Abstract
Factor XII (FXII) is a coagulation protein that is essential for surface-activated blood coagulation tests but whose deficiency is not associated with bleeding. For over forty years, investigators in hemostasis have not considered FXII important because its deficiency is not associated with bleeding. It is because there is a dichotomy between abnormal laboratory assay findings due to FXII deficiency and clinical hemostasis that investigators sought explanations for physiologic hemostasis independent of FXII. FXII is a multidomain protein that contains two fibronectin binding consensual sequences, two epidermal growth factor regions, a kringle region, a proline-rich domain, and a catalytic domain that when proteolyzed turns into a plasma serine protease. Recent investigations with FXII deleted mice that are protected from thrombosis indicate that it contributes to the extent of developing thrombus in the intravascular compartment. These findings suggest that it has a role in thrombus formation without influencing hemostasis. Last, FXII has been newly appreciated to be a growth factor that may influence tissue injury repair and angiogenesis. These combined studies suggest that FXII may become a pharmacologic target to reduce arterial thrombosis risk and promote cell repair after injury, without influencing hemostasis.
In 1955, Oscar Ratnoff and Joan Colopy described a patient, 37 year old John Hageman, who was found to have a prolonged Lee-White clotting time that was obtained during routine preoperative screening. The patient had no hemorrhagic symptoms even though he had a remarkably prolonged whole blood and plasma clotting times in glass and silicone-coated glass tubes. The prolonged clotting time was corrected by small amounts of plasma from each of the other known clotting factor deficiencies. Ratnoff concluded that his patient was deficient in an unrecognized clotting factor which he named Hageman factor, later known as Factor XII (FXII) [1]. Further experiments indicated that Hageman factor (FXII) circulates as an inactive precursor (zymogen) that becomes “activated” (FXIIa) as clotting commenced.
In 1961, Ratnoff and Davie demonstrated that Factor XI (FXI) was activated by FXIIa, contributing to the presentation of their waterfall cascade hypothesis for the blood coagulation system [2]. These studies encompass the major known properties of Factor XII, a protein that autoactivates upon exposure to negatively charged surfaces to become the enzyme Factor XIIa (α-FXIIa), which then activates FXI, prekallikrein (PK), and C1 esterases (C1r, C1s), the first components of the macromolecular complex of C1 and the classic complement cascade. FXIIa activation of PK forms plasma kallikrein that reciprocally activates FXII and liberates bradykinin (BK) from high molecular weight kininogen (HK). Bradykinin reproduces many aspects of an inflammatory state, such as vasodilation and increased capillary permeability, leading to edema and changes in arterial blood pressure. Plasma kallikrein also cleaves α-FXIIa to form β-FXIIa (FXII fragment or Hageman factor fragment) a 28 kDa catalytic domain alone that also activates the first component of complement [3]. Activation of FXI leads to a series of proteolytic reactions resulting in thrombin generation and the hemostatic pathway. Although it has been known that FXIIa activates FXI in vitro, the fact that FXII-deficient patients, along with PK- and HK-deficient individuals, do not have hemostatic defects indicates that FXII is not a physiologic hemostatic protein. However, knowledge about FXII is clinically significant since over 200 million activated partial thromboplastin times (APTT), a routine screening test for bleeding disorders, are performed annually in United States requires its presence to be normal. Further, as will be discussed below, FXII has recently been shown to have a role in intravascular thrombus formation and cell growth. This review will be organized on basis of FXII structure and what is known about its function. Additional sections will discuss the role of FXII in hemostasis and thrombosis.
