LJ. Lax M. Relationship between prenatal anxiety and perinatal outcome in nulliparous women: A prospective study. J Natl Med Assoc. 1997;89:93–98. [PMC free article] [PubMed] [Google Scholar]
- 17.DiRenzo G. Polito P. Volpe A. Anceschi M. Guidetti R. A multicentric study on fear of childbirth in pregnant women at term. J Psychosom Obstet Gynecol. 1984;3:155–163. [Google Scholar]
- 18.Hatton DC. Harrison-Hohner J. Coste S, et al. Symptoms of postpartum depression and breastfeeding. J Hum Lactation. 2005;21:444–449. doi: 10.1177/0890334405280947. [DOI] [PubMed] [Google Scholar]
- 19.Henderson JJ. Evans SF. Straton JA. Priest SR. Hagan R. Impact of postnatal depression on breastfeeding duration. Birth. 2003;30:175–189. doi: 10.1046/j.1523-536x.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 20.Taveras EM. Capra AM. Braveman PA, et al. Clinician support and psychosocial risk factors associated with breastfeeding discontinuation. Pediatrics. 2003;112:108–115. doi: 10.1542/peds.112.1.108. [DOI] [PubMed] [Google Scholar]
- 21.Mezzacappa ES. Katlin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychol. 2002;21:187–193. [PubMed] [Google Scholar]
- 22.Gröer MW. Differences between exclusive breastfeeders, formula-feeders, and controls: A study of stress, mood, and endocrine variables. Biol Res Nurs. 2005;7:106–117. doi: 10.1177/1099800405280936. [DOI] [PubMed] [Google Scholar]
- 23.Abou-Saleh MT. Ghubash R. Karim L. Krymski M. Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23:465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 24.Barnes J. Stein A. Smith T. Pollock JI. Extreme attitudes to body shape, social and psychological factors, and a reluctance to breastfeed. J R Soc Med. 1997;90:551–559. doi: 10.1177/014107689709001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ. Rubio MR. Elo IT, et al. Factors associated with intention to breastfeed among low income, inner-city pregnant women. Matern Child Health J. 2005;9:253–261. doi: 10.1007/s10995-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 26.Chung EK McCollum KF. Elo IT, et al. Maternal depressive symptoms and infant health practices among low-income women. Pediatrics. 2004;113:e523–529. doi: 10.1542/peds.113.6.e523. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon AJ. Leduc G. Waghorn K. Yang H. Platt RW. In-hospital formula supplementation of healthy breastfeeding newborns. J Hum Lactation. 2005;21:397–405. doi: 10.1177/0890334405280835. [DOI] [PubMed] [Google Scholar]
- 28.Mitra AK. Khoury AJ. Hinton AW. Carothers C. Predictors of breastfeeding intention among low-income women. Matern Child Health J. 2004;8:65–70. doi: 10.1023/b:maci.0000025728.54271.27. [DOI] [PubMed] [Google Scholar]
- 29.Anderson AK. Damio G. Himmelgreen DA, et al. Social capital, acculturation, and breastfeeding initiation among Puerto Rican women in the United States. J Hum Lactation. 2004;20:39–45. doi: 10.1177/0890334403261129. [DOI] [PubMed] [Google Scholar]
- 30.Heck KE. Braveman P. Cubbin C, et al. Socioeconomic status and breastfeeding initiation among California mothers. Public Health Rep. 2006;121:51–59. doi: 10.1177/003335490612100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phares TM. Morrow B. Lansky A, et al. Surveillance for disparities in maternal health-related behaviors—Selected states, (PRAMS), 2000–2001. MMWR Surveill Summ. 2004;53:1–13. [PubMed] [Google Scholar]
- 32.Perez-Escamilla R. Maulen-Radovan I. Dewey KG. The association between cesarean delivery and breast-feeding outcomes among Mexican women. Am J Public Health. 1996;86:832–836. doi: 10.2105/ajph.86.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard C. Howard F. Lawrence R, et al. Office prenatal formula advertising and its effect on breast-feeding patterns. Obstet Gynecol. 2000;95:296–303. doi: 10.1016/s0029-7844(99)00555-4. [DOI] [PubMed] [Google Scholar]
- 34.Lu MC. Lange L. Slusser W. Hamilton J. Halfon N. Provider encouragement of breastfeeding: Evidence from a national survey. Obstet Gynecol. 2001;97:290–295. doi: 10.1016/s0029-7844(00)01116-9. [DOI] [PubMed] [Google Scholar]
