Abstract
Chronic systemic inflammation links periodontal disease and diabetes to increased incidence of serious comorbidities. Activation of TLRs, particularly TLR2 and TLR4, promotes chronic systemic inflammation. Human B cells have been generally thought to lack these TLRs. However, recent work showed that an increased percentage of circulating B cells from inflammatory disease patients express TLR2 and TLR4, and that TLR engagement on B cells resulted in unexpected changes in gene expression. New data show that B cells from inflammatory disease patients secrete multiple cytokines in response to different classes of TLR ligands. Furthermore, the B cell response to combinations of TLR ligands is cytokine- and ligand-specific. Some cytokines (IL-1β and IL-10) are predominantly regulated by TLR4, but others (IL-8 and TNF-α) are predominantly regulated by TLR2, due in part to TLR-dictated changes in transcription factor/promoter association. TLR2 and TLR9 also regulate B cell TLR4 expression, demonstrating that TLR cross-talk controls B cell responses at multiple levels. Parallel examination of B cells from periodontal disease and diabetes patients suggested that outcomes of TLR cross-talk are influenced by disease pathology. We conclude that disease-associated alteration of B cell TLR responses specifically regulates cytokine production and may influence chronic inflammation.
Chronic inflammation underlies many diseases, including periodontal disease and diabetes mellitus (1, 2). These diseases are generally categorized by pathological changes, but many factors mediating morbidity likely stem from the systemic inflammation shared among these diseases (3, 4). In general, pathological inflammation results from inappropriate immune responses to seemingly innocuous stimuli, such as commensal microbes or host-derived ligands. Some chronic inflammatory diseases, such as periodontal disease, are thought to initiate as a failure in homeostasis with commensal bacteria. The resulting local inflammation is mirrored by increased systemic inflammation, which likely explains the link between periodontal disease and cardiovascular disease (5). Similarly, the systemic inflammatory environment in diabetes patients probably stems from elevated levels of endogenous ligands, such as free fatty acids or advanced glycation end products, that can stimulate innate immune cells to produce proinflammatory cytokines (6–8). Elevated circulating endotoxin levels in diabetes patients (9), perhaps originating from compromised mucosal surfaces, may further exacerbate the systemic inflammatory response implicated in the most serious complications of this disease.
TLRs are involved in mounting pathogenic inflammatory responses to commensal organisms and host-derived ligands in chronic inflammatory diseases, for instance periodontal disease and diabetes (10–12). The likely role of TLRs in human systemic inflammatory disease is supported by multiple reports. First, both TLR2 and TLR4 are receptors for products of major periodontal pathogens, including Porphyromonas gingivalis (13). Second, TLR4 polymorphisms that alter TLR4 function associate with occurrence of periodontal disease and diabetes, at least in some cohorts (14, 15). Third, TLR2 and TLR4 are strongly implicated in diabetes by demonstrations that either inhibiting or genetically deleting each receptor in mice protects against a key characteristic of type 2 diabetes, insulin resistance (6, 16, 17). These observations suggest that altering expression, and thus function, of TLRs can promote inflammation in chronic diseases. Interestingly, multiple studies, including analyses of TLR4+ B cells from periodontal disease patients, show that cellular responses to TLR4 ligands can be a mixture of prototypic pro- and anti-inflammatory responses (18, 19).
The concept that cross-talk among TLR family members defines the innate immune response has emerged from studies on immune system sentinel cells, especially dendritic cells and macrophages. TLR2 and TLR4 activate these cells through a MyD88-dependent pathway. TLR4 also activates the TRIF/TRAM pathway in response to selected ligands. Pathway cross talk explains why in at least some cases, TLR4 engagement has the same biological outcome as coengagement of TLR2 and TLR4 (20, 21). However, TLR2 and TLR4 ligands can also synergize to activate production of proinflammatory cytokines under some conditions (22, 23). Engagement of other TLR family members, such as TLR9, can alternatively alter cellular responses to either TLR2 or TLR4 ligands in myeloid cells (23, 24). Appropriate TLR cross-talk therefore plays an important role in mounting an effective immune response to the complex combinations of TLR ligands presented by pathogens, commensal bacteria, and endogenous ligands.
Studies aimed at understanding the role of innate immune cells and TLR function in systemic inflammatory disease, with the exception of studies on TLR9, have largely focused on myeloid cells. However, B cells also function as a critical arm of the innate immune system, in part due to their ability to respond to TLR ligands and secrete cytokines (25). The role of B cell TLR engagement and subsequent cytokine production in chronic inflammatory diseases, including periodontal disease and diabetes, is poorly characterized. Our studies suggest that activated human B cells can circulate throughout the body (26); therefore, B cells may play an ongoing role in systemic manifestations of inflammatory diseases. Periodontal disease patients vs healthy donors have an elevated percentage of TLR2- and TLR4-positive B cells. New data show that these B cells constitutively and inducibly secrete elevated levels of cytokines, the latter in response to TLR ligands. These results also uncovered a high degree of specificity in B cell cytokine production in response to combinations of TLR ligands. Finally, B cells from periodontal disease and diabetes patients responded differently to combinations of TLR ligands. Based on this analysis of biologically important outcomes of TLR pathway cross-talk in human inflammatory disease patients, we conclude that clinical treatments and vaccines aimed at regulating immune responses through TLRs must test the complex response of B cells to combinations of TLR ligands.
Materials and Methods
Cells
Human samples were obtained following informed consent under a Boston University Institutional Review Board-approved protocol. Peripheral blood was collected into heparinized tubes by venous puncture. B cells were purified from whole blood using Histopaque 1077 to isolate the peripheral blood mononuclear layer, then negatively selected with magnetic beads to isolate CD19+ B cells according to the manufacturer’s protocol (Miltenyi Biotec). Only B cell preparations that were >95% pure were used in cytokine analyses. Most contaminating cells in all preparations were CD3+ T cells; monocyte contamination was <1%. B cells were stimulated for 24 h before analysis of secreted cytokines. The initial B cell concentration for all cultures was 106/ml, and all cultures were incubated in U-bottom wells. Nondiabetic periodontal disease (PD)4 patients had a diagnosis of localized aggressive periodontitis (27) but no other known disease. The PD patients that provided B cells for cytokine analyses are summarized in supplemental Table S1.5 In brief, the PD patients were characterized by periodontal infection with multiple organisms including P. gingivalis and Actinobacillus actinomycetemcomitans. Clinical and radiographic criteria of PD were: age of onset around the circumpubertal period (<13 years old), and alveolar bone loss localized around the first permanent molars and incisors (27). Additionally, a subject’s periodontal diagnosis was further confirmed by neutrophil functional analysis (28). Systemically healthy donors who had no sign of periodontal disease other than mild gingivitis were matched to PD patients based on age, sex, and race when possible. Patients and healthy donors were recruited from the Clinical Research Center and the Boston University School of Medicine community and were nonsmokers. Gingival tissue was collected from patients with PD as part of their routine periodontal surgical treatment. Human Mono Mac-6 monocytes were cultured and stimulated as published (29). Patients with diabetes (DM) were recruited from the Endocrinology, Diabetes and Nutrition Clinic at Boston University Medical Campus. Supplemental Table S2 outlines parameters for the diabetes patients (n = 11) that provided peripheral blood B cells for cytokine analyses. Periodontal status of these patients is unknown.
Flow cytometry
B cells in whole blood were prepared and analyzed as published (18, 30). Intracellular IL-8 was labeled with FastImmune (BD Pharmingen). Alternatively, purified B cells from healthy donors were incubated for 24 h in the presence of the TLR9 ligand CpG (ODN 2006, 1 µg/ml) or IL-4 (10 ng/ml), alone or in combination for 72 h, before staining for surface TLR4 expression using standard procedures (30).
