Abstract
Objective
The primary objective of this study was to characterize the association between cyclic alternating pattern (CAP) and neurocognitive performance in a group of normal subjects before and after two nights of experimentally-induced sleep fragmentation.
Subjects and Methods
Fifteen healthy subjects underwent one night of uninterrupted and two sequential nights of experimental sleep fragmentation achieved by auditory and mechanical stimuli. Eight subjects were re-examined using a similar paradigm with three nights of uninterrupted sleep. Sleep was polygraphically recorded and CAP analysis was performed for all recordings. A battery of neurocognitive tests was performed for spatial attention, inhibition of return, mental rotation, and Stroop color word test in the afternoon following the first and third night of sleep under fragmented and non-fragmented conditions.
Results
With sleep fragmentation, the percentage of slow-wave sleep was dramatically reduced and there was a two-fold increase in total CAP rate across all NREM sleep stages. Moreover, the number of all CAP A subtypes/hour of sleep (index) was significantly increased. Total CAP rate during the non-fragmented night correlated with reaction times. Similarly, the percentages of A1 and A3 subtypes were negatively and positively correlated with reaction times, respectively. Of the neurocognitive test battery, however, only values obtained from some subtests of the Mental Rotation test showed a significant improvement after sleep fragmentation.
Conclusions
The results of this study suggest that CAP A1 subtypes are associated with higher cognitive functioning, whereas CAP A3 subtypes are associated with lower cognitive functioning in young healthy subjects. The lack of cognitive functioning impairment after sleep fragmentation may be due to persistence and even enhancement of transient slow-wave activity contained in CAP A1 subtypes which also caused a significant enhancement of the EEG power spectrum in the lower frequencies.
Keywords: Cyclic Alternating Pattern, neurocognitive function, sleep fragmentation, slow-wave sleep, sleep instability, arousals
Introduction
There is increasing evidence that sleep is fundamental for learning and memory [1]. In fact, it is hypothesized that during sleep memory traces might be replayed [2], modified [3], stabilized [4] or even enhanced [5]. Specific stages of sleep, such as slow-wave sleep, may be critical for some forms of memory consolidation [3,6]. Huber et al. [5], for example, reported that a motor learning task performed during the day was followed by an increase in EEG slow-wave activity (SWA) during the night and that SWA correlated with an improvement in subsequent performance.
SWA, which is homeostatically regulated [7], appears to be an important determinant of the restorative aspect of sleep and any associated synaptic changes [8]. The “synaptic homeostasis hypothesis” [8] suggests that learning during wakefulness is associated with synaptic potentiation in the relevant cortical circuits and that SWA is associated with synaptic downscaling in these circuits. It is speculated that this downscaling is most likely responsible for the beneficial effects of sleep on cognitive performance. Moreover, according to the “synaptic-strength hypothesis” [9], changes in the synaptic connectivity during learning might lead to a greater need of space and energy to accommodate growth. Downscaling synaptic strength would make efficient use of gray matter networks, and thereby save energy and clear unnecessary “noise” from the previous day. The resulting renewal of synaptic networks may have favorable effects for subsequent learning and memory.
Transcranial application of slow (0.75 Hz) oscillating potentials during the first part of sleep can induce an immediate increase in slow-wave sleep, endogenous cortical slow oscillations and slow spindle activity in the frontal cortex which parallels improved memory [10]. Thus, endogenous slow EEG oscillations may have a causal role in the sleep-associated consolidation of memory.
Over the last few years, we have sought to determine the biological role of SWA, which is a central component of the Cyclic Alternating Pattern (CAP) [11,12]. CAP reflects periodic EEG activity during NREM sleep which is characterized by repeated spontaneous sequences of transient events (phase A) with some characteristic features. These include a distinct pattern that is different from the background rhythm of the underlying sleep stage, an abrupt frequency shift and variation in amplitude, and a pattern which recurs at intervals up to 2 min in duration. The return to background activity identifies the interval that separates the repetitive elements (i.e., phase B). The alternation between the transient events and the background electrical activity is most likely an expression of arousal instability/stability [13].
The relative proportions of SWA and faster EEG rhythms allow the subdivision of the A-phase of CAP into the A1, A2 and A3 subtypes. The A1 subtype is characterized by a predominance of high-voltage slow waves (EEG synchrony), whereas the A3 subtype has a predominance of fast low-amplitude rhythms (EEG desynchrony). The A2 subtype is a mixture of slow and fast EEG rhythms. The A1 is the most common subtype of CAP, normally accounting for the majority of all CAP A-phases during normal sleep, occurring approximately 200 – 400 times per night [14,15].
The A1 subtype of CAP is typically recorded over the frontal and prefrontal regions of the scalp. The distribution of A1 subtypes and the predominance of slow waves in the A1 subtype suggest that they may have a role in cognitive processing during sleep [16–19]. In fact, Huber et al. [5] reported that after learning a motor task during the day, performance the following day significantly improves with an increase in EEG slow-wave activity during the night. Furthermore, SWA activity and CAP slow components, in particular, are increased by a learning task during the day [20].
We have recently shown that CAP rate and A1 subtypes influence cognitive function in healthy subjects, following undisturbed sleep. A positive correlation between A1 subtypes and neurocognitive tests that characterize frontal lobe cognitive functions (e.g., verbal fluency, working memory, verbal learning) was identified [21]. Furthermore, cognitive performance was negatively correlated with A2 subtypes, whereas A3 subtypes were negatively correlated with planning and motor sequencing. These results indicate that the A1 subtypes are associated with higher cognitive functioning, whereas the A2 and A3 subtypes are associated with impaired neurocognitive functioning.
The primary objective of the current study was to characterize the association between CAP features recorded during the night and neurocognitive functioning in a group of normal subjects and to assess the association between CAP and neurocognitive functioning after two nights of experimentally-induced sleep fragmentation [22].
