Abstract
Rationale
By acting on peripheral opioid receptors, opioid agonists can attenuate nociceptive responses induced by a variety of agents.
Objectives
This study was conducted to characterize capsaicin-induced thermal hyperalgesia in rats and to evaluate the hypothesis that local administration of either mu or kappa opioid agonists (fentanyl and U50, 488, respectively) can attenuate capsaicin-induced nociception.
Methods
Capsaicin was administered s.c. in the tail of rats to evoke a nociceptive response, which was measured by the warm-water tail-withdrawal procedure. Either fentanyl or U50,488 was co-administered with capsaicin in the tail to evaluate local antinociceptive effects. In addition, the local antagonism study was performed to confirm the site of action of both opioid agonists.
Results
Capsaicin (0.3–10 μg) dose dependently produced thermal hyperalgesia manifested as reduced tail-withdrawal latencies in 45°C water. Co-administration of either fentanyl (0.32–3.2 μg) or U50,488 (10–100 μg) with capsaicin (3 μg) attenuated capsaicin-induced hyperalgesia in a dose-dependent manner. Furthermore, this local antinociception was antagonized by small doses (10–100 μg) of an opioid antagonist, quadazocine, applied s.c. in the tail. However, the locally effective doses of quadazocine, when applied s.c. in the back (i.e., around the scapular region), did not antagonize either fentanyl or U50,488.
Conclusions
In this experimental pain model, activation of peripheral mu or kappa opioid receptors can attenuate capsaicin-induced thermal hyperalgesia in rats. It supports the notion that peripheral antinociception can be achieved by local administration of analgesics into the injured tissue without producing central side effects.
Keywords: Capsaicin, Peripheral opioid receptor, Antinociception, Neurogenic inflammation, Hyperalgesia
Introduction
It has been shown that locally administered opioid agonists produce antinociceptive effects by interacting with peripheral opioid receptors in inflamed tissues (Stein et al. 1989; Nagasaka et al. 1996; Wilson et al. 1996). This discovery has stimulated research into treatments that minimize central side effects. One approach is the application of small, systemically inactive doses of analgesics directly into the injured tissue (Stein et al. 1991; Joshi et al. 1993). This may be useful particularly under localized pathologic conditions. Another approach is the use of peripherally selective compounds with a reduced ability to cross the blood–brain barrier (Junien and Riviere 1995; Read et al. 1997). This allows for systemic administration and may be beneficial in conditions where pain is less localized. In the clinic, there are many nociceptive conditions, such as postoperative pain, cancer and arthritis. It will be valuable to develop different types of experimental pain models and evaluate the antinociceptive efficacy of locally applied analgesics.
Capsaicin, the pungent ingredient in hot chili peppers, has been used to evoke nociceptive responses for evaluating analgesics in primates including humans (Park et al. 1995; Eisenach et al. 1997; Kinnman et al. 1997; Ko et al. 1998). Exposure of nociceptor terminals such as C-fibers to capsaicin initially leads to excitation of the neuron and the subsequent perception of pain associated with local release of inflammatory pain mediators such as substance P and calcitonin gene-related peptide (CGRP) (Holzer 1991; Winter et al. 1995; Caterina et al. 1997; Kilo et al. 1997). It has been reported that intradermal administration of capsaicin to human skin produces burning pain and allodynic/hyperalgesic responses (Simone et al. 1989; LaMotte et al. 1992). In a previous study, we found that capsaicin dose-dependently produced thermal nociception after it was administered s.c. in the tail of rhesus monkeys (Ko et al. 1998). More interestingly, when small, systemically inactive doses of mu or kappa opioid agonists were co-administered with capsaicin in the tail, they locally attenuated nociceptive responses (Ko et al. 1998, 1999). These observations strengthen the notion that peripheral antinociception can be achieved by local administration of opioids into the injured tissue without producing central side effects (Stein 1995; Wilson et al. 1996).