Biochemistry
B cells were isolated by negatively selecting magnetic beads and were >95% pure as assayed by flow cytometry with anti-CD11b, anti-CD3, and anti-CD19 (Fig. 1). Rested B cells (1 h at 37°C) were stimulated with ligands at 1 µg/µl (approximately equimolar amounts) for 6–24 h for chromatin immunoprecipitation (ChIP) or cytokine analyses, respectively. ChIPs were completed as published (18). Alternatively, DNA was amplified with IL-8 primers: 5′-TGGGCCATCAGTTGCAAA-3′ 5′-ACTTAT GCACCCTCATCTTTTCATT-3′. Cytokines were quantified on a Luminex 200 using a 10-plex detection kit that measured IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFN-γ, TNF-α, and GM-CSF (Invitrogen). TLR4 ligands have been described (31, 32).
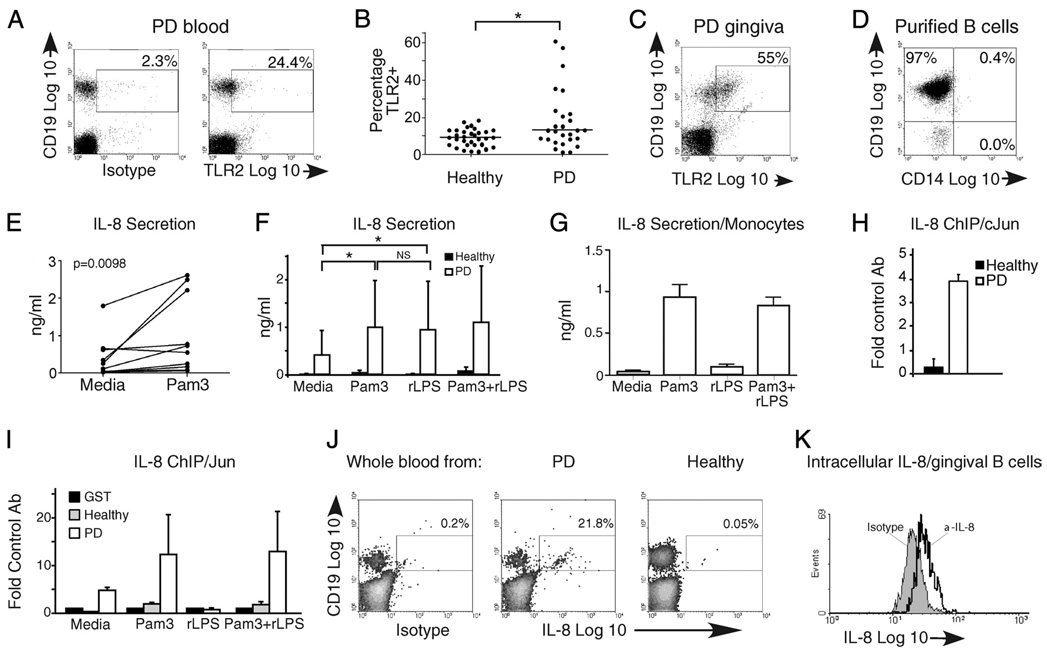
FIGURE 1.
An elevated percentage of CD19+ B cells from PD patients express functional TLR2 and secrete IL-8. A, Representative flow cytometric plot of B cells in peripheral blood from a PD patient stained for surface TLR2 expression relative to isotype control. A low percentage of CD19+TLR2+ B cells in blood of healthy donors has been published (30). B, Analysis of percentage and median percentage (horizontal line) of TLR2+ B cells in 32 healthy and 27 PD donors. Each point represents one donor. The percentages of TLR2+ B cells are significantly different (p= 0.048 by Mann-Whitney U test) between the two cohorts. Medians were: healthy = 8.97%, PD = 12.5% TLR2+ B cells. C, Flow cytometric plot showing percentage of CD19+TLR2+ B cells in surgically excised gingival tissue from PD patients. One of three similar results is shown. D, Representative reanalysis of isolated B cells from peripheral blood to demonstrate purity in samples subjected to biochemical analyses. The CD19−CD14− population (lower left) was predominantly CD3+ T cells (not shown). E, IL-8 secretion by peripheral blood B cells from 10 PD patients incubated in the absence vs presence of the TLR2 ligand Pam3CSK4. A paired t test established significance (p= 0.0098, shown in upper left). Each set of paired points shows results from a single donor under unstimulated (left) or stimulated (right) conditions. F, IL-8 secretion in B cells from PD (open bars) and healthy (filled bars) donors in the absence or presence of TLR2 (Pam3) and/or TLR4 (rLPS) ligand. Shown is average and SEM of 11 PD donors. *p < 0.05; NS, p > 0.1. G, IL-8 secretion by human monocytes stimulated for 24 h as indicated. Shown is average and SD of determinations from three donor samples. H, ChIP measuring constitutive association between the IL-8-activating transcription factor c-Jun and the IL-8 promoter in fresh ex vivo B cells from healthy (filled bar) or PD (open bar) donors. I, ChIP measuring c-Jun association with the IL-8 promoter in B cells from healthy (gray bars) or PD (open bars) donors incubated for 6 h with stimulus indicated on the x-axis. H and I show fold increase in DNA amplification in samples precipitated with the indicated Ab compared with an irrelevant Ab (anti-GST). Error bars show range from two to three donors for each treatment. J, Flow cytometry measuring intracellular IL-8 in B cells from PD or healthy donors as indicated. Isotype control was performed on the PD sample shown in the middle panel. Note that permeabilization decreases the CD19 signal nonspecifically. Percentage of IL-8-positive cells in the CD19+ gate is indicated. K, Flow cytometry measuring intracellular IL-8 in B cells from surgically excised gingiva from PD patients. Cells were gated on CD19. Forty-four percent of the 31,000 cells analyzed were IL-8+ in this sample. J and K represent independent determinations from three donors.
Statistical analyses
The Mann-Whitney U test was used for nonparametric comparisons of group means for B cells from peripheral blood. Wilcoxon matched-pairs tests were used for cytokine analyses. Linear associations of continuous variables were assessed with Spearman’s rank correlation coefficient (Prism). ChIPs were analyzed by Student’s t test. All statistical tests were two-tailed, and p values of <0.05 defined statistical significance.
Results
B cells from PD patients express functional TLR2
Our work on B cells from inflamed tonsil and inflammatory bowel disease patients demonstrated that B cells from inflammatory environments are surface TLR2-positive and respond to TLR2 ligand by secreting IL-8 (26, 30). To test whether B cells from another chronic inflammatory disease, aggressive periodontitis, are similarly altered, we measured the percentage of TLR2-positive B cells in whole blood and inflamed gingiva from PD patients (Fig. 1A–C). PD patients had a modest but statistically significant increased percentage of TLR2-positive B cells compared with healthy donors (Fig. 1B). A high percentage of B cells in inflamed gingiva also express TLR2 (Fig. 1C), but the absence of healthy B cells from noninflamed gingiva prevented a comparison to gingival B cells from PD patients (18). TLR2-positive B cells were equivalently represented in the CD19+/CD38+, CD19+/CD38−, and CD19+/CD27+ populations (not shown). This finding is consistent with data showing similar expression levels of TLRs 1, 2, 7, 9, and 10 among naive, germinal center, and memory B cells (33).