Methods
Study Sample
Healthy volunteers were recruited from the general community. To participate, volunteers had to be less than 40 years of age, have a body mass index <30 kg/m2, consume less than two alcoholic or three caffeinated beverages per day, habitually sleep for at least 7 hours/night, have a usual bedtime before midnight and not work at night or on a rotating shift schedule. Additional exclusion criteria included cigarette smoking and use of any prescription medications or non-prescription anti-inflammatory agents. Eligibility for participation also required absence of the following conditions: type 2 diabetes mellitus, angina, myocardial infarction, coronary revascularization, congestive heart failure, stroke, obstructive lung disease, renal or hepatic dysfunction, psychiatric or neurological disease. After an initial telephone screening, eligible volunteers were required to complete a serologic screen and an overnight polysomnogram to rule out obstructive sleep apnea as previously described [23]. Usual sleep habits were also objectively assessed with a wrist activity monitor that was worn for at least five days, including one weekend. A normal polysomnogram, demonstration of habitual bedtime by midnight and an average of at least seven hours of sleep on actigraphy, and normal serum chemistries were required for enrolment. Most subjects had at least a college education with exception of one subject who had only completed high school.
After enrolment, multiple contacts were made to counsel each volunteer on maintaining at least seven hours of sleep per night. Ambulatory monitoring of sleep habits was repeated for three nights prior to the baseline evaluation to confirm that habitual sleep patterns remain unchanged. Volunteers were excluded from participating in the study if sleep duration on any one night was less than six hours or the average sleep duration was less than seven hours preceding admission to the clinical research unit (CRU). Female volunteers were scheduled for the study protocol during the follicular phase (day 4 – 10) of the menstrual cycle. The experiments were entirely conducted at the Johns Hopkins University, School of Medicine, Baltimore, MD, U.S.A. Informed consent was obtained from each volunteer and the study protocol was approved by the local institutional review board.
Study Protocol
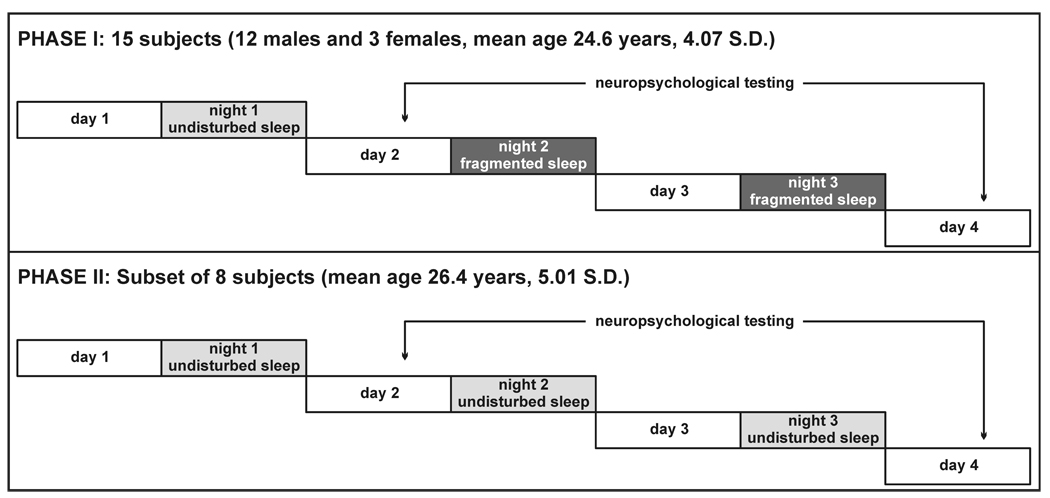
The current study was conducted in two phases. In the first phase each subject was admitted to the CRU and underwent one night of uninterrupted (night 1) and two sequential nights of experimental sleep fragmentation (nights 2 and 3). In the second phase of the study, a subset of the subjects was re-examined in a similar paradigm but with three consecutive nights of uninterrupted sleep (Figure 1).
Fig. 1.
Schematic diagram of the study protocol.
Polysomnographic Recordings
The overnight recording included the following montage: EEG (4 channels, including C3, C4, O1, and O2, referred to the contralateral mastoid); bilateral electrooculograms (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to the left mastoid), electromyogram (EMG) of the submentalis muscle, and ECG (CM4 derivation: anode in position V6 and cathode attached to the manubrium of the sternum). Sleep signals were digitized using the Somnologica software (Embla Systems) and converted to European data format for further analysis.
Sleep Fragmentation
Continuous polysomnographic monitoring was performed on each of the three nights in the CRU. Lights out and morning wake times for each subject were matched to their usual bedtimes and wake times and kept constant throughout the three study nights. During the day, subjects were ambulatory in the CRU but were not allowed to sleep. Sleep fragmentation was achieved by auditory and mechanical stimuli in anticipation of habituation that may occur with a single repeated auditory stimulus type. Auditory tones were broadcast through two speakers placed 12 inches from the head of the bed. Mechanical stimuli were administered using a commercially-available mechanical vibrator. Four such devices were placed underneath the mattress. The aim was to elicit EEG micro-arousals (>3 seconds), as defined by standard criteria [24], at a frequency of 30 or more events/hour using the following guidelines. Following lights out, two minutes of continuous stage 2 sleep (or higher) were observed before applying the first auditory stimulus, a sine-wave auditory tone of 500 ms duration and 57 dB. If an EEG micro-arousal was not elicited, subsequent stimuli were varied by increasing tone volume in 5 – 10 dB increments up to a maximum of 100 dB, modulating the frequency of the auditory tone and applying the mechanical stimulus alone or in combination with the auditory tone. Once an arousal was elicited, at least 30 seconds of stimulus-free interval of sleep were required before applying a subsequent stimulus. Arousals were elicited irrespective of sleep stage.
Sleep scoring and CAP analysis
Sleep stages were scored following standard criteria on 30-second epochs [25]. Subsequently, each CAP A phase was detected in each recording during NREM sleep and classified into three subtypes (A1, A2, and A3) according to Terzano et al. [11].
CAP was detected by means of the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy) which allows computer-assisted detection of CAP A phase subtypes. CAP scoring was performed by a validated computerized algorithm. For the purpose of the current investigation, the automatic scoring was visually edited for the final determination of various CAP parameters Polysomnographic recordings obtained during nights 1 and 3 were used for the current analysis.