Capsaicin-sensitive nerve fibers play an important role in many types of nociceptive conditions, such as arthritis and nerve injury (Barthó et al. 1990; Kim et al. 1995; Winter et al. 1995; Abbadie and Basbaum 1998). Many findings have supported the feasibility of pharmacological studies of capsaicin-induced pain to investigate different types of potential analgesics (Park et al. 1995; Sakurada et al. 1996; Eisenach et al. 1997; Sluka 1997). Nevertheless, there was no rodent study exploring the possibility of peripheral antinociceptive actions in the capsaicin-induced pain model. The aim of this study, therefore, was to develop an experimental pain model in rats by applying capsaicin in the tail to induce locally transient nociception. Then, the peripheral antinociception of locally administered fentanyl (a selective mu opioid agonist) and U50,488 (a selective kappa opioid agonist) were evaluated. In particular, local administration of an opioid antagonist, quadazocine, was conducted to confirm the site of action of both agonists.
Methods
Animals
Adult male Wistar rats, approximately 300–350 g (Harlan, Indianapolis, Ind.), were maintained on a 12-h.12-h light/dark cycle (lights on at 6:30 am) with free access to food and water in a temperature-controlled (22±1°C) room. Each animal was used only once, except in the temperature–threshold experiment (described below). Animals were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals (7th edn.) by the Institute of Laboratory Animal Resources (Natl. Academic Press, Washington D.C., revised 1996).
Procedure
Antinociceptive effects were measured by a warm-water tail-withdrawal assay, which was modified from the study by Walker et al. (1994). Each rat was hand-held in a towel with its tail hanging freely. The distal 6–8 cm of the tail was immersed into water baths set at different temperatures. The latency to remove the tail from water was measured via a hand-held timer; a 15-s maximum latency was used as the cutoff latency. Normally, at least one day before the test session, naive rats were habituated to the experimenter’s handling. During habituation, the rat was wrapped in a towel and the tail was immersed into the room-temperature water for 10 s; this procedure took place at least twice. If a rat kept struggling and failed to maintain its tail in room-temperature water for over 10 s, it was not used. During this study, only 3–5% of all the rats failed to satisfy this criterion. A single dosing procedure was used in all test sessions. Each experimental session began with control determinations at each temperature. Subsequent tail-withdrawal latencies were determined at 15, 30, 45 and 60 min following the injection.
Experimental designs
Temperature threshold
The effect of different temperatures on the tail-withdrawal latency was determined in one group of rats (n=12). At the first test session, rats were exposed to water at two different temperatures (45°C and 55°C). At the second test session (24-h interval), the rats were exposed to another two temperatures (40°C and 50°C). The interval between the two immersions was approximately 10 min and the order in which two stimuli were presented was determined randomly. The tail-withdrawal latency for each temperature was measured twice for each rat.
Capsaicin-induced thermal hyperalgesia
Based on a study by Gilchrist et al. (1996), the dose range of capsaicin (0.3–10 μg) was chosen to study the thermal hyperalgesic effect in 45°C and 50°C water. Rats were lightly anesthetized with isoflurane; then capsaicin was injected s.c. in the terminal 3–4 cm of the tail, in a constant 0.1-ml volume. Rats recover within 2 min from this procedure. After injection, the time course of nociceptive responses induced by capsaicin was determined every 15 min for 1 h.
Local antinociceptive effects of opioid agonists
From the protocol described above, 3 μg of capsaicin was chosen as a standard noxious stimulus for further studies in 45°C water. Either a selective mu opioid agonist, fentanyl (0.32–3.2 μg), or a selective kappa opioid agonist, U50,488 (10–100 μg), was co-administered with capsaicin in the tail to assess local antinociceptive effects against capsaicin in 45°C water. The maximum locally effective dose of both agonists was also administered s.c. in the back (i.e., around the scapular region), immediately following capsaicin injection, in order to evaluate its site of action.
Local antagonism of quadazocine
Given that onset and distribution factors may be minimized with local administration, an opioid antagonist, quadazocine (1–100 μg), was co-administered with capsaicin and the opioid agonist in the tail, in order to investigate local antagonist effects. Likewise, the highest locally effective dose of quadazocine was injected s.c. in the back, immediately following capsaicin injection, to verify whether the antagonist effects were localized in the tail.
Data analysis
Except for the temperature and time-course study, the 15-min time point was used for analysis because this was the time of peak effects of both capsaicin and opioid agonists. The dose-dependent effects of the agonist and antagonist were analyzed with one-way analysis of variance (ANOVA) followed by the Newman-Keuls test (*P<0.05; **P<0.01). In addition, in the time-course study, a significant reduction in tail-withdrawal latency was also determined using the Newman-Keuls test.