To determine whether the modest elevation of circulating TLR2-positive B cells in PD patients correlated with increased TLR2 responsiveness, we stimulated highly purified CD19+ B cells from PD patients (Fig. 1D) with the prototypic TLR2 ligand Pam3CSK4 (Pam3). B cells from PD patients secrete IL-8 in response to TLR2 engagement (Fig. 1, E and F). This contrasts with the lack of IL-8 production by B cells from healthy donors (Fig. 1F). Differences in IL-8 secretion between B cells from healthy vs PD donors were statistically significant (Fig. 1F; compare open and filled bars; p < 0.05 under all conditions), including media incubation. These data suggest that B cells from PD patients are constitutively activated, perhaps by in vivo ligands from common periodontal pathogens, such as P. gingivalis (34). We conclude that modest TLR2 elevation on B cells from PD patients confers significant increases in TLR2 ligand response.
A theoretical source of IL-8 in our cultures is the small number of contaminating cells, especially monocytes, that secrete high levels of cytokines in response to TLR2 (and TLR4) ligand. The lack of IL-8 in samples from healthy donors (Fig. 1F) discounts this possibility. However, to further test the role of contaminating non-B cells in constitutive and inducible IL-8 production, we determined the relationship between B cell purity (% CD19+) and cytokine production (ng/ml) by Spearman’s rank correlation. No correlation emerged (p > 0.1; not shown), confirming that contaminating cells are unlikely sources of IL-8 or other cytokines in these cultures. Furthermore, preliminary examination of monocyte-contaminated B cell preparations indicated that < 1% contaminating monocytes would insignificantly contribute to overall cytokine levels measured in our highly purified B cell populations (<10% of total cytokine production, data not shown). We conclude that contaminating monocytes are unlikely sources of TLR2 (and TLR4)-induced IL-8 in our purified B cell cultures. These findings support our conclusion that the relatively modest increase in TLR2-positive B cells in PD patients confers robust responsiveness to TLR2 ligand as measured by IL-8 secretion.
To identify mechanisms driving IL-8 production by B cells from PD patients, we examined IL-8 promoter association with a transcription factor that activates IL-8 in myeloid cells, c-Jun (35). c-Jun constitutively associated with the IL-8 promoter in freshly isolated B cells from PD patients (Fig. 1H), and association was maintained after 24 h in culture (Fig. 1I, leftmost open bars). c-Jun/IL-8 association is further increased in B cells activated through TLR2 (Pam3; Fig. 1I). These data suggest that TLR2 activates c-Jun, resulting in constitutive and inducible IL-8 production in B cells from PD patients.
To confirm that B cells from PD patients produce IL-8 in vivo, and to further test similarities between circulating and gingival B cells (Fig. 1, A and C), we measured intracellular IL-8 expression in fresh ex vivo peripheral blood and gingival B cells from PD patients. Blood B cells from PD patients, but not healthy donors, were intracellular IL-8-positive (Fig. 1J). Likewise, B cells isolated from inflamed gingiva produced IL-8 (Fig. 1K). These data confirm that B cells, rather than contaminating cells, are the source of IL-8 in our ex vivo experiments, and they support the likelihood that B cells produce IL-8 in vivo. We conclude that activation of the IL-8 promoter by TLR2 engagement and c-Jun/IL-8 association drives IL-8 production by B cells in PD patients.
B cells from PD patients constitutively secrete IL-1β
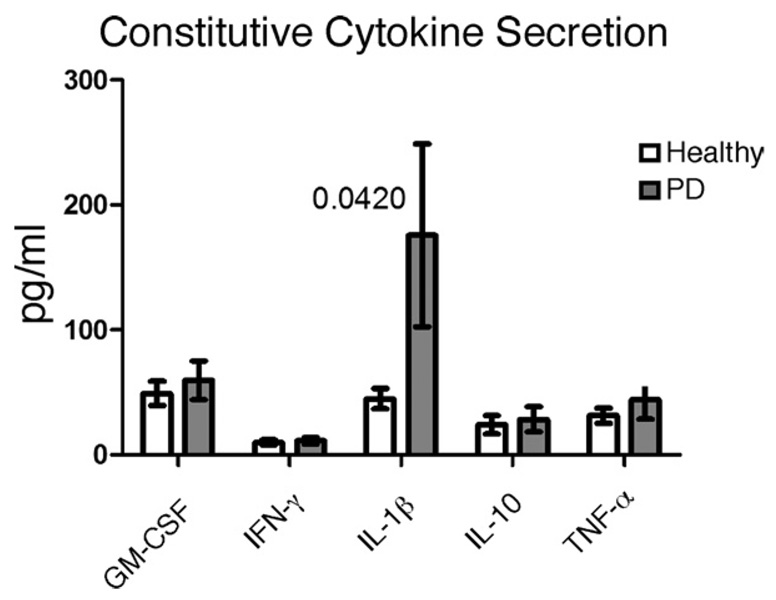
In addition to IL-8, multiple proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, have been implicated in PD and in a second inflammatory disease that is a strong risk factor for PD, diabetes (36–39). To more comprehensively identify the role B cells play in chronic inflammatory disease, we first measured secretion of additional cytokines by fresh ex vivo B cells from healthy or PD patients. On average, B cells from PD patients vs healthy donors constitutively secreted elevated levels of IL-1β (Fig. 2). All B cells constitutively secreted low levels of GM-CSF, IFN-γ, IL-10, and TNF-α (Fig. 2) and undetectable levels of IL-2, IL-4, and IL-5 (not shown). IL-6 production was highly variable in all assays hence uninterpretable (not shown). We conclude that B cells from PD patients may contribute to chronic systemic inflammation through constitutive IL-8 and IL-1β secretion.
FIGURE 2.
B cells from PD patients constitutively secrete IL-1β. Cytokine production by purified B cells from PD patients was quantified after 24 h in media. Differences in cytokine production between B cells from healthy (open bars) and PD (filled bars) donors were analyzed by Wilcoxon matched pairs tests. Values of p were >0.05, except as indicated for IL-1β (p= 0.042); n= 13 for GM-CSF and IL-1β; n= 12 for IFN-γ, IL-10, and TNF-α.
B cell TLR2 engagement activates selected cytokines and other signatures of inflammatory cells
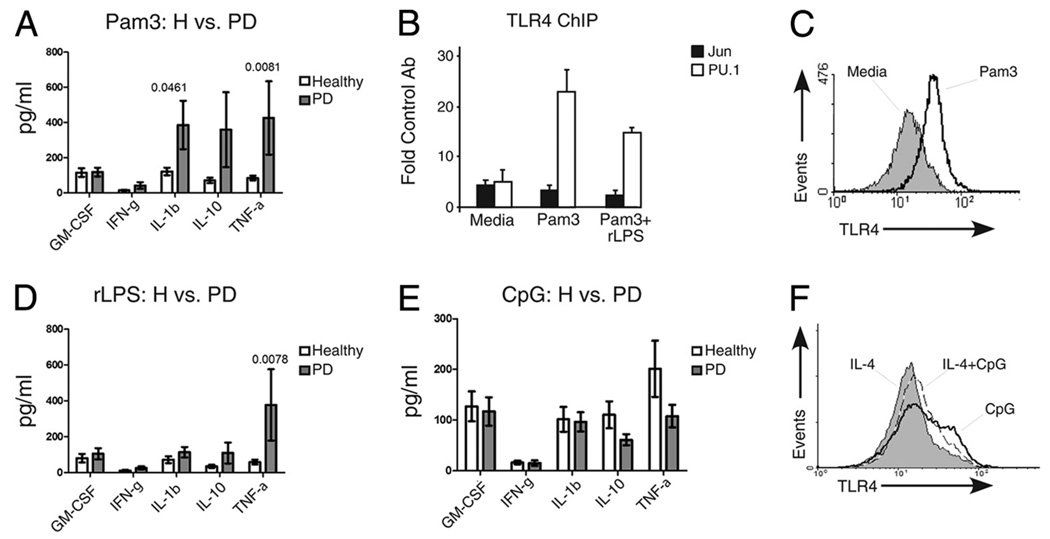
To more broadly evaluate the role of increased B cell TLR expression (Fig. 1 and Ref. 18) in chronic inflammatory disease, we measured secretion of additional cytokines by B cells responding to TLR ligands. The first series of analyses focused on cytokine secretion by B cells responding to single TLR ligands. B cells from PD patients responded to the TLR2 ligand Pam3 by significantly increasing production of all cytokines (supplemental Fig. S1), although some cytokine levels were indistinguishable, on average, from levels produced by TLR2-stimulated B cells from healthy donors (Fig. 3A; GM-CSF and IFN-γ). A weak TLR2 response in B cells from healthy donors is consistent with published data (30, 33). However, a subset of cytokines, specifically IL-1β and TNF-α, are secreted at significantly elevated levels (p < 0.05) by TLR2-stimulated B cells from PD vs healthy donors (Fig. 3A). IL-10 secretion was also preferentially induced in B cells from PD patients, but this increase was statistically insignificant (p > 0.05), due to greater variability in inducible IL-10 secretion. IL-2, IL-4, and IL-5 were undetectably secreted by all B cells responding to all stimuli tested (not shown). These data further support the conclusion that elevated surface TLR2 expression on B cells from PD patients may have proinflammatory outcomes, and they suggest that constitutively elevated IL-1β production by B cells from PD patients (Fig. 2) could result from TLR2 engagement by in vivo ligands.