Spectral EEG Analysis
Each polysomnogram was visually edited to remove muscle or movement artifacts prior to subjecting the EEG to power spectral analysis. The EEG recording used for the detection of CAP A phases (C3/A2) was initially subdivided into 4-s mini-epochs. Each mini-epoch was assigned to a sleep stage based on the sleep scoring previously performed, and epochs of sleep stages 1, 2 and slow-wave sleep were subjected to power spectral analysis.
Power spectra were calculated for each 4-s epoch using the Hypnolab 1.2 software (SWS Soft, Italy), by means of the Fast Fourier Transform after Welch windowing, to minimize the truncation error and reduce spectral leakage [26,27]. The power spectrum was calculated for frequencies between 0.25 and 25 Hz with a frequency step of 0.25 Hz. Average spectra were obtained for all of NREM sleep. Differences in power spectra were computed by subtracting the power between nights 1 and 3 and used to calculate group differences.
Neurocognitive Testing
A battery of neurocognitive tests was performed on each subject and included assessments of spatial attention, inhibition of return, mental rotation, and Stroop color word test, as described below. The aforementioned tests were administered in the afternoon following the first and third night of fragmented and non-fragmented sleep. The order in which these tests were administered was random.
Spatial Attention
A version of the Posner & Cohen precuing paradigm was employed[28]. During each trial, two boxes appear on a computer screen, one to each side of a central fixation cross. Subjects were asked to quickly press the correct one of two keys in response to the appearance of an “X” in one of the two boxes. If the X appears in the left box, the subjects had to respond by pressing the “Z” key and, if the X appeared in the right box the subjects had to respond by pressing the “/” key. Before presentation of the stimulus, the subjects received one of three hints: (1) a VALID cue, (2) an INVALID cue, or (3) a NEUTRAL cue. The cue was in the form of one or both of the boxes flickering. If both boxes flickered, then no information was provided by the cue (a neutral cue). If a box flickered, then it was 80% likely that the stimulus appeared in that box. The remaining 20% of the cues were misleading and the stimulus actually appeared in the other box (invalid trials). For the misleading trials, as with the other trials, subjects were required to press the key corresponding to the stimulus. If the response was incorrect or if no key was pressed or if the latency was less than 100 milliseconds a short tone was presented. Each of the three trial types (i.e., valid, invalid, and neutral) were presented at three different cue-to-stimulus intervals (the delay between the flickering of the box and the appearance of the stimulus X), in order to test for inhibition of return.
Inhibition of return (IOR)
When the cue-target onset asynchrony (CTOA; the interval between the onset of the cue and the target) is less than 250 ms, responses to targets are faster to cued targets (i.e., those targets occupying space previously occupied by the cue) than to uncued targets (i.e., targets appearing at a new location). This facilitative effect of the cue for targets at the cued versus uncued locations has been attributed the benefit of attention being at the location of cued targets and the cost of having to reorient attention away from cued locations toward uncued targets. When the cue is uninformative about the location of the target the capture of attention by the cue is transient. Posner and Cohen [28] have previously described that when the CTOA is increased beyond 250 ms, this facilitative effect reverses, and responses to cued targets become slower than responses to uncued targets. This latter effect of the cue has been called inhibition of return.
Mental Rotation
A version of the Shepard and Cooper mental rotation paradigm was utilized [29]. For each trial, a symbol resembling an upper-case “F” was presented. The symbol was presented at one of eight orientations, and is either a letter “F” or its mirror-image. The task was to determine whether the symbol is a normal or a reversed 'F', and to respond as quickly as possible by pushing the “Z” key if the symbol is reversed, or the “/” key if the symbol is not reversed. If the response was incorrect, or if an invalid key was pressed, a brief tone was presented
Stroop Color Word test
A version of the Stroop paradigm was utilized [30]. For each trial, the subject was presented with a string of letters printed in color. The task was to respond to the COLOR in which the word is printed by quickly pressing the correct key. If the response was incorrect, or if an invalid key was pressed, a short tone was presented. Three conditions were presented in separate blocks. In the first condition the letter string was composed of X’s of a given color. In the second condition 2, the letter string was the word “red,” “green,” “blue,” or “yellow” printed in a color different from the named color. For the third condition 3, the letter string was the name of the color that the letters are printed in.
Statistical analysis
The repertoire of sleep parameters and the neurocognitive testing were compared using the non parametric Wilcoxon test for paired datasets. To examine the associations between CAP and neurocognitive functioning a series of partial correlations were performed between selected CAP parameters obtained at baseline (Night 1) and a number of indices of neurocognitive functioning obtained from the aforementioned tests the day after (Day 2). Partial correlations were conducted adjusting for age and educational status. Analyses were conducted using the Statistica software package (StatSoft, Inc., 2001. STATISTICA data analysis software system, version 6, www.statsoft.com). Statistical significance was determined using a Type I error threshold of 0.05. A Bonferroni correction was used to account for multiple comparisons artifact.
Results
The study sample included 15 subjects (12 males and 3 females, mean age 24.6 years, 4.07 S.D.) that completed the sleep fragmentation phase of the study. Of these, 8 subjects returned for the second phase with three sequential nights of non-fragmented sleep (mean age 26.4 years, 5.01 S.D.).
Table 1 displays the parameters of sleep architecture obtained from the two study nights (i.e., nights 1 and 3) with and without sleep fragmentation. With sleep fragmentation, sleep latency decreased, the number of stage shifts and the percentage of sleep stage 1 increased, and the percentage of slow-wave sleep was reduced. In contrast, non-fragmented sleep was not associated with any significant changes in sleep structure. Comparisons of the baseline night between fragmented and non-fragmented conditions showed no significant differences across all parameters of sleep structure. In contrast, night 3 was markedly different between the fragmented and non-fragmented subjects with evidence of more stage shifts (p <0.002) and awakenings (p <0.014), increased wakefulness after sleep onset (p <0.042), higher percentage of sleep stage 1 (p <0.002), and decreased percentages of slow-wave (p <0.039) and REM sleep (p <0.007).