Structural Basis for Factor XII function
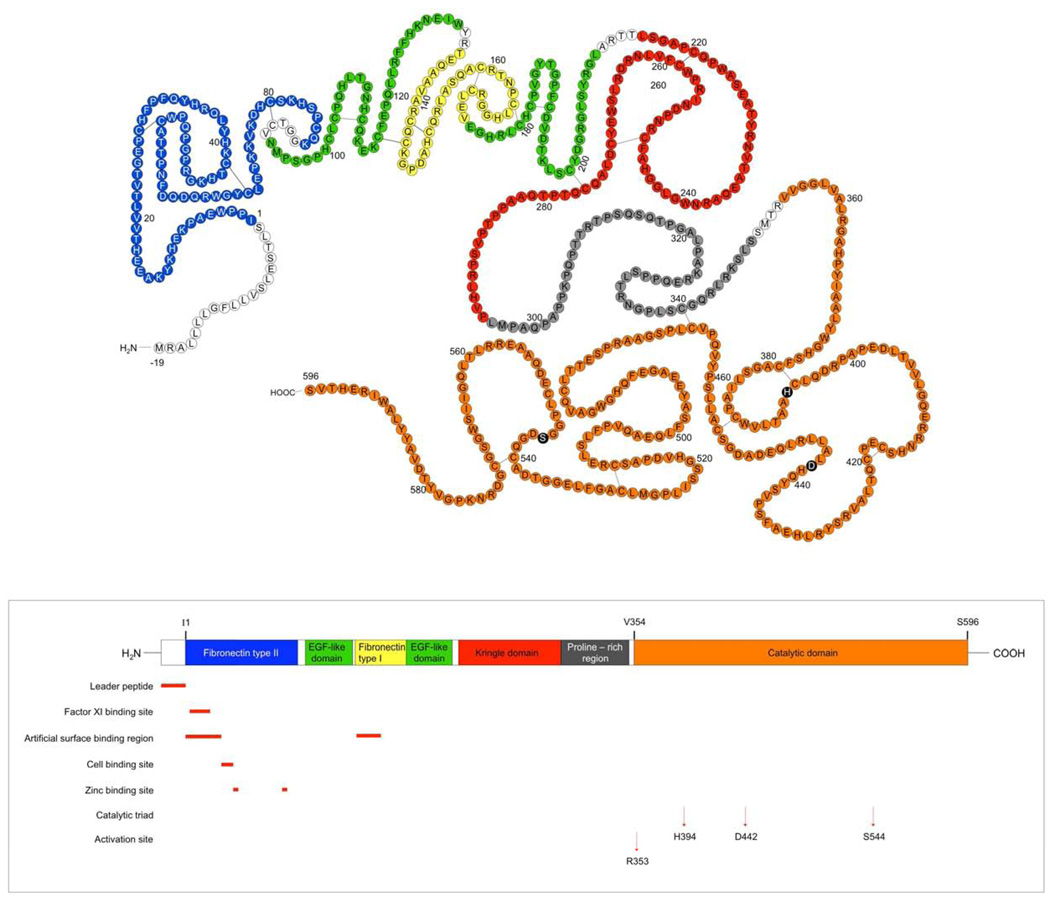
Coagulation Factor XII (Hageman factor, FXII) is produced and secreted by the liver. It is the product of a single gene that maps to chromosome 5 [4]. The gene for Factor XII is 12kb and is composed of 13 introns and 14 exons [5]. The FXII zymogen, consists of a heavy chain (353 residues) and a light chain (243 residues) held together by a disulfide bond (Figure 1), (Table 1). FXII consists of several structural domains. Starting from the N-terminus, the domains are a leader peptide, a fibronectin domain type II, an epidermal-growth-factor-like (EGF-like)domain, a fibronectin domain type I, a second EGF-like domain, a kringle domain, a proline-rich region and the catalytic domain (Figure 1). These domains are homologous to those found in other serine proteases, except for the proline-rich region which is unique to FXII. Each of these six regions of FXII are discussed in detail in the following sections.
Figure 1.
Structural Basis of FXII Function: FXII is divided into several domains. Top structure: amino acid sequence; bottom structure, linear diagram color coding each of the regions on the protein. Amino acids -19-1: leader peptide, 1–88: fibronectin type II domain, 94–131: EGF-like domain, 133–173: fibronectin type I domain, 174–210: EGF-like domain, 215–295: kringle domain, 296–349: proline-rich region, 354–596: catalytic domain or light chain. Amino acids 1–353 are the so-called heavy chain. Each of these areas is highlighted in the same color as the linear cartoon below it. This figure is adapted from Cool and MacGillivray [5].