FIGURE 3.
B cells from PD patients activate multiple inflammatory modulators in response to single TLR ligands. A, TLR2 ligation with Pam3CSK4 (Pam3) induces a subset of cytokines in B cells from PD patients. Shown in all bars graphs are average ± SEM. N values are listed in Fig. 2. Nonparametric two-tailed t test established significance of the Pam3-mediated increase in cytokine production by B cells from PD vs healthy donors (filled or open bars, respectively). Values of p for tests that showed statistical significance are listed above the results for all bar graphs. Although IL-10 was, on average, more highly expressed by B cells from PD patients (supplemental Fig. S1), variability prevented statistical significance. B, ChIP showing association of the TLR4-activating transcription factors c-Jun (filled bars) or PU.1 (open bars) in B cells from PD patients treated for 6 h as indicated. Shown is average fold increase in DNA amplification in samples precipitated with the indicated Ab compared with a control Ab (anti-GST). Error shows range from three PD donors. C, Pam3 treatment up-regulates surface TLR4 expression on B cells. Flow cytometric analysis of B cells from a DM donor treated as indicated for 72 h and then stained for surface TLR4. Similar results were obtained with B cells from healthy donors (not shown). D, Cytokine production by B cells from healthy or PD patients treated with the TLR4 ligand rLPS (Rhodobacter sphaeroids LPS). Details are as in A; n= 11 for GM-CSF and IL-1β; n= 10 for IFN-γ, IL-10 and TNF-α. Data for individual donors is in supplemental Fig. S2. E, Cytokine production by B cells from PD or healthy donors treated with the TLR9 ligand CpG. Details are as in A; n= 6 for GM-CSF and IL-1β; n= 5 for IFN-γ, IL-10, and TNF-α. F, CpG treatment up-regulates surface TLR4 expression on B cells from healthy donors. Flow cytometric analysis of B cells from a healthy donor treated as indicated for 72 h and then stained for surface TLR4. IL-4 was added as indicated to enhance survival. C and F represent similar results from three different donors.
TLR2 and TLR4 regulate each other in mouse B cells (40); therefore, we asked how elevated TLR2 activity might affect TLR4 in B cells from PD patients. To test whether TLR2 engagement affected TLR4 gene activation in B cells from PD patients, we treated B cells from PD patients with TLR2 ligand (Pam3) and measured inducible association of the TLR4-activating transcription factor PU.1 (41) with the TLR4 promoter (Fig. 3B). PU.1 association with the TLR4 promoter increased ~5-fold in TLR2-stimulated B cells, and it increased somewhat less (3-fold) in cells stimulated with TLR2 ligand (Pam3) plus TLR4 ligand (rLPS). As expected, TLR2 ligand also up-regulated surface TLR4 expression as assayed by flow cytometry (Fig. 3C). We conclude that TLR2 ligand activates TLR4 expression in B cells from PD patients. Interestingly, these findings identify a mechanism by which in vivo TLR2 ligands may regulate TLR4-mediated B cell activation in PD (18).
B cell TLR4 engagement activates a subset of cytokines activated by TLR2
To further explore potential physiological outcomes of TLR2-mediated TLR4 activation in B cells from PD patients, we measured B cell responses to TLR4 ligands. We measured cytokine responses to rLPS, the TLR4 ligand that alters gene expression in B cells from PD patients (18). Although, on average, rLPS increased production of most cytokines in PD B cells (GM-CSF, IFN-γ, IL-1β, and IL-10; supplemental Fig. S2), only IL-8 and TNF-α production was significantly higher in B cells from PD vs healthy donors (Fig. 1F and Fig. 3D). Interestingly, increased IL-8 secretion in response to rLPS did not correlate with inducible c-Jun/IL-8 promoter association (Fig. 1I), indicating that, at least in some cases, IL-8 can be activated in the absence of c-Jun binding. Note that rLPS failed to activate IL-8 production by monocytes (Fig. 1G), indicating that TLR4 may function differently on B cells compared to monocytes. We conclude that TLR4 significantly activates only a subset of TLR2-activatable cytokines in B cells from PD vs healthy donors.
TLR9 engagement activates cytokine and TLR4 expression by B cells from healthy and PD donors
To more comprehensively identify outcomes of TLR activation in B cells from inflammatory disease patients compared with healthy donors, we stimulated B cells with the TLR9 ligand CpG and measured cytokine production. CpG-mediated TLR9 activation up-regulated multiple cytokines in B cells from both PD and healthy donors, on average, to an equivalent level (Fig. 3E and supplemental Fig. S3). Differences in cytokine production between PD and healthy donors were not significant (p > 0.1). These data suggest that the MyD88-dependent TLR pathway, presumably activated by all three TLR ligands tested, is not fundamentally hyperactive in B cells from PD patients. Furthermore, as with the TLR2 ligand, the TLR9 ligand activated TLR4 expression, as measured by an increased mean fluorescence intensity of TLR4 staining on CpG-treated B cells (Fig. 3F). We conclude that activation of TLRs up-regulated on B cells from PD patients (TLR2 and TLR4) selectively increases cytokine production, but that activation of TLRs expressed on B cells from healthy donors (i.e., TLR9) generally increases cytokine production in all B cells. Furthermore, our demonstration that TLR2 and TLR9 activate TLR4 expression predicts that the outcome of B cell TLR engagement may be influenced by cross-talk between members of the TLR family.