Table 1.
Comparison between sleep architecture parameters obtained from the 2 study nights with and without sleep fragmentation.
| night 1 | night 3 | Wilcoxon test | |||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | p< | |
| Fragmented Sleep (n=15) | |||||
| TIB, min | 483.0 | 16.00 | 485.1 | 19.47 | NS |
| SPT, min | 473.3 | 16.62 | 478.4 | 17.42 | NS |
| TST, min | 449.6 | 18.41 | 437.7 | 56.86 | NS |
| SOL, min | 9.7 | 10.51 | 7.1 | 8.03 | 0.03 |
| FRL, min | 91.5 | 50.02 | 103.5 | 65.76 | NS |
| SS/hour | 14.8 | 6.59 | 23.9 | 8.13 | 0.0012* |
| AWN/hour | 4.1 | 2.27 | 5.8 | 2.95 | NS |
| SE, % | 93.1 | 4.04 | 90.3 | 11.97 | NS |
| WASO, % | 5.0 | 3.11 | 8.4 | 12.09 | NS |
| S1, % | 7.0 | 6.19 | 13.8 | 5.28 | 0.004 |
| S2, % | 53.7 | 7.56 | 53.0 | 10.03 | NS |
| SWS, % | 12.3 | 6.49 | 5.4 | 6.97 | 0.0032* |
| REM, % | 22.0 | 5.11 | 19.4 | 5.13 | NS |
| Non-Fragmented Sleep (n=8) | |||||
| TIB, min | 484.5 | 10.99 | 485.4 | 13.26 | NS |
| SPT, min | 471.1 | 22.87 | 464.6 | 36.58 | NS |
| TST, min | 455.5 | 26.62 | 452.4 | 38.44 | NS |
| SOL, min | 13.5 | 18.09 | 21.2 | 29.85 | NS |
| FRL, min | 80.6 | 36.81 | 86.4 | 47.27 | NS |
| SS/hour | 12.4 | 4.19 | 12.0 | 3.37 | NS |
| AWN/hour | 3.3 | 1.44 | 3.3 | 1.53 | NS |
| SE, % | 94.0 | 4.44 | 93.2 | 7.01 | NS |
| WASO, % | 3.3 | 2.22 | 2.7 | 1.95 | NS |
| S1, % | 5.1 | 2.89 | 5.7 | 4.91 | NS |
| S2, % | 52.9 | 9.61 | 53.1 | 5.85 | NS |
| SWS, % | 13.0 | 7.84 | 12.3 | 7.13 | NS |
| REM, % | 25.8 | 5.66 | 26.3 | 7.66 | NS |
TIB: time in bed; SPT: sleep period time; TST: total sleep time; SL: sleep latency; FRL: first REM latency; SS: stage shifts; AWN: awakenings number; SE: sleep efficiency; WASO: wakefulness after sleep onset; S1: stage 1; S2: stage 2; SWS: slow-wave sleep; REM: REM sleep.
Significant after Bonferroni correction.
Table 2 displays the CAP parameters obtained from the two nights under the sleep fragmented and non-fragmented conditions. As expected, several differences were observed with sleep fragmentation. There was a two-fold increase in total CAP rate across all NREM sleep stages. The relative percentage of A1 subtypes decreased, whereas the percentage of A2 subtypes increased. In contrast, the number of all CAP A-phase subtypes/hour of sleep (index) was significantly increased with sleep fragmentation. The B-phase duration was decreased and the length of CAP sequences was increased with sleep fragmentation. The two groups differed only on one parameter (A2 duration, p <0.001) at baseline. For Night 3, however, sleep fragmentation was associated with an increase in total CAP rate (p <0.0013), as well as in each NREM sleep stage, decrease in percentage of A1 (p <0.038) and increased percentage of A2 subtypes (p <0.022), increased A1 (p<0.005), A2 (p<0.004), and A3 index (p <0.009), shorter phase B duration (p <0.026) and increased duration of CAP sequences (p <0.002).
Table 2.
Comparison between CAP parameters obtained from the 2 study nights with fragmented and non-fragmented sleep.
| night 1 | night 3 | Wilcoxon test | |||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | p< | |
| Fragmented Sleep (n=15) | |||||
| CAP_Rate% | 37.8 | 13.15 | 66.7 | 15.04 | 0.00065* |
| in S1 | 16.3 | 13.08 | 37.7 | 20.36 | 0.0015* |
| in S2 | 36.3 | 16.09 | 72.5 | 16.09 | 0.00065* |
| in SWS | 61.6 | 24.05 | 94.4 | 11.85 | 0.00065* |
| A1% | 73.1 | 14.41 | 61.0 | 14.30 | 0.03 |
| A2% | 15.1 | 11.44 | 27.0 | 12.17 | 0.0012* |
| A3% | 11.8 | 8.29 | 11.9 | 7.12 | NS |
| A1 duration, s | 6.4 | 0.70 | 6.3 | 0.70 | NS |
| A2 duration, s | 9.9 | 2.80 | 8.1 | 1.23 | 0.009 |
| A3 duration, s | 10.7 | 1.49 | 10.5 | 1.55 | NS |
| A1 Index | 38.8 | 14.26 | 56.2 | 16.89 | 0.003* |
| A2 Index | 8.7 | 8.17 | 26.2 | 14.99 | 0.00065* |
| A3 Index | 4.1 | 3.36 | 8.9 | 6.51 | 0.01 |
| B duration, s | 23.5 | 2.36 | 21.7 | 2.79 | 0.0055 |
| Sequence duration, s | 235.7 | 61.25 | 488.6 | 165.11 | 0.00065* |
| No. of sequences | 33.2 | 9.00 | 30.1 | 8.03 | NS |
| Non-Fragmented Sleep (n=8) | |||||
| CAP_Rate% | 35.4 | 14.48 | 35.2 | 15.34 | NS |
| in S1 | 8.3 | 3.89 | 17.6 | 18.41 | NS |
| in S2 | 29.7 | 14.51 | 29.3 | 14.55 | NS |
| in SWS | 69.7 | 17.13 | 64.2 | 22.35 | NS |
| A1% | 73.2 | 13.59 | 74.5 | 9.29 | NS |
| A2% | 17.7 | 10.65 | 15.5 | 5.11 | NS |
| A3% | 9.2 | 6.45 | 9.9 | 7.44 | NS |
| A1 duration, s | 6.3 | 0.54 | 6.7 | 0.71 | NS |
| A2 duration, s | 8.0 | 0.89 | 8.4 | 1.44 | NS |
| A3 duration, s | 10.9 | 2.85 | 10.8 | 1.43 | NS |
| A1 Index | 36.7 | 15.83 | 37.2 | 17.47 | NS |
| A2 Index | 9.7 | 9.95 | 7.4 | 6.31 | NS |
| A3 Index | 2.4 | 1.57 | 2.7 | 1.90 | NS |
| B duration, s | 24.2 | 3.07 | 25.2 | 3.61 | NS |
| Sequence duration, s | 237.3 | 78.18 | 239.0 | 83.78 | NS |
| No. of sequences | 30.1 | 5.79 | 29.3 | 6.45 | NS |
A1, A2, and A3 index: number of A1, A2 or A3 subtypes per hour of NREM; A1%, A2%, and A3%: percentage of A1, A2 or A3 subtypes.