Drugs
Fentanyl hydrochloride (National Institute on Drug Abuse, Bethesda, Md.), U50,488 (Upjohn Co., Kalamazoo, Mich.) and quadazocine methanesulfonate (Sanofi, Malvern, Pa.) were dissolved in sterile water. Capsaicin (Sigma, St. Louis, Mo.) was initially dissolved in a solution of Tween 80/95%ethanol/saline in a ratio of 1/1/8, and was subsequently diluted to lower concentrations with saline. For local administration, all compounds were mixed in the capsaicin solution and injected as a 0.1-ml volume in the tail.
Results
Control tail-withdrawal latencies
After habituation, rats showed a consistent profile in tail-withdrawal responses. Normally, rats kept their tails in 40°C and 45°C water for 15 s (cutoff latency) and removed their tails from 50°C and 55°C water rapidly. In 50°C water, the average tail-withdrawal latency was 5.0±0.8 s (n=12); in 55°C water, the latency was 2.4± 0.3 s. Although most of rats kept their tails in 40°C and 45°C water for 15 s, it should be noted that approximately 10% of rats in this study had 12–14 s latency in 45°C water, possibly indicating that 45°C water was a thermal threshold for rats in this procedure.
Capsaicin-induced thermal hyperalgesia
After capsaicin (0.3–10 μg) was injected into the tail, it produced transiently nociceptive responses, such as thermal hyperalgesia, which were indicated as reduced tail-withdrawal latencies in both 45°C and 50°C water. Figure 1 illustrates the time course of the capsaicin-evoked nociceptive responses in 45°C water in a dose-dependent manner. In particular, both 3 μg and 10 μg of capsaicin caused rapid tail-withdrawal latencies of approximately 5.2 s in 45°C water at 15 min post-injection, and nociceptive responses gradually disappeared over the course of an hour. This quick-onset and short-duration nociceptive response was mediated locally in the tail, because the same amount of capsaicin (3 μg and 10 μg), applied s.c. in the back, had no effect on the tail-withdrawal latency (data not shown). For our purpose, the dose of 3 μg capsaicin was chosen to induce thermal hyperalgesia in 45°C water for further studies.