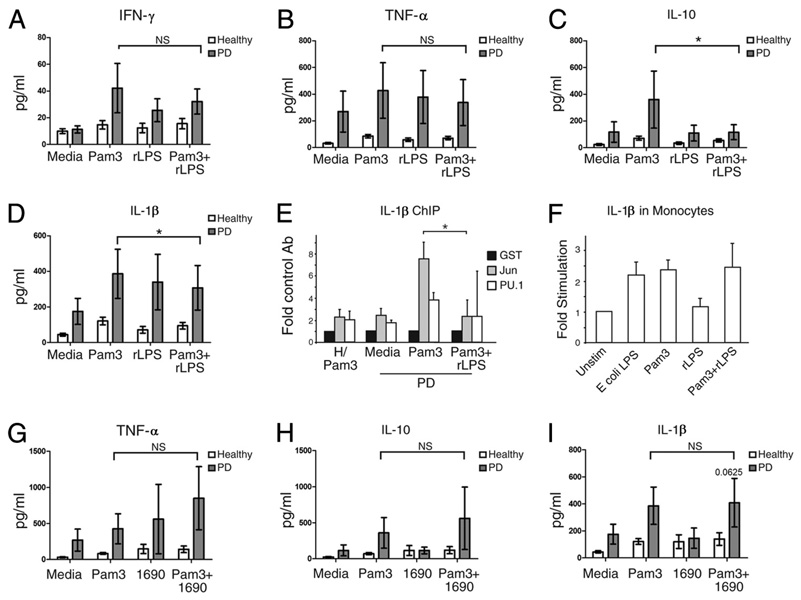
Cytokine-specific outcomes of TLR costimulation identify B cell cytokines regulated predominantly by TLR2 vs TLR4
TLR family cross-talk results in alteration of cytokine production, compared with cytokine production in the presence of single TLR ligands (42). The paucity of TLR2 and TLR4 expression on B cells from healthy donors (43) had previously made TLR2/4/9 cross-talk studies irrelevant in B cells. However, the demonstrations that (1) TLR2 and TLR4 are up-regulated on B cells from inflammatory disease patients (18, 26) (Fig. 1) and (2) TLR2 ligand activates TLR4 (Fig. 3, B and C) make investigation of TLR2/4 cross-talk critical for identifying roles for B cells in disease pathogenesis. Understanding up-regulated TLR cross-talk is also essential for safe vaccine design for the large number of individuals affected by inflammatory diseases. To begin testing how TLR cross-talk affects B cell cytokine production, we stimulated these cells in vitro with TLR2 ligand (Pam3) in combination with the TLR4 ligand rLPS. Levels of some cytokines, specifically IFN-γ, TNF-α, and IL-8, were indistinguishable in B cells stimulated with Pam3 with or without rLPS (Fig. 1F and Fig. 4, A and B). The lack of an effect of rLPS on Pam3-induced IL-8 production (Fig. 1F) is consistent with the indistinguishable change in c-Jun/IL-8 promoter association in Pam3 with or without rLPS-stimulated B cells (Fig. 1I). In contrast, Fig. 4C shows that B cells from PD patients stimulated with Pam3 plus rLPS secreted significantly lower levels of IL-10, on average, compared with B cells stimulated with TLR2 ligand (Pam 3) alone (p = 0.037). IL-1β was modestly, but statistically significantly lower in B cells stimulated with TLR2 plus TLR4 ligand (Pam3 plus rLPS) vs TLR2 ligand (Pam 3) alone (Fig. 4D; p = 0.027; 10 of 12 samples had lower IL-1β levels in Pam3 plus rLPS vs Pam3-stimulated cultures). The biological significance of this modest IL-1β decrease remains to be determined. However, decreased IL-1β protein secretion corresponds to significantly decreased association between the IL-1β promoter and an IL-1β activating transcription factor, c-Jun (44), in cells treated with Pam3 plus rLPS vs Pam3 alone (Fig. 4E). The ability of rLPS to decrease Pam3-mediated IL-10 and IL-1β production in B cells contrasts with the inability of rLPS to affect Pam3-induced IL-1β mRNA production by monocytes (Fig. 4F). This finding further supports the conclusion that TLR4 has different functional consequences in B cells vs monocytes. As expected, B cells from healthy donors produced low amounts of cytokines in response to all stimuli (Fig. 4, A–D, open bars). We conclude that rLPS predominantly regulates both IL-1β and IL-10 genes in the presence of TLR2 ligand (Pam3). This result contrasts with similar levels of TNF-α production by B cells stimulated with TLR2 alone or in combination with TLR4 ligand, and suggests that TLR2- and TLR4-activated pathways are redundant for only a subset of cytokines.
FIGURE 4.
A selected TLR4 ligand decreases TLR2-mediated activation of a restricted set of cytokines. A–D, B cells from PD patients were stimulated with the TLR2 ligand Pam3 in the presence or absence of the TLR4 ligand rLPS. Shown is average and SEM of (A) IFN-γ, (B) TNF-α, (C) IL-10, and (D) IL-1β production. Analysis was described in Fig. 3. Values of p > 0.1 for all comparisons are emphasized by NS; *, p < 0.05. The number of Pam3-stimulated samples is as in Fig. 3. For rLPS, n = 11 for IL-1β, and n = 10 for IFN-γ, TNF-α, and IL-10. For Pam3 plus rLPS, n = 12 for IL-1β, and n = 11 for IFN-γ, TNF-α, and IL-10. E, ChIP analysis of transcription factor association with the IL-1β promoter in B cells from healthy (left) or PD (right) donors stimulated as indicated. *, p = 0.04 (by paired Student’s t test) in c-Jun/IL-1β promoter association in B cells from PD patients stimulated with Pam3 alone or in combination with rLPS. Shown are averages and SEM from two to three healthy and three PD donors. F, IL-1β mRNA production by monocytes stimulated with the indicated ligand(s) for 20 h. mRNA was normalized to GAPDH and quantified by the ΔΔCt method. The y-axis shows mRNA in stimulated vs unstimulated monocytes. The latter value was set to equal 1. Shown are averages and SD of three independent experiments. G-I, B cells from healthy or PD donors were stimulated with TLR2 (Pam3) or TLR4 (LPS 1690) ligand alone and in combination as indicated. NS emphasizes no significant difference between Pam3 and Pam3 plus 1690-stimulated B cells from PD patients. Results for (G) TNF-α, (H) IL-10, or (I) IL-1β are shown. Bars show average of n = 4 for 1690-stimulated cells, and n = 6 for Pam3 plus 1690-stimulated cells.
To determine how TLR4 ligand from a periodontal pathogen (P. gingivalis LPS 1690) influences B cell activation, we stimulated purified B cells from PD or healthy donors with LPS 1690 in the presence or absence of TLR2 ligand (Pam3). LPS 1690 alone had little effect on cytokine production (Fig. 4, G–I and supplemental Fig. S4). Furthermore, LPS 1690 insignificantly changed Pam3-induced cytokine production (Fig. 4, G–I). Overall, data in Fig. 4 indicate that coengagement of TLR2 and TLR4 on B cells from PD patients has cytokine-specific (IL-10 and IL-1β vs TNF-α) and ligand-specific (LPS 1690 vs rLPS) outcomes.