Significant after Bonferroni correction.
Table 3 displays the partial correlation coefficients between CAP parameters obtained from the baseline night (night 1) and results of neurocognitive tests obtained on the following day (day 2) with adjustments for age and educational status. Total amount of CAP rate during the preceding undisturbed night was correlated with reaction times but was statistically significant for only one out of 12 items. Nevertheless, after correcting for multiple comparisons this isolated correlation was no longer significant. Similarly, the percentages of A1 and A3 subtypes were negatively and positively correlated with reaction times, respectively, despite correction for multiple comparisons. In contrast, no association was identified between the percentage of A2 subtypes and reaction times or between the percentage of A2 subtypes and error rates.
Table 3.
Partial correlation coefficients between selected CAP parameters obtained from the baseline night (night 1) of all subjects included in this study and neurocognitive testing results (time to complete the test, for the Stroop test, and reaction times, for the other tests) obtained on the following day (day 2).
| CAP Rate, % | A1,% | A2, % | A3, % | |||||
|---|---|---|---|---|---|---|---|---|
| Coeff. | p< | Coeff. | p< | Coeff. | p< | Coeff. | p< | |
| Spatial attention | ||||||||
| Cue 1 | −0.365 | NS | −0.659 | NS | 0.302 | NS | 0.551 | NS |
| Cue 2 | −0.586 | NS | −0.571 | NS | −0.06 | NS | 0.862 | 0.003 * |
| Cue 3 | −0.288 | NS | −0.713 | 0.031 | 0.338 | NS | 0.581 | NS |
| Inhibition of return | ||||||||
| Cue 1 | −0.542 | NS | −0.413 | NS | −0.046 | NS | 0.625 | NS |
| Cue 3 | −0.557 | NS | −0.354 | NS | −0.126 | NS | 0.641 | NS |
| Mental Rotation | ||||||||
| No Rotation / No Reverse | −0.524 | NS | −0.309 | NS | −0.129 | NS | 0.585 | NS |
| No Rotation / Mirror image | −0.721 | 0.028 | −0.379 | NS | −0.296 | NS | 0.882 | 0.002* |
| Rotation / No Reverse | −0.164 | NS | −0.751 | 0.020 | 0.497 | NS | 0.446 | NS |
| Rotation / Mirror image | −0.564 | NS | −0.606 | NS | 0.060 | NS | 0.766 | 0.016 |
| Stroop test | ||||||||
| Task 1 (compatible) | −0.435 | NS | −0.657 | NS | 0.243 | NS | 0.620 | NS |
| Task 2 (incompatible) | −0.598 | NS | −0.555 | NS | −0.094 | NS | 0.881 | 0.002* |
| Task 3 (neutral) | −0.295 | NS | −0.721 | 0.028 | 0.333 | NS | 0.598 | NS |
Coeff. = correlation coefficient.
Significant after Bonferroni correction.
Table 4 compares the neurocognitive test results obtained at baseline (after night 1) and after fragmented and non-fragmented sleep. Only values obtained from some subtests of the Mental Rotation test showed a significant improvement after sleep fragmentation.
Table 4.
Comparison between neurocognitive test results (time to complete the test, for the Stroop test, and reaction times, for the other tests) obtained after the 2 study nights (days 2 and 4) with fragmented and non-fragmented sleep.
| day 2 | day 4 | Wilcoxon test | |||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | p< | |
| Fragmented Sleep (n=15) | |||||
| Spatial attention | |||||
| Cue 1 | 616.8 | 81.49 | 614.7 | 83.98 | NS |
| Cue 2 | 682.8 | 118.62 | 675.5 | 115.75 | NS |
| Cue 3 | 601.0 | 107.48 | 591.0 | 79.22 | NS |
| Inhibition of return | |||||
| Cue 1 | 365.8 | 67.09 | 377.1 | 101.38 | NS |
| Cue 3 | 348.4 | 73.53 | 359.5 | 124.33 | NS |
| Mental Rotation | |||||
| No Rotation / No Reverse | 485.1 | 85.49 | 458.4 | 82.63 | 0.023 |
| No Rotation / Mirror image | 589.7 | 168.20 | 546.6 | 78.19 | NS |
| Rotation / No Reverse | 626.4 | 64.39 | 593.2 | 103.80 | NS |
| Rotation / Mirror image | 731.2 | 162.28 | 645.8 | 100.25 | 0.009 |
| Stroop test | |||||
| Task 1 (compatible) | 44053.2 | 5766.47 | 44470.3 | 6625.62 | NS |
| Task 2 (incompatible) | 49372.5 | 9807.34 | 48529.5 | 9109.85 | NS |
| Task 3 (neutral) | 43291.2 | 8137.06 | 41974.5 | 5919.92 | NS |
| Non-Fragmented Sleep (n=8) | |||||
| Spatial attention | |||||
| Cue 1 | 623.3 | 101.64 | 593.4 | 78.46 | NS |
| Cue 2 | 671.0 | 133.78 | 627.3 | 83.97 | NS |
| Cue 3 | 604.1 | 104.50 | 569.9 | 89.38 | NS |
| Inhibition of return | |||||
| Cue 1 | 372.9 | 42.82 | 412.3 | 87.84 | NS |
| Cue 3 | 357.8 | 39.07 | 369.3 | 79.92 | NS |
| Mental Rotation | |||||
| No Rotation / No Reverse | 494.4 | 82.72 | 460.6 | 67.80 | NS |
| No Rotation / Mirror image | 546.0 | 112.08 | 527.1 | 129.69 | NS |
| Rotation / No Reverse | 606.1 | 68.85 | 561.8 | 58.86 | 0.017 |
| Rotation / Mirror image | 672.3 | 117.38 | 605.2 | 107.73 | 0.017 |
| Stroop test | |||||
| Task 1 (compatible) | 46893.1 | 7677.91 | 43981.1 | 5320.76 | NS |
| Task 2 (incompatible) | 50137.1 | 9784.66 | 46453.3 | 5729.41 | NS |
| Task 3 (neutral) | 44697.3 | 7543.81 | 42216.3 | 6164.34 | NS |
All values are expressed in milliseconds. None of the comparison significant after Bonferroni correction.