- 35.DiGirolamo AM. Grummer-Strawn LM. Fein S. Maternity care practices: Implications for breastfeeding. Birth. 2001;28:94–100. doi: 10.1046/j.1523-536x.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 36.Scott JA. Landers MC. Hughes RM. Binns CW. Factors associated with breastfeeding at discharge and duration of breastfeeding. J Paediatr Child Health. 2001;37:254–261. doi: 10.1046/j.1440-1754.2001.00646.x. [DOI] [PubMed] [Google Scholar]
- 37.Philipp BL. Merewood A. Miller LW, et al. Baby-friendly hospital initiative improves breastfeeding initiation rates in a U.S. hospital setting. Pediatrics. 2001;108:677–681. doi: 10.1542/peds.108.3.677. [DOI] [PubMed] [Google Scholar]
- 38.Cregan MD. De Mello TR. Kershaw D. McDougall K. Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81:870–877. doi: 10.1034/j.1600-0412.2002.810913.x. [DOI] [PubMed] [Google Scholar]
- 39.Arora S. McJunkin C. Wehrer J, et al. Attitude and milk supply major factors influencing breastfeeding rates: Mother's perception of father's attitude. Pediatrics. 2000;106:67–71. doi: 10.1542/peds.106.5.e67. [DOI] [PubMed] [Google Scholar]
- 40.Donath SM. Amir LH ALSPAC Study Team. Relationship between prenatal infant feeding intention and initiation and duration of breastfeeding: A cohort study. Acta Paediatr. 2003;92:352–356. [PubMed] [Google Scholar]
- 41.Donath SM. Amir LH ALSPAC Study Team. The relationship between maternal smoking and breastfeeding duration after adjustment for maternal infant feeding intention. Acta Paediatr. 2004;93:1514–1518. doi: 10.1080/08035250410022125. [DOI] [PubMed] [Google Scholar]
- 42.DiGirolamo A. Thompson N. Martorell R. Fein S. Grummer-Strawn LM. Intention or experience? Health Educ Behav. 2005;32:208–226. doi: 10.1177/1090198104271971. [DOI] [PubMed] [Google Scholar]
- 43.Gillman MW. Rich-Edwards J. Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 44.Rini CK. Dunbel-Schetter C. Wadhwa PD, et al. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 45.Roesch S. Dunkel-Schetter C. Woo G. Hobel C. Modeling the types and timing of stress in pregnancy. Anxiety Stress Coping. 2004;17:87–102. [Google Scholar]
- 46.Lobel M. Dunbel-Schetter C. Scrimshaw SCM. Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- 47.Cox JL. Holden JM. Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Depression Scale. Br J Psychiatry. 1987;150:782–788. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 48.Rich-Edwards JW. Kleinman K. Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60:221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray L. Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS) J Reprod Inf Psychol. 1990;8:99–107. [Google Scholar]
- 50.Eberhard-Gran M. Esbrld A. Tambs K. Opjordsmoen S. Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104:243–249. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson SW. Jacobson JL. Frye KF. Incidence and correlates of breast-feeding in socioeconomically disadvantaged women. Pediatrics. 1991;88:728–736. [PubMed] [Google Scholar]
- 52.Papinczak TA. Turner TC. An analysis of personal and social factors influencing initiation and duration of breastfeeding in a large Queensland maternity hospital. Breastfeed Rev. 2000;8:25–33. [PubMed] [Google Scholar]
- 53.Thome M. Adler EM. Ramel A. A population-based study of exclusive breastfeeding in Icelandic women. Int J Nurs Stud. 2006;43:11–20. doi: 10.1016/j.ijnurstu.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Dunn S. Devres B. McCleary L, et al. The relationship between vulnerability factors and breastfeeding outcome. J Obstet Gynecol Neonat Nurs. 2006;35:87–97. doi: 10.1111/j.1552-6909.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- 55.McLearn KT. Minbovitz CS. Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adoles Med. 2006;160:279–284. doi: 10.1001/archpedi.160.3.279. [DOI] [PubMed] [Google Scholar]
- 56.Celi A. Rich-Edwards JW. Gillman M, et al. Immigration, race/ethnicity, and social and economic factors as predictors of breastfeeding initiation. Arch Pediatr Adolesc Med. 2005;159:255–260. doi: 10.1001/archpedi.159.3.255. [DOI] [PubMed] [Google Scholar]