TLR2 and TLR4 insignificantly affect TLR9-mediated cytokine production by B cells from healthy and PD donors
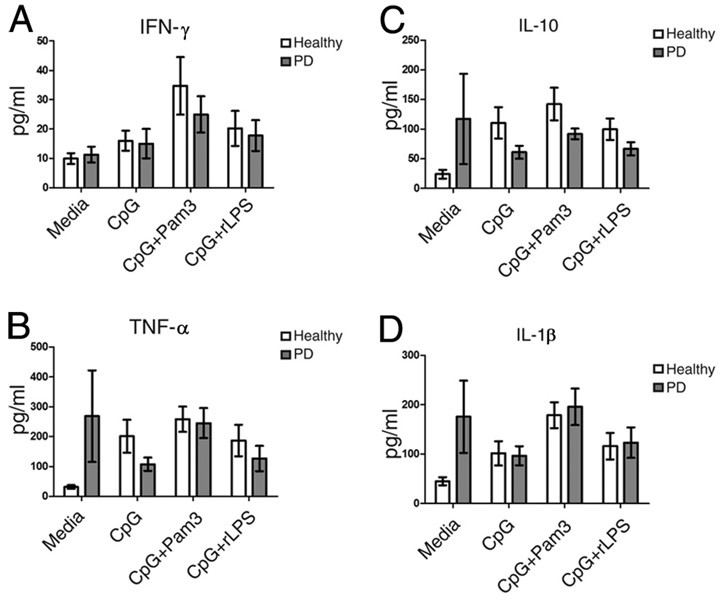
To further test the possibility that TLR cross-talk regulates cytokine production by B cells, we stimulated cells with CpG alone or in combination with Pam3 or rLPS to activate TLR9, TLR2, or TLR4, respectively, and measured cytokine production. B cells from PD and healthy donors produced similar levels of cytokines in response to all stimulation conditions (Fig. 5). Note that cytokine values in Fig. 5 cannot be quantitatively compared with values from Fig. 3 (single TLR ligand stimulations) because not all samples in Fig. 3 yielded a sufficient number of cells for additional analysis. Furthermore, insufficient numbers and variability of measurements for IL-8 (n = 3–4) made any conclusions on IL-8 production in response to TLR9 ligand unreliable. Regardless of these caveats, multiple conclusions are supported by these data. First, the TLR-triggered MyD88-dependent pathway that lies downstream of TLR2, TLR4, and TLR9 (45, 46) is probably fundamentally similar in B cells from PD and healthy donors. This finding strengthens our conclusion that cytokine production is regulated through ligand-specific (unknown) mechanisms. Second, these data show that TLR2 or TLR4 up-regulation does not alter response to a TLR normally expressed in human B cells, TLR9 (43, 47, 48). The precise response of B cells is therefore determined by the availability of specific TLR ligands. Finally, the similar response of B cells from healthy and PD donors indicates that B cell TLR9-mediated cytokine production plays no critical role in these patients.
FIGURE 5.
TLR9 equivalently activates cytokine production by B cells from PD and healthy donors. A–D, B cells from PD patients were stimulated with the TLR9 ligand CpG ODN 2006 in the presence or absence of the TLR2 ligand Pam3, or the TLR4 ligand rLPS as indicated. Values of p for comparison of results from B cells from PD vs healthy donors (filled or open bars, respectively) were all >0.1. No significant difference was detected between TLR9 and TLR9 plus TLR2- or TLR9 plus TLR4-stimulated cells (p > 0.1). Cytokines measured were (A) IFN-γ, (B) TNF-α, (C) IL-10, and (D) IL-1β. Bar shows average of n= 6 (for IL-1β) or n= 5 (for IFN-γ, TNF-α, and IL-10) for CpG- or CpG plus rLPS-treated samples; n= 5 (for IL-1β) or n= 4 (for IFN-γ, TNF-α and IL-10) for CpG plus Pam3-treated samples.
IL-1β production uncovers differences in the TLR responses of B cells from PD vs DM patients
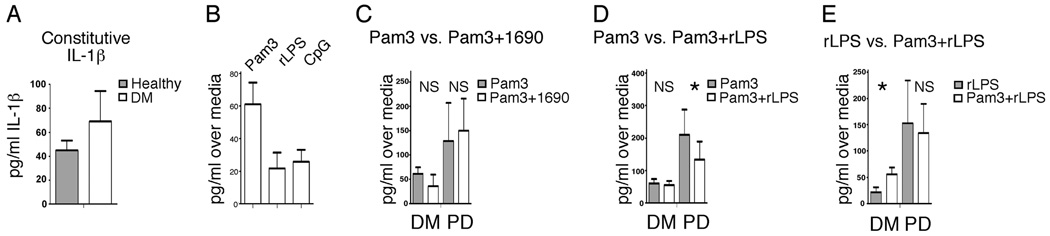
IL-1β is a key proinflammatory cytokine in multiple chronic inflammatory diseases with systemic manifestations, including PD and DM (49, 50). Because IL-1β secretion is constitutive by B cells from PD patients (Fig. 2), but is further regulated by TLR2 and TLR4 ligands (Fig. 3A and Fig. 4D), we asked whether IL-1β is similarly regulated in B cells from DM patients, who often have confounding oral inflammation. In contrast to B cells from PD patients, B cells from DM patients do not constitutively secrete IL-1β at levels that are significantly greater, on average, than levels secreted by B cells from healthy donors (Fig. 6A). We next considered the possibility that TLR ligands regulate inducible IL-1β production by B cells from DM patients. Ligands to TLR2, TLR4, and TLR9 modestly (albeit statistically significantly) increased IL-1β secretion (Fig. 6B). This result contrasts with the significant increase in IL-1β production by B cells from PD patients responding to TLR2 ligand (Pam3; Fig. 3A). We conclude that TLR-mediated IL-1β regulation differs in B cells from DM vs PD patients.
FIGURE 6.
TLR cross-talk differentially activates IL-1β production by B cells from diabetes patients compared with PD patients. A, Constitutive IL-1β production by B cells from DM or healthy donors as indicated. Difference was not significant (p= 0.2; n= 12). B, IL-1β production by B cells from DM patients stimulated as indicated. Average IL-1β production over media controls is shown. All conditions significantly elevated IL-1β levels compared with media (p < 0.05); n= 12 for Pam3 and CpG; n= 8 for rLPS. C–E, B cells from DM or PD patients stimulated with ligands as shown. Production over media controls is shown. Statistical differences between cytokine production in B cells stimulated with one vs two TLR ligands (as labeled) are highlighted with an asterisk (p < 0.05). The number of samples analyzed from DM patients was: C, 5; D, 8; E, 8. Number of PD samples analyzed are as listed in other figures.
To test whether B cells from DM patients, as with other innate immune cell types (but in contrast to B cells from PD patients), synergistically activate cytokine production in response to combinations of TLR ligands, we measured responses of cells stimulated with multiple TLR ligands. Fig. 6C shows that the oral pathogen TLR4 ligand LPS 1690 insignificantly altered IL-1β production by B cells from either DM or PD patients (p > 0.1 in Pam3 vs Pam3 plus LPS 1690 samples, NS). Although the TLR4 ligand rLPS modestly decreased IL-1β TLR2 (Pam3)-activated IL-1β production in B cells from PD patients (Fig. 6D), TLR4 ligand did not affect IL-β production by TLR2-stimulated B cells from DM patients (Fig. 6D, NS). Similarly, Pam3 further activated TLR4-mediated IL-1β production by B cells from DM patients, but not PD patients (Fig. 6E). Under all stimulation conditions, IL-1β production by B cells from DM patients was low. However, the data support the idea of fundamental differences in TLR pathway “wiring” in B cells from DM vs PD patients. Preliminary analysis of additional cytokines produced by B cells from DM patients is consistent with this interpretation (not shown). Taken together, these new findings on B cell cytokine production in PD and DM raise the possibility that alteration of TLR pathways, and therefore outcomes of TLR activation, significantly influence systemic inflammatory responses by altering B cell cytokine production, most potently in PD patients.
Discussion
Our data identify disease-, stimulus-, and cytokine-specific B cell responses to combinations of TLR ligands. Findings herein also suggest mechanistic differences to explain the elegant specificity of human B cell responses to disease-associated combinations of TLR ligands. This response occurs despite modest elevation of surface TLRs, indicating surface expression may underestimate ligand responsiveness. Many pathogens, including the periodontal pathogen P. gingivalis, produce ligands that activate TLR2 and TLR4 (13, 51–53), two TLRs elevated on the surface of B cells from PD patients. Similarly, multiple TLRs would be engaged in B cells responding to TLR-activating vaccine adjuvant or confounding PD in the multitude of diabetic individuals with naturally elevated levels of TLR4 ligands, including free fatty acids and/or systemic endotoxin (7, 54, 55). Understanding the response of B cells to combinations of TLR ligands will aid in rational vaccine strategies that use TLR ligands as adjuvants and are targeted to a broad cross-section of individuals, inevitably including PD and DM patients.