Table 5 displays the comparison between error rates in neurocognitive tests obtained before and after fragmented and non-fragmented sleep. Error rates were consistently low throughout and showed statistically significant differences between the two test sessions with fragmented and non-fragmented sleep.
Table 5.
Comparison between neurocognitive test results (error rates) obtained after the 2 study nights (days 2 and 4) with fragmented and non-fragmented sleep.
| dDay 2 | day 4 | Wilcoxon test | |||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | p< | |
| Fragmented Sleep (n=15) | |||||
| Spatial attention | |||||
| Cue 1 | 0.02 | 0.034 | 0.02 | 0.039 | NS |
| Cue 2 | 0.01 | 0.040 | 0.02 | 0.049 | NS |
| Cue 3 | 0.03 | 0.042 | 0.04 | 0.047 | NS |
| Inhibition of return | |||||
| Cue 1 | 0.00 | 0.007 | 0.00 | 0.005 | NS |
| Cue 3 | 0.01 | 0.017 | 0.01 | 0.014 | NS |
| Mental Rotation | |||||
| No Rotation / No Reverse | 0.02 | 0.035 | 0.01 | 0.030 | NS |
| No Rotation / Mirror image | 0.01 | 0.022 | 0.02 | 0.035 | NS |
| Rotation / No Reverse | 0.03 | 0.039 | 0.04 | 0.044 | NS |
| Rotation / Mirror image | 0.02 | 0.022 | 0.03 | 0.033 | NS |
| Stroop test | |||||
| Task 1 (compatible) | 0.02 | 0.025 | 0.05 | 0.063 | NS |
| Task 2 (incompatible) | 0.06 | 0.100 | 0.05 | 0.064 | NS |
| Task 3 (neutral) | 0.03 | 0.041 | 0.04 | 0.047 | NS |
| Non-Fragmented Sleep (n=8) | |||||
| Spatial attention | |||||
| Cue 1 | 0.00 | 0.002 | 0.01 | 0.006 | NS |
| Cue 2 | 0.00 | 0.006 | 0.00 | 0.012 | NS |
| Cue 3 | 0.01 | 0.009 | 0.02 | 0.019 | NS |
| Inhibition of return | |||||
| Cue 1 | 0.00 | 0.005 | 0.00 | 0.004 | NS |
| Cue 3 | 0.00 | 0.007 | 0.00 | 0.004 | NS |
| Mental Rotation | |||||
| No Rotation / No Reverse | 0.01 | 0.029 | 0.02 | 0.039 | NS |
| No Rotation / Mirror image | 0.01 | 0.029 | 0.01 | 0.029 | NS |
| Rotation / No Reverse | 0.01 | 0.008 | 0.02 | 0.016 | NS |
| Rotation / Mirror image | 0.01 | 0.008 | 0.01 | 0.012 | NS |
| Stroop test | |||||
| Task 1 (compatible) | 0.04 | 0.025 | 0.03 | 0.033 | NS |
| Task 2 (incompatible) | 0.04 | 0.034 | 0.04 | 0.023 | NS |
| Task 3 (neutral) | 0.02 | 0.007 | 0.03 | 0.018 | NS |
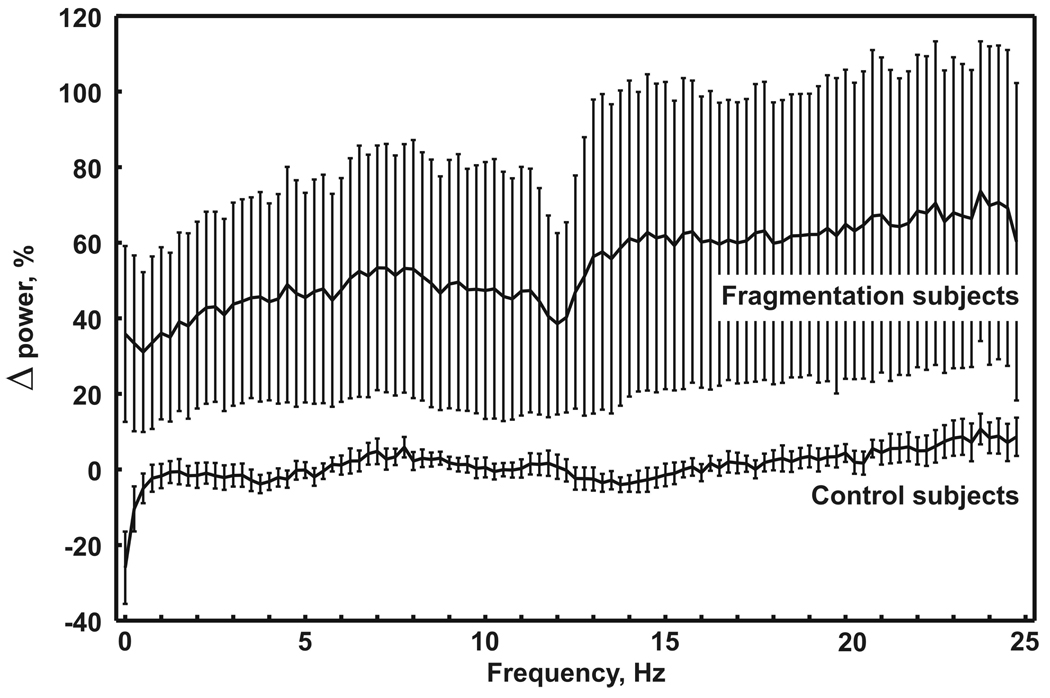
Figure 2 displays the mean difference of all-night EEG power spectra during NREM sleep, obtained from the two study nights with and without sleep fragmentation. A substantial difference was evident between fragmented and non-fragmented sleep with a significant increase in EEG spectral power at all frequencies (0.25–25 Hz), ranging from approximately 40% for the lower frequencies to approximately 60% for higher frequencies up to 25 Hz.