Fig. 1.
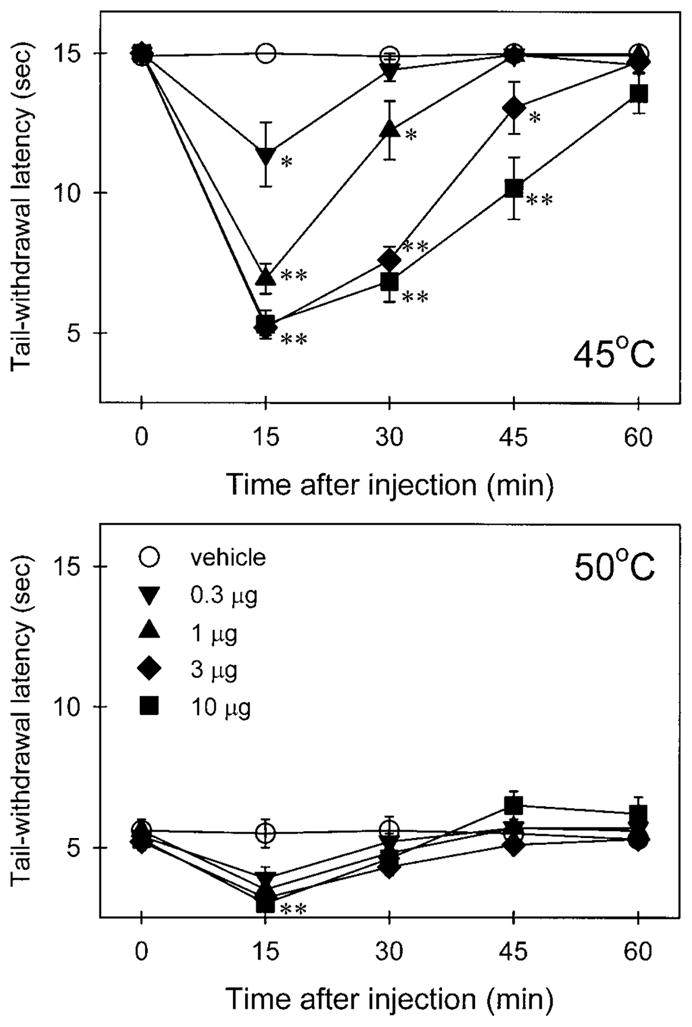
Time course of capsaicin-induced thermal hyperalgesia in 45°C (upper panel) and 50°C water (lower panel). Each symbol represents the condition induced by different doses of capsaicin injected into the tail. Each symbol represents the mean±SEM (n=8). Abscissae: time after injection (min). Ordinates: tail-withdrawal latency (s). Asterisks represent significant differences from the vehicle group at the same time point (*P<0.05; **P<0.01)
Local antinociceptive effects of opioid agonists
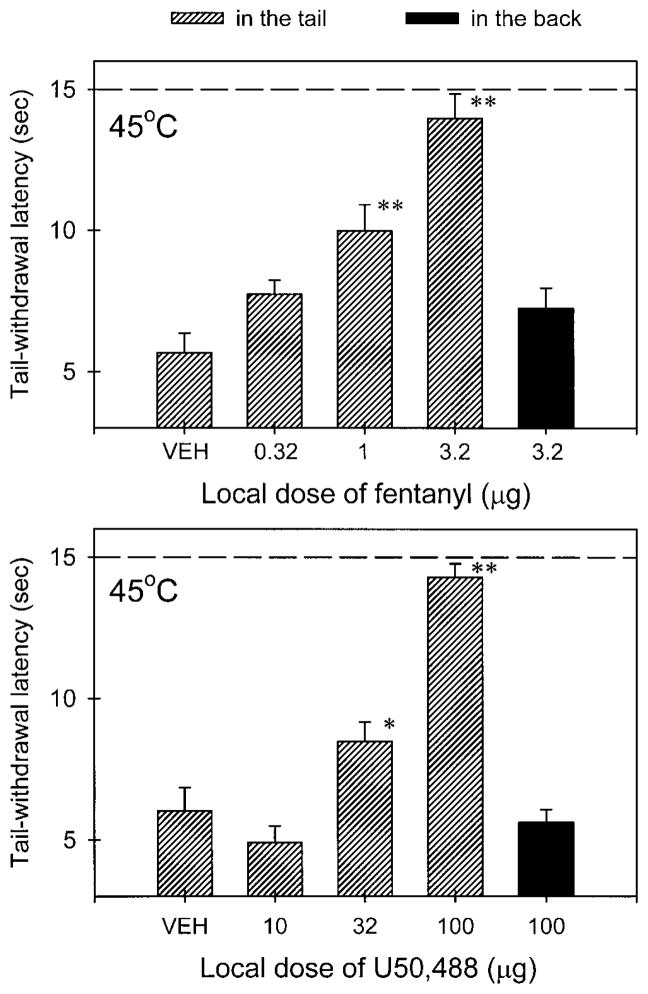
Figure 2 compares the local antinociceptive effects of fentanyl and U50,488 following co-administration with capsaicin (3 μg). Local administration of fentanyl (0.32–3.2 μg) dose dependently inhibited capsaicin-induced thermal hyperalgesia in 45°C water (Fig. 2, upper panel). Both doses of fentanyl (i.e., 1 μg and 3.2 μg) significantly elevated the tail-withdrawal latency in the presence of capsaicin compared with the vehicle group (P<0.01). However, the highest dose of fentanyl (3.2 μg), when injected s.c. in the back, was not effective against capsaicin. On the other hand, local administration of U50, 488 (10–100 μg) also dose dependently inhibited capsaicin-induced hyperalgesia (Fig. 2, lower panel). Similarly, the antinociceptive effect of U50, 488 was not observed when the high dose of U50,488 (100 μg) was applied in the back. Although the 15-min time point was used to analyze the data, it was worth noting that this ineffectiveness was observed throughout one hour of the test session. In addition, the locally effective doses of both agonists did not cause any behavioral changes such as sedation or reduction of locomotion following injections. It is worth noting that locally effective doses of fentanyl (3.2 μg) and U50,488 (100 μg) were not effective against a noxious stimulus of 50°C water in the absence of capsaicin (data not shown).
Fig. 2.