Both TLR expression and TLR function differ between B cells from inflammatory disease patients and human myeloid cells. One interpretation of these findings is that B cell TLRs are “hard wired” differently from myeloid cell TLRs. A second possibility is that mechanisms driving cytokine activation are fundamentally different in B cells vs myeloid cells. The results from ChIP assays and the relatively “normal” activation of the MyD88-dependent pathway in B cells preliminarily discount this explanation. A third possibility is that TLR function differs, regardless of cell type, in patients with inflammatory disease compared with healthy individuals. This scenario emphasizes the importance of testing responses to TLR ligands in primary cells from patients before clinical trials to identify disease-specific outcomes that cannot be predicted by analysis of cells from healthy donors. Additionally, the demonstration that B cells from individual donors with PD tend to exhibit similar cytokine responses (supplemental Fig. S5) suggests that efforts to further understand and then harness TLR crosstalk to regulate cytokine production by B cells are likely to have widespread efficacy.
The change in cytokine production by B cells was low for some cytokines, regardless of statistically significance. A modest change in average cytokine production may hold higher significance in the subset of patients with more responsive B cells, and it therefore may identify subsets of patients within our cross-sectional cohort. Regardless, cytokines often function through feedback loops that can amplify the short-term signals measured in our 24-h cultures. Low levels of IL-1β are particularly effective in activating a positive feedback loop to achieve robust IL-1β production in an autocrine/paracrine mechanism (56). Changes in the absolute value of cytokine production may therefore inaccurately estimate the physiological relevance of such changes in the complex in vivo environment. Future studies would benefit from a model organism that, unlike mice, generally houses a low percentage of TLR2+/TLR4+ B cells. This model remains to be identified.
Periodontal disease is a common confounder of diabetes, and therefore the response of B cells in diabetic patients could result solely from oral inflammation. However, the distinct differences in IL-1β response in B cells from PD vs DM patients demonstrates that the B cells are functionally different, regardless of the cooccurrence of PD in diabetics. One possible explanation is that PD can be divided into multiple classes, based on a series of >100 measurements of the oral cavity. B cells from the localized aggressive periodontitis patients comprising the PD cohort herein may be different from the chronic periodontitis more common in DM patients. A direct comparison of the role of PD vs diabetes in B cell function will require analysis of additional DM patients with healthy gingiva, who represent only ~30% of our clinical population. Alternatively, analysis of chronic periodontitis patients without confounding DM would determine the role that this type of PD might play in B cells responses in DM patients with confounding oral inflammation. These comparisons will be important to determine whether B cells in PD patients are likely to respond uniquely to treatments developed to counter TLR activation in diabetes.
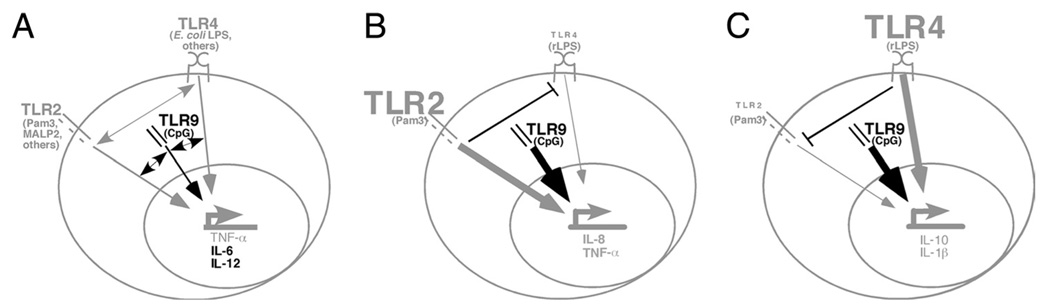
The paradigm for TLR cross-talk summarized from various studies on myeloid lineage cells is shown in Fig. 7A. Based on our data, we propose a revised model to describe the effect of TLR cross-talk through surface molecules up-regulated in B cells from inflammatory disease patients (Fig. 7, B and C). Our data support the conclusion that selected cytokines (in gray) are predominantly regulated by TLR2 (B) or TLR4 (C). TLR responses are influenced by idiosyncrasies of the inflammatory disease (PD vs DM, not shown in model). TLR2 and TLR4 insignificantly influence TLR9-mediated cytokine production (thick black arrow), in contrast to the generally additive effects of multiple TLR ligands on cytokine production in dendritic cells (24, 57, 58). This model makes testable predictions for understanding mechanisms responsible for the high specificity of TLR-triggered cellular responses likely to play important roles in disease pathogenesis.
FIGURE 7.
Models explaining biological outcomes of TLR cross-talk in hematopoietic cells. A, TLR cross-talk in myeloid cells is complex. Numerous studies show myeloid cells, predominantly dendritic cells and macrophages, respond to TLR2/4 coengagement by synergistically increasing IL-6 and TNF-α production (22, 23), although some studies indicated cytokine production decreases (20) or does not change in human monocytes responding to a combination of TLR2 and TLR4 vs a single TLR ligand alone (21). TLR2/4 cross-talk is represented by a gray double-headed arrow. TLR2 and TLR9 (leftmost double-headed black arrow) also cooperate to increase cytokine responses, although synergy in TNF-α production (in gray) is minimal (23). Coengagement of TLR4 and TLR9 (rightmost black double-headed arrow) resulted in additive/ synergistic activation of IL-12 and IL-6 (in black) but not other cytokines (24). At least some of the apparent inconsistencies in these results are likely due to different outcomes triggered by different ligands that bind the same TLR. B and C, Model for TLR cross-talk in B cells from inflammatory disease patients. Our studies show that B cells from these patients express TLR2 and TLR4 and regulate select cytokines using mechanisms than likely vary from those used by myeloid cells. TLR2/4 cross-talk did not result in additive cytokine activation in B cells. We instead identified cytokines that are predominantly regulated by (B) TLR2 (IL-8, TNF-α, gray) or (C) TLR4 (IL-1β and IL-10, gray) even in the presence of ligands for both. ⊥, Indicates that dominant regulation of cytokine production by TLR2 or TLR4 may involve direct interference of TLR-activated pathways, although this possibility remains to be tested. Decreased cytokine production was demonstrated for only one of two TLR4 ligands tested. Our data also show that TLR2 and TLR4 ligands do not detectably change TLR9-mediated B cell cytokine production (thick black arrow). The response of inflammatory disease patient B cells to other TLR2, TLR4, or TLR9 ligands remains to be determined. Interestingly, studies in wild-derived mouse strains support our conclusion that TLR responses differ between B cells and myeloid cells in outbred populations (59).
Supplementary Material
Acknowledgments
We thank Matt Rarick and Dr. Paul Skolnik from the Center for HIV/AIDS at Boston University Medical Center for use of, and technical expertise with, the multiplex analyzer. Drs. Ann Marshak-Rothstein, Ron Corley, Andy Henderson, Michael Clare-Salzler, and Greg Viglianti provided valuable comments on the manuscript.
Footnotes
This work was supported in part by National Institutes of Health Grant AI54611 and a Research Grant from the American Diabetes Association (to B.N.); by the Evans Medical Foundation, Broad Medical Research Program of The Broad Foundation, and a Becton Dickinson Grant Award 2007 (to L.M.G.); by U.S. Public Health Service Grants DE018917 (to H.H.) and by National Institutes of Health Grants P50 DE16191 and RR00533 (to T.V.D.).