Fig. 2.
Mean difference of all-night EEG power spectra during NREM sleep, obtained in the 2 study nights from the two groups of subjects. Each frequency bin is expressed as a percentage of night 3 vs. night 1 (whiskers represent standard error of mean).
Discussion
Recent data suggest that in young healthy adults the amount of CAP A1 subtypes may be associated with better neurocognitive functioning, whereas that of A2 and A3 subtypes is associated with worse neurocognitive functioning (e.g., verbal fluency, working memory, and verbal learning) during the day following undisturbed sleep [21]. The results of the current study suggest a potential role for CAP in additional functions involving not only frontal cortical areas but also activity of broader cortical networks. The present study employed a set of neurocognitive tests known to involve the activity of several brain areas. A fronto-parietal network is activated when visual information is expected to appear at a specific spatial location, with involvement of the posterior parietal cortex [31]. The Stroop task is associated with the activity of the anterior cingulate cortex, a part of the limbic system, within the frontal lobes [32], whereas rotation tasks activate the Brodmann's areas 7A and 7B, the middle frontal gyrus, extra-striate cortex, the hand somatosensory cortex, and the frontal cortex [33]. Moreover, because the tasks employed in this study required a motor response, an important involvement of the supplementary motor areas in the posterior parts of frontal lobes cannot be excluded.
The present study reinforces previous findings on the positive effects of CAP A1 subtypes on neurocognitive functioning. The A1 subtypes are characterized by a power spectral peak in the frequency range of 0.25–2.5 Hz [12,34] and the results provided herein support the notion that SWA during sleep is correlated with neurocognitive functioning [1,3,5,8–10,35]. But the EEG signal of slow waves occurring during A1 subtypes is characterized by nonlinear dynamics which cannot be detected during continuous slow-wave sleep [36], and they are accompanied by high levels of synchronization between different scalp areas [19], higher than that found during continuous slow-wave sleep, showing its maximum for the interactions between the frontal areas [37]. The data presented herein also indicate that perhaps not all slow waves have the same neurophysiological implications and that those included in the transient A1 components of CAP may have features distinct from those included in long subcontinuous SWA during NREM slow-wave sleep. These differences may involve the respective role of transient and subcontinuous SWA in neurocognitive processing during sleep.
The notion that transient bouts of SWA have a more prominent role than subcontinuous SWA in sleep-related neurocognitive processing is also supported by the results of our study. In fact, sleep fragmentation induced a substantial reduction of slow-wave sleep but an enhancement of the number of A1 subtypes per hour of sleep (as well as an enhancement of A2 and A3 subtypes), paralleled by a significant increase in power spectrum at all frequencies, including the SWA frequency range. Interestingly, we did not observe a decrease in neurocognitive performance but did observe a maintenance or an improvement (reduction) in reaction times, accompanied by stable error rates. These findings can be explained by the persistence and even enhancement of transient SWA contained in CAP A1 subtypes, which also caused a significant enhancement of the power spectrum in the low-frequency band of the EEG.
Data derived from our study on short-term sleep fragmentation suggest that the A1 subtype is positively associated with higher neurocognitive functioning, whereas the A3 subtype (i.e., arousal-related CAP) is associated with lower neurocognitive functioning. Although both of these components were enhanced with sleep fragmentation, overall neurocognitive function was unchanged. The lack of significant change with short-term sleep fragmentation could be due to positive effects of A1 counterbalancing the negative effects of the A3 subtypes. Whether recurrent and sustained disruption of sleep, as seen in patients with obstructive sleep apnea and insomnia, has similar effects is not entirely clear. Experimental modeling of chronic sleep disruption would be necessary to further elucidate this question but is not possible due to participant burden and the possibility of untoward effects.
Recently, Landsness et al. [35] examined the effects of slow-wave sleep suppression on performance and contrasted it with control acoustic stimulation which had little effect on spontaneous slow wave activity. While total sleep time and sleep efficiency were not affected, SWA and the number of slow waves decreased with slow-wave deprivation whereas low-frequency EEG power increased with the control acoustic condition. Visuomotor performance significantly improved with control acoustic stimulation condition but not with slow-wave suppression leading to the conclusion that slow wave activity may have a causal role in sleep-dependent improvement of visuomotor performance. The results presented herein are in line with the report by Landsness et al. [35] because the increase in SWA, induced by acoustic stimulation, was accompanied by improvement in visuomotor performance. We speculate that the control acoustic stimulation most likely enhanced CAP A1 subtypes because these events represent the initial response evoked by increasing levels of noise at night, whereas overt arousals are evoked with higher levels of stimulation [38].
There are several limitations that should be considered in interpreting the results of our study. First, the sample size was relatively modest, thus limiting the potential generalizability of our results. Second, the restriction to a younger cohort and the limited number of women in the sample precluded stratified analyses by age and sex. It is certainly possible that the implications of sleep fragmentation on neurocognitive functioning are determined by several subject factors. Third, sleep fragmentation for only two nights does not provide information on how disruption of sleep and poor sleep quality in chronic conditions alters neurocognitive functioning. Nonetheless, the study has several important strengths including the use of a healthy cohort of subjects free of confounding conditions and a rigorous experimental paradigm that is difficult to model in a clinical sample.