Local antinociceptive effects of fentanyl (upper panel) and U50,488 (lower panel) administered in the tail (hashed bars) and in the back (filled bars) against capsaicin (3 μg) in 45°C water. Each value represents the mean±SEM (n=7–9). Abscissae: dose of agonists injected locally (μg). Ordinates: tail-withdrawal latency (s). Each data point was obtained 15 min after injection. Asterisks represent significant differences from the vehicle group (*P<0.05; **P<0.01). A stippled line represents a cut-off time. Other details as in Fig. 1
Local antagonism of quadazocine
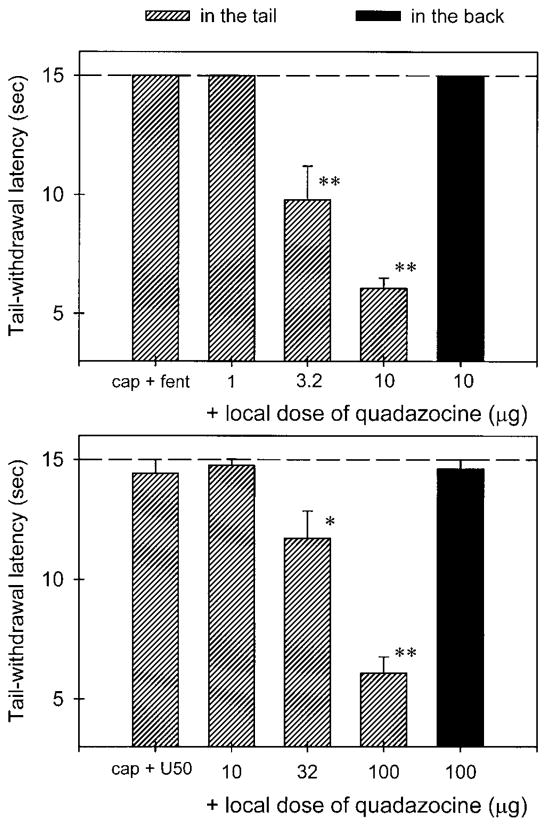
Local administration of quadazocine (1–10 μg) antagonized the local antinociception of fentanyl (3.2 μg) against capsaicin in a dose-dependent manner (Fig. 3, upper panel). Both doses of quadazocine (3.2 μg and 10 μg) significantly suppressed local actions of fentanyl (P<0.01). After the locally effective dose of quadazocine (10 μg) was applied in the back, it did not antagonize local fentanyl. On the other hand, local administration of quadazocine (10–100 μg) also antagonized the local actions of U50,488 (100 μg) in a dose-dependent manner (Fig. 3, lower panel). Similarly, the highest effective dose of quadazocine (100 μg), when applied in the back, did not antagonize local U50,488. Nevertheless, local administration of quadazocine displayed a different antagonist potency against fentanyl and U50,488, in which 10 μg of quadazocine significantly antagonized local fentanyl, but not U50,488. In addition, the high dose of quadazocine (100 μg) alone did not change the rat’s nociceptive responses in this procedure (data not shown).
Fig. 3.
Local antagonist effects of quadazocine administered in the tail (hashed bars) and in the back (filled bars) against local antinociceptive effects of fentanyl and U50,488 in capsaicin-induced hyperalgesia in 45°C water. Each value represents the mean±SEM (n=7–9). Abscissae: dose of quadazocine injected locally (μg). The labels cap+fent and cap+U50 represent the effects of co-administration of capsaicin (3 μg) with fentanyl (3.2 μg) and U50,488 (100 μg) in the tail, respectively. Other details as in Fig. 2
Discussion
The present study illustrated that local administration of opioid agonists significantly diminished capsaicin-induced thermal hyperalgesia in a dose-dependent manner. The antagonist study confirmed that this local anti-nociception was in the tail and was mediated by different opioid receptors. These results support the notion that activation of peripheral opioid receptors can relieve nociception mediated by different mechanisms (Stein et al. 1989; Stein 1995; Nagasaka et al. 1996; Wilson et al. 1996; Ko et al. 1998, 1999).