Abbreviations used in this paper: PD, periodontal disease; ChIP, chromatin immunoprecipitation; DM, diabetes.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ford PJ, Yamazaki K, Seymour GJ. Cardiovascular and oral disease interactions: what is the evidence? Prim. Dent. Care. 2007;14:59–66. doi: 10.1308/135576107780556806. [DOI] [PubMed] [Google Scholar]
- 2.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 3.Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann. NY Acad. Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 4.Castoldi G, Galimberti S, Riva C, Papagna R, Querci F, Casati M, Zerbini G, Caccianiga G, Ferrarese C, Baldoni M, et al. Association between serum values of C-reactive protein and cytokine production in whole blood of patients with type 2 diabetes. Clin. Sci. (Lond) 2007;113:103–108. doi: 10.1042/CS20060338. [DOI] [PubMed] [Google Scholar]
- 5.Offenbacher S, Beck JD. A perspective on the potential cardioprotective benefits of periodontal therapy. Am. Heart J. 2005;149:950–954. doi: 10.1016/j.ahj.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 6.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 7.Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J. Clin. Endocrinol. Metab. 1985;61:807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- 8.Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, Mori Y, Okigaki M, Toyoda N, Masaki H, et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:5423–5428. doi: 10.1210/jc.2003-032223. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 12.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of Toll-like receptors 1–10 in periodontitis. Oral Microbiol. Immunol. 2008;23:425–431. doi: 10.1111/j.1399-302X.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 13.Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ. Activation of Toll-like receptors 2 and 4 by Gram-negative periodontal bacteria. Oral Microbiol. Immunol. 2007;22:145–151. doi: 10.1111/j.1399-302X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 14.Schroder NW, Meister D, Wolff V, Christan C, Kaner D, Haban V, Purucker P, Hermann C, Moter A, Gobel UB, Schumann RR. Chronic periodontal disease is associated with single-nucleotide polymorphisms of the human TLR-4 gene. Genes Immun. 2005;6:448–451. doi: 10.1038/sj.gene.6364221. [DOI] [PubMed] [Google Scholar]
- 15.Kolek MJ, Carlquist JF, Muhlestein JB, Whiting BM, Horne BD, Bair TL, Anderson JL. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am. Heart J. 2004;148:1034–1040. doi: 10.1016/j.ahj.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 16.Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, Saad MJ. Inhibition of Toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J. Endocrinol. 2008;199:399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 17.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 18.Shin H, Zhang Y, Jagannathan M, Hasturk H, Kantarci A, Liu H, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. B cells from periodontal disease patients express surface Toll-like receptor 4. J. Leukocyte Biol. 2009;85:648–655. doi: 10.1189/jlb.0708428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Shu R, Zhang MZ, Wu AP. Toll-like receptor 4 signaling plays a role in triggering periodontal infection. FEMS Immunol. Med. Microbiol. 2008;52:362–369. doi: 10.1111/j.1574-695X.2008.00386.x. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 21.Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J. Immunol. 2006;176:7438–7446. doi: 10.4049/jimmunol.176.12.7438. [DOI] [PubMed] [Google Scholar]
- 22.Liang MD, Bagchi A, Warren HS, Tehan MM, Trigilio JA, Beasley-Topliffe LK, Tesini BL, Lazzaroni JC, Fenton MJ, Hellman J. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring Toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J. Infect. Dis. 2005;191:939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- 23.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 24.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr. Dir. Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 26.Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J. Leukocyte Biol. 2009;86:1006–1016. doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J. Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 29.Liang M, Zhang Y, McDevit M, Marecki S, Nikolajczyk B. The IL-1β gene is transcribed from a poised promoter architecture in monocytes. J. Biol. Chem. 2006;281:9227–9237. doi: 10.1074/jbc.M510700200. [DOI] [PubMed] [Google Scholar]
- 30.Ganley-Leal LM, Liu X, Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin. Immunol. 2006;120:272–284. doi: 10.1016/j.clim.2006.04.571. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Coats SR, Bumgarner RE, Darveau RP. Hierarchical gene expression profiles of HUVEC stimulated by different lipid A structures obtained from Porphyromonas gingivalis and Escherichia coli. Cell. Microbiol. 2007;9:1028–1038. doi: 10.1111/j.1462-5822.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 32.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J. Biol. Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 33.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Tolllike receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–548. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajishengallis G, Martin M, Sojar HT, Sharma A, Schifferle RE, DeNardin E, Russell MW, Genco RJ. Dependence of bacterial protein adhesins on Toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab. Immunol. 2002;9:403–411. doi: 10.1128/CDLI.9.2.403-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agelopoulos M, Thanos D. Epigenetic determination of a cell-specific gene expression program by ATF-2 and the histone variant macroH2A. EMBO J. 2006;25:4843–4853. doi: 10.1038/sj.emboj.7601364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin CR, Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998;4:43–47. doi: 10.1111/j.1601-0825.1998.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 37.Graves DT, Delima AJ, Assuma R, Amar S, Oates T, Cochran D. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J. Periodontol. 1998;69:1419–1425. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- 38.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 39.Gainet J, Chollet-Martin S, Brion M, Hakim J, Gougerot-Pocidalo MA, Elbim C. Interleukin-8 production by polymorphonuclear neutrophils in patients with rapidly progressive periodontitis: an amplifying loop of polymorphonuclear neutrophil activation. Lab. Invest. 1998;78:755–762. [PubMed] [Google Scholar]
- 40.Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J. Immunol. 2005;174:6639–6647. doi: 10.4049/jimmunol.174.11.6639. [DOI] [PubMed] [Google Scholar]
- 41.Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J. Biol. Chem. 2000;275:9773–9781. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- 42.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between Toll-like receptor (TLR) 2-and TLR4-mediated signaling pathways. J. Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 43.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 44.Grondin B, Lefrancois M, Tremblay M, Saint-Denis M, Haman A, Waga K, Bedard A, Tenen DG, Hoang T. c-Jun homodimers can function as a context-specific coactivator. Mol. Cell. Biol. 2007;27:2919–2933. doi: 10.1128/MCB.00936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 46.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 2000;10:1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 47.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of Toll-like receptors on B lymphocytes. Cell. Immunol. 2005;236:140–145. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The Toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 49.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 50.Delima AJ, Karatzas S, Amar S, Graves DT. Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J. Infect. Dis. 2002;186:511–516. doi: 10.1086/341778. [DOI] [PubMed] [Google Scholar]
- 51.Tabeta K, Yamazaki K, Akashi S, Miyake K, Kumada H, Umemoto T, Yoshie H. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect. Immun. 2000;68:3731–3735. doi: 10.1128/iai.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davey M, Liu X, Ukai T, Jain V, Gudino C, Gibson FC, 3rd, Golenbock D, Visintin A, Genco CA. Bacterial fimbriae stimulate proinflammatory activation in the endothelium through distinct TLRs. J. Immunol. 2008;180:2187–2195. doi: 10.4049/jimmunol.180.4.2187. [DOI] [PubMed] [Google Scholar]
- 53.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect. Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seghrouchni I, Drai J, Bannier E, Riviere J, Calmard P, Garcia I, Orgiazzi J, Revol A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin. Chim. Acta. 2002;321:89–96. doi: 10.1016/s0009-8981(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 55.Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 56.Hiscott J, Marois J, Garoufalis J, D’Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, et al. Characterization of a functional NF-κB site in the human interleukin 1β promoter: evidence for a positive autoregulatory loop. Mol. Cell. Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theiner G, Rossner S, Dalpke A, Bode K, Berger T, Gessner A, Lutz MB. TLR9 cooperates with TLR4 to increase IL-12 release by murine dendritic cells. Mol. Immunol. 2008;45:244–252. doi: 10.1016/j.molimm.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J. Immunol. 2006;177:7551–7558. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 59.Thiriot A, Drapier AM, Memet S, Fitting C, Sturny-Leclere A, Cavaillon JM, Cazenave PA, Freitas AA, Rueff-Juy D. Wild-derived mouse strains, a valuable model to study B cell responses. Mol. Immunol. 2009;46:601–612. doi: 10.1016/j.molimm.2008.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.