In conclusion, the results of this study expand and further confirm our previous work on the positive correlation between CAP A1 components and neurocognitive functioning [21]. Future research might be directed to the assessment of eventual age-related differences in the relative neurocognitive functioning maintenance observed in this study in young adults. It is certainly likely that older subjects are unable to compensate for the level of imposed disturbance and may thus respond differently in neurophysiological and neurocognitive outcomes. The same considerations might also apply to chronic sleep disorders such as obstructive sleep apnea syndrome and insomnia.
Acknowledgements
The research was supported by National Institutes of Health Grants HL075078, HL086862, HL089467.
The work was performed at the Johns Hopkins University, School of Medicine, Baltimore, MD, U.S.A., and at the Sleep Research Centre, Department of Neurology I.C., Oasi Institute (IRCCS), Troina, Italy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005;28:51–64. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- 7.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 11.Terzano MG, Parrino L, Smerieri A, Chervin R, Chokroverty S, Guilleminault C, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2:537–553. doi: 10.1016/s1389-9457(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 12.Ferri R, Bruni O, Miano S, Plazzi G, Terzano MG. All-night EEG power spectral analysis of the cyclic alternating pattern components in young adult subjects. Clin Neurophysiol. 2005;116:2429–2440. doi: 10.1016/j.clinph.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8:137–145. doi: 10.1093/sleep/8.2.137. [DOI] [PubMed] [Google Scholar]
- 14.Bruni O, Ferri R, Miano S, Verrillo E, Vittori E, Della MG, et al. Sleep cyclic alternating pattern in normal school-age children. Clin Neurophysiol. 2002;113:1806–1814. doi: 10.1016/s1388-2457(02)00265-1. [DOI] [PubMed] [Google Scholar]
- 15.Bruni O, Ferri R, Miano S, Verrillo E, Vittori E, Farina B, et al. Sleep cyclic alternating pattern in normal preschool-aged children. Sleep. 2005;28:220–230. doi: 10.1093/sleep/28.2.220. [DOI] [PubMed] [Google Scholar]
- 16.Ferini-Strambi L, Ortelli P, Castronovo V, Cappa S. Increased periodic arousal fluctuations during non-REM sleep are associated to superior memory. Brain Res Bull. 2004;63:439–442. doi: 10.1016/j.brainresbull.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Miano S, Donfrancesco R, Bruni O, Ferri R, Galiffa S, Pagani J, et al. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder. Sleep. 2006;29:797–803. [PubMed] [Google Scholar]
- 18.Miano S, Bruni O, Elia M, Trovato A, Smerieri A, Verrillo E, et al. Sleep in children with autistic spectrum disorder: A questionnaire and polysomnographic study. Sleep Med. 2007;9:64–70. doi: 10.1016/j.sleep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Dynamics of the EEG slow-wave synchronization during sleep. Clin Neurophysiol. 2005;116:2783–2795. doi: 10.1016/j.clinph.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Ferri R, Huber R, Aricò D, Drago V, Rundo F, Ghilardi MF, et al. The slow-wave components of the Cyclic Alternating Pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett. 2008;432:228–231. doi: 10.1016/j.neulet.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Aricò D, Drago V, Foster PS, Heilman KM, Williamson J, Ferri R. Effects of NREM sleep instability on cognitive processing. 2009 doi: 10.1016/j.sleep.2010.02.009. Submitted. [DOI] [PubMed] [Google Scholar]
- 22.Parrino L, Smerieri A, Rossi M, Terzano MG. Relationship of slow and rapid EEG components of CAP to ASDA arousals in normal sleep. Sleep. 2001;24:881–885. doi: 10.1093/sleep/24.8.881. [DOI] [PubMed] [Google Scholar]
- 23.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington: Washington Public Health service; US Government Printing Office; 1968. [Google Scholar]
- 26.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. The Art of Scientific Computing. Cambridge: Press Syndicate of the University of Cambridge; 1989. Numerical Recipes. [Google Scholar]
- 27.Cooley JW, Tukey OW. An algorithm for the machine calculation of complex Fourier series. Math Comput. 1965;19:297–301. [Google Scholar]
- 28.Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D-G, editors. Attention and performance X: Control of language processes. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- 29.Shepard R, Cooper L. Mental images and their transformations. Cambridge, MA: MIT Press; 1982. [Google Scholar]
- 30.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 31.Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132:645–660. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, et al. Changes in cortical activity during mental rotation. A mapping study using functional MRI. Brain. 1996;119:89–100. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- 34.De Carli F, Nobili L, Beelke M, Watanabe T, Smerieri A, Parrino L, et al. Quantitative analysis of sleep EEG microstructure in the time-frequency domain. Brain Res Bull. 2004;63:399–405. doi: 10.1016/j.brainresbull.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, et al. Sleep-dependent improvement in visuo-motor learning: A causal role for slow waves. Sleep. 2009 doi: 10.1093/sleep/32.10.1273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri R, Parrino L, Smerieri A, Terzano MG, Elia M, Musumeci SA, et al. Non-linear EEG measures during sleep: effects of the different sleep stages and cyclic alternating pattern. Int J Psychophysiol. 2002;43:273–286. doi: 10.1016/s0167-8760(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 37.Ferri R, Rundo F, Bruni O, Terzano MG, Stam CJ. Regional scalp EEG slow-wave synchronization during sleep cyclic alternating pattern A1 subtypes. Neurosci Lett. 2006;404:352–357. doi: 10.1016/j.neulet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Terzano MG, Parrino L, Fioriti B, Orofiamma H, Depoortere H. Modifications of sleep structure induced by increasing levels of acoustic perturbation in normal subjects. Electroencephalogr Clin Neurophysiol. 1990:29–38. doi: 10.1016/0013-4694(90)90055-o. [DOI] [PubMed] [Google Scholar]