Local application of capsaicin into the tail of rats caused transient thermal hyperalgesia, manifested as reduced tail-withdrawal latencies in 45°C water (Fig. 1). In the similar dose range, intraplantar injection of capsaicin in rats also produced hyperalgesia to heat and mechanical stimuli (Gilchrist et al. 1996). The duration of thermal hyperalgesia in both studies is also similar, in that its effect only lasts for 15–30 min and disappears within an hour. In contrast, the duration of mechanical hyperalgesia seems to be longer, approximately 2 h (Gilchrist et al. 1996). Capsaicin has been widely used in clinical studies for evaluating the antinociceptive efficacy of different analgesics (Park et al. 1995; Eisenach et al. 1997; Kinnman et al. 1997). Given the evidence that capsaicin-sensitive nerve fibers are involved in a variety of nociceptive conditions (Barthó et al. 1990; Kim et al. 1995; Winter et al. 1995; Abbadie and Basbaum 1998), it is valuable to develop capsaicin-induced pain models in animals for evaluating different compounds and clarifying the underlying mechanisms (Sakurada et al. 1996; Sluka et al. 1997; Ko et al. 1998, 1999).
Capsaicin evokes pain sensations by activating C-fibers and stimulating the release of neuropeptides, such as substance P and CGRP, from primary nociceptive afferents (Holzer 1991; Winter et al. 1995; Caterina et al. 1997; Kilo et al. 1997). Both substance P and CGRP play an important role in neurogenic inflammation and contribute to the transmission of nociceptive information (McDonald et al. 1996; Kilo et al. 1997; Cao et al. 1998). It has been shown that activation of peripheral mu or kappa opioid receptors can inhibit the excitability of primary afferent neurons (Russell et al. 1987; Haley et al. 1990; Andreev et al. 1994) and reduce the release of substance P from peripheral sensory endings (Yonehara et al. 1992). In addition, in vitro evidence also supports the inhibitory effects of mu and kappa opioid receptors (Levine et al. 1993; Schafer et al. 1994; Minami et al. 1995). Thus, the observation that local administration of opioid agonists can attenuate capsaicin-induced thermal hyperalgesia (Fig. 2) could be explained by the evidence that peripheral mu and kappa opioid receptors may act on primary afferents to inhibit nociceptive transmission by C-fibers (Russell et al. 1987; Haley et al. 1990; Andreev et al. 1994; Zhou et al. 1998). Furthermore, the current findings are consistent with results observed in rhesus monkeys (Ko et al. 1998, 1999). The procedure described in this rodent study could serve as a valuable tool for evaluating analgesics or peripherally antinociceptive actions. Compared with the non-human primate model, the rodent model is inexpensive and less labor intensive, and allows for rapid acquisition of data.
The expression of hyperalgesia involves complicated mechanisms in both peripheral and central nervous systems (Dougherty and Willis 1992; Treede et al. 1992; Stanfa and Dickenson 1995). That the site of antinociceptive action of locally applied opioid agonists is located in the tail is substantiated by antagonist studies. Local administration of quadazocine, an opioid antagonist, dose-dependently antagonized the local inhibition of fentanyl against capsaicin-induced hyperalgesia in the tail (Fig. 3). More importantly, the locally effective dose of quadazocine, when applied in the back (i.e., around the scapular region), did not antagonize local fentanyl. This observation confirms the local agonist study, which indicates that the site of action of a locally applied mu opioid agonist is in the tail. A similar result for a kappa opioid agonist, U50,488, indicated that the site of action of locally applied U50,488 is also in the tail. Quadazocine is more potent in antagonizing fentanyl than U50,488. This different antagonist potency of quadazocine against mu versus kappa agonists is expected, since quadazocine has a higher affinity for mu receptors than kappa receptors in opioid-binding preparations (Negus et al. 1993). Quadazocine has been previously used to differentiate mu and kappa receptor-mediated effects in vivo in a variety of preparations (Bertalmio and Woods 1987; Negus et al. 1993; Ko et al. 1998, 1999).
Taken together, this report provides the functional evidence that local administration of mu or kappa opioid agonists can attenuate capsaicin-induced thermal hyperalgesia in rats. The antagonist study confirms that this local anti-hyperalgesia is in the tail and is mediated by opioid receptor subtypes. This experimental pain model could be a useful tool for evaluating newly developed analgesics and peripherally antinociceptive actions. It indicates the possibility of local administration of opioids for pain medication of localized nociceptive origin.
Acknowledgments
This study was supported by USPHS Grant DA00254. The authors express their gratitude to Brendan Mc-Ginty for initiating this experiment and Beck McLaughlin for help in preparing this manuscript.
