Abstract
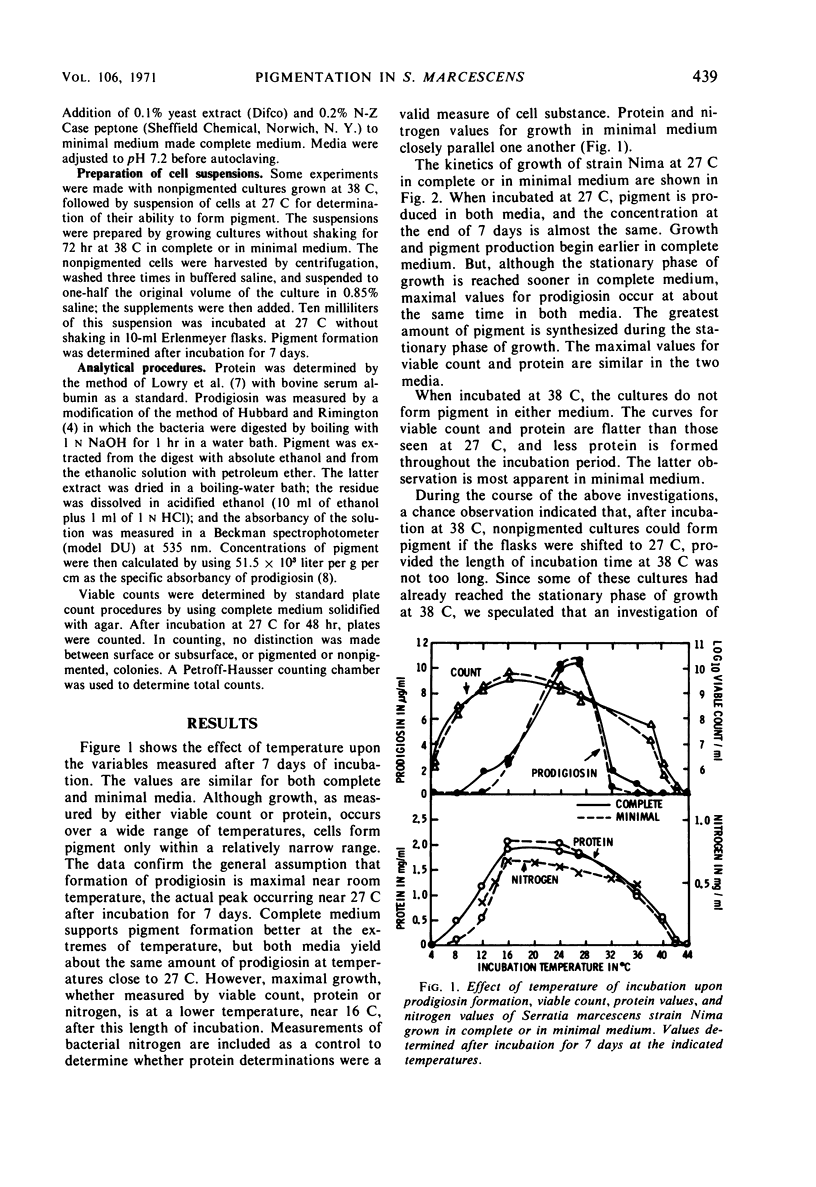
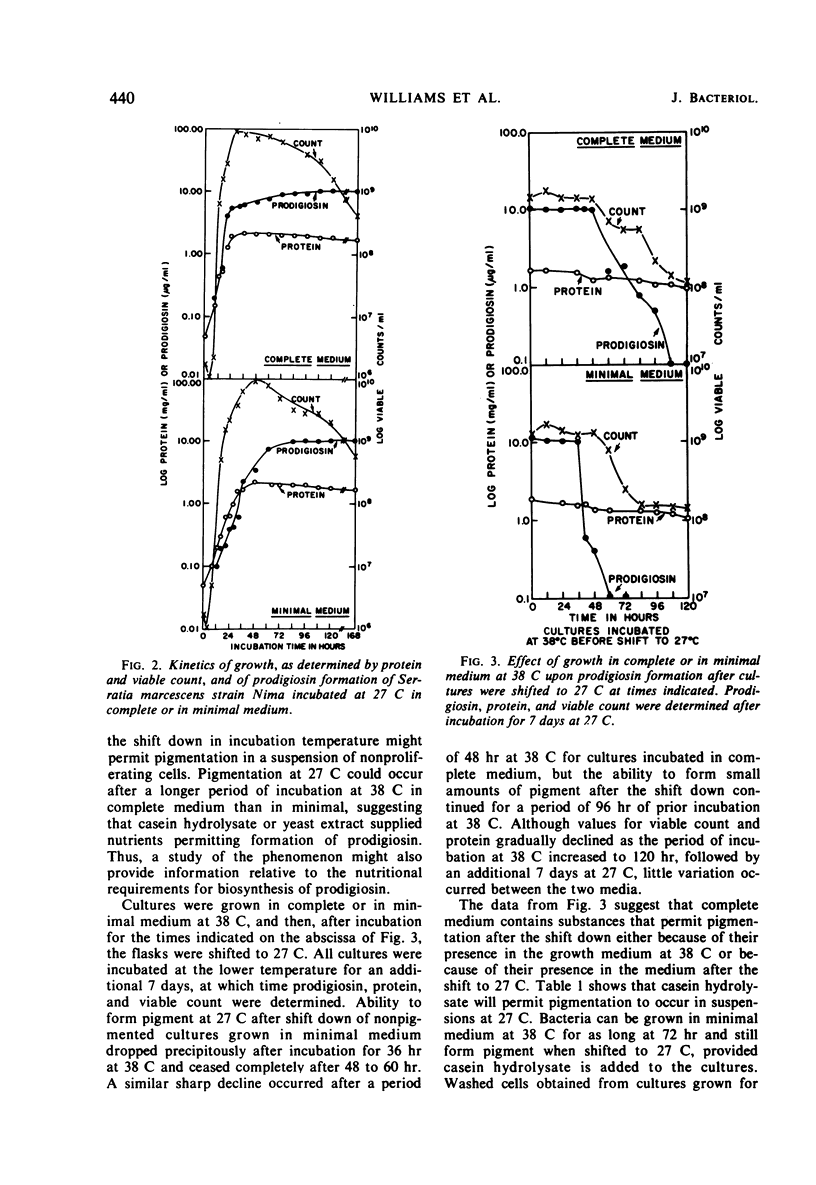
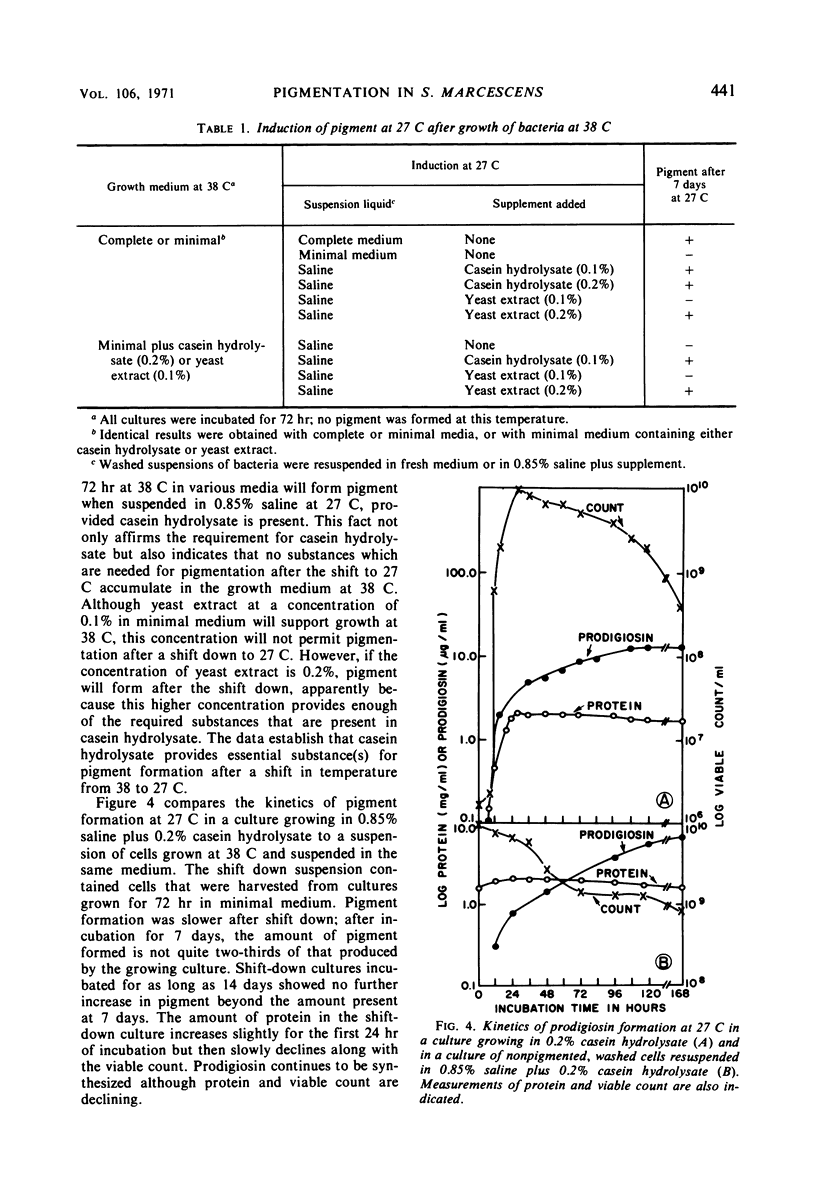
Maximal amounts of prodigiosin were synthesized in either minimal or complete medium after incubation of cultures at 27 C for 7 days. Biosynthesis of prodigiosin began earlier and the range of temperature for formation was greater in complete medium. No prodigiosin was formed in either medium when cultures were incubated at 38 C; however, after a shift to 27 C, pigmentation ensued, provided the period of incubation at 38 C was not longer than 36 hr for minimal medium or 48 hr for complete medium. Washed, nonpigmented cells grown in either medium at 38 C for 72 hr could synthesize prodigiosin when suspended in saline at 27 C when casein hydrolysate was added. These suspensions produced less prodigiosin at a slower rate than did cultures growing in casein hydrolysate at 27 C without prior incubation at 38 C. Optimal concentration of casein hydrolysate for pigment formation by suspensions was 0.4%; optimal temperature was 27 C. Anaerobic