References
- Abbadie C, Basbaum AI. The contribution of capsaicin-sensitive afferents to the dorsal root ganglion sprouting of sympathetic axons after peripheral nerve injury in the rat. Neurosci Lett. 1998;253:143–146. doi: 10.1016/s0304-3940(98)00642-9. [DOI] [PubMed] [Google Scholar]
- Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuroscience. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Barthó L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid anti-nociception in inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol. 1990;342:666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- Bertalmio AJ, Woods JH. Differentiation between mu and kappa receptor-mediated effects in opioid drug discrimination: apparent pA2 analysis. J Pharmacol Exp Ther. 1987;243:591–597. [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie A-M, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Haley J, Ketchum S, Dickenson A. Peripheral κ-opioid modulation of the formalin response: an electrophysiological study in the rat. Eur J Pharmacol. 1990;191:437–446. doi: 10.1016/0014-2999(90)94178-z. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Joshi GP, McCarroll SM, O’Brien TM, Lenane P. Intraarticular analgesia following knee arthroscopy. Anesth Analg. 1993;76:333–336. [PubMed] [Google Scholar]
- Junien JL, Riviere P. Review article: the hypersensitive gut – peripheral kappa agonists as a new pharmacological approach. Aliment Pharmacol Ther. 1995;9:117–126. doi: 10.1111/j.1365-2036.1995.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kim YI, Na HS, Han JS, Hong SK. Critical role of the capsaicin-sensitive nerve fibers in the development of the causalgic symptoms produced by transecting some but not all of the nerves innervating the rat tail. J Neurosci. 1995;15:4133–4139. doi: 10.1523/JNEUROSCI.15-06-04133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnman E, Nygards EB, Hansson P. Peripherally administrated morphine attenuates capsaicin-induced mechanical hypersensitivity in humans. Anesth Analg. 1997;84:595–599. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:150–156. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. Activation of peripheral kappa opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1999;289:378–385. [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LER, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–462. [PubMed] [Google Scholar]
- Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of μ-, δ- and κ-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- Nagasaka H, Awad H, Yaksh TL. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology. 1996;85:808–816. doi: 10.1097/00000542-199610000-00016. [DOI] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- Read NW, Abitbol JL, Bardhan KD, Whorwell PJ, Fraitag B. Efficacy and safety of the peripheral kappa agonist fedotozine versus placebo in the treatment of functional dyspepsia. Gut. 1997;41:664–668. doi: 10.1136/gut.41.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NJW, Schaible H-G, Schmidt RF. Opiates inhibit the discharges of fine afferent units from inflamed knee joint of the cat. Neurosci Lett. 1987;76:107–112. doi: 10.1016/0304-3940(87)90201-1. [DOI] [PubMed] [Google Scholar]
- Sakurada T, Sugiyama A, Sakurada C, Tan-No K, Yonezawa A, Sakurada S, Kisara K. Effect of spinal nitric oxide inhibition on capsaicin-induced nociceptive response. Life Sci. 1996;59:921–930. doi: 10.1016/0024-3205(96)00390-6. [DOI] [PubMed] [Google Scholar]
- Schafer MK-H, Bette M, Romeo H, Schwaeble W, Weihe E. Localization of κ-opioid receptor mRNA in neuronal subpopulations of rat sensory ganglia and spinal cord. Neurosci Lett. 1994;167:137–140. doi: 10.1016/0304-3940(94)91046-4. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Blockade of calcium channels can prevent the onset of secondary hyperalgesia and allodynia induced by intradermal injection of capsaicin in rats. Pain. 1997;71:157–164. doi: 10.1016/s0304-3959(97)03354-x. [DOI] [PubMed] [Google Scholar]
- Stanfa L, Dickenson A. Spinal opioid systems in inflammation. Inflamm Res. 1995;44:231–241. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Wilson JL, Nayanar V, Walker JS. The site of anti-arthritic action of the κ-opioid, U-50, 488H, in adjuvant arthritis: importance of local administration. Br J Pharmacol. 1996;118:1754–1760. doi: 10.1111/j.1476-5381.1996.tb15601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Yonehara N, Imai Y, Chen J-Q, Takiuchi S, Inoki R. Influence of opioids on substance P release evoked by antidromic stimulation of primary afferent fibers in the hind instep of rats. Regul Pept. 1992;38:13–22. doi: 10.1016/0167-0115(92)90068-6. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang Q, Stein C, Schafer M. Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther. 1998;286:1000–1006. [PubMed] [Google Scholar]