incubation, shift back to 38 C, killing cells by heating, or chloramphenicol (25 μg/ml) inhibited pigmentation. Suspensions of washed cells forming pigment reached pH 8.0 to 8.3 rapidly and maintained this pH throughout incubation for 7 days. Measurements of viable count and of protein, plus other data, indicated that cellular multiplication did not occur in suspensions of washed cells during pigment formation. By this procedure utilizing a shift down in temperature, biosynthesis of prodigiosin by washed cells could be separated from multiplication of bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIZZARD J. L., PETERSON G. E. SELECTIVE INHIBITION OF PROLINE-INDUCED PIGMENTATION IN WASHED CELLS OF SERRATIA MARCESCENS. J Bacteriol. 1963 May;85:1136–1140. doi: 10.1128/jb.85.5.1136-1140.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting M. I. A Description of Some Color Variants Produced by Serratia marcescens, Strain 274. J Bacteriol. 1940 Jul;40(1):57–68. doi: 10.1128/jb.40.1.57-68.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Rimington C. The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens). Biochem J. 1950 Feb;46(2):220–225. doi: 10.1042/bj0460220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. M., Campbell J. J. A new resuspension medium for pyocyanine production. Can J Microbiol. 1969 Jun;15(6):595–598. doi: 10.1139/m69-101. [DOI] [PubMed] [Google Scholar]
- Ingledew W. M., Campbell J. J. Evaluation of shikimic acid as a precursor of pyocyanine. Can J Microbiol. 1969 Jun;15(6):535–541. doi: 10.1139/m69-092. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- WILLIAMS R. P., GOTT C. L., GREEN J. A. Studies on pigmentation of Serratia marcescens. V. Accumulation of pigment fractions with respect to length of incubation time. J Bacteriol. 1961 Mar;81:376–379. doi: 10.1128/jb.81.3.376-379.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L., Qadri S. M. Induction of pigmentation in nonproliferating cells of Serratia marcescens by addition of single amino acids. J Bacteriol. 1971 May;106(2):444–448. doi: 10.1128/jb.106.2.444-448.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