Table 1.
The Regions of Factor XII: Structure/Function
| Domains | Amino Acid sequence |
Subdomains of known functional activity |
Proposed Role | Citation |
|---|---|---|---|---|
| Leader peptide | −19-1 | |||
| Fibronectin type II | 1–88 | [6, 20, 64] | ||
| 3–19 | Interaction with FXI |
[12, 13] | ||
| 1–28 | Artificial surface- binding region |
[8, 10, 20] | ||
| 39–47 | HUVEC binding region |
[14] | ||
| 40–44, 78–82 | Zinc-binding sites | [65] | ||
| EGF-like domain | 94–131 | Zinc binding site | [19] | |
| Fibronectin type I | 133–173 | Fibrin and heparin binding |
[7] | |
| 134–153 | Artificial surface binding region |
[10, 26] | ||
| EGF-like domain | 174–210 | Putative artificial surface binding region |
[11, 64] | |
| 174–146 | Putative Zinc binding site |
[19] | ||
| Kringle domain | 215–295 | Putative artificial surface binding region |
[11, 64] | |
| 193–276 | May enhance susceptibility to cleavage by kallikrein |
[66] | ||
| Proline-rich region | 296–349 | [64] | ||
| Catalytic domain | 354–596 | |||
| 353–354 | α-FXIIa fragment formation site |
[5, 20, 64] | ||
| H394, D442, S544 | Catalytic triad of active site |
[5] |
Fibronectin domain, type II homology
The amino-terminal region of FXII (Figure 1) shares sequence homology with the type II homology regions of fibronectin. Residues 13–69 of FXII share 39% sequence homology (22/57 residues are identical) and 40% sequence homology (23/57 residues are identical) with the two fibronectin sequences, respectively, including the four half-cysteine residues [6]. The type II homologies that comprise the collagen-binding site in fibronectin may be responsible for the artificial surface-binding properties of Factor XII [7]. A putative binding site for negatively charged surfaces, has been mapped at the N-terminus of FXII that consists of residues 1–28, located in the fibronectin type II [8, 9] and residues 134–153 in the fibronectin type I domain (Table 1) [10]. Structure/function analysis of human FXII using recombinant deletion mutants confirmed that the N-terminus of FXII contains a binding site for negatively charged activating surfaces [11].
In addition to an artificial surface binding site, the FXII fibronectin type II region contains a binding site for FXI that spans amino acid sequences 3–19 (Table 1) [12]. The FXII binding site on FXI is two-fold: a region on apple domain 4 (A4) and substrate recognition site at the FXI cleavage site (Arg369-Ile370) [13]. In addition to protein-protein interactions in solution, FXII has been demonstrated to bind to endothelial cells, neutrophils, and platelets [14–16]. On endothelial cells, a peptide of amino acids 39–47 block FXII binding to cultured endothelial cells (Table 1) [14]. On endothelial cells, FXII has been demonstrated to interact with the urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1, the same receptors that have been demonstrated to be HK binding sites [17, 18]. On platelets, FXII has been demonstrated to bind to the GPIba-IX-V complex [16]. FXII’s interaction with human umbilical vein endothelial cells requires a free zinc ion concentration to be 30-fold the constitutive level in plasma [14]. Thus in vivo, FXII is not constitutively bound to cells in the intravascular compartment. It binds cells only when the local zinc ion concentration is sufficiently high. In vivo, collagen-activated platelets liberate granule zinc ion sufficiently to support its cell binding interaction [14]. On FXII there are two recognized zinc binding sites in the fibronectin type II region, residues 40–44 and 78–82 [19]. Two other zinc ion binding sites are also postulated for residues 94–131 in the first EGF-like domain and residues 174–176 in the second EGF-like domain [19]. The localization of the two zinc-binding loci in the N-terminal region of FXII and the finding that β-FXIIa does not contain any zinc-binding sites indicate that the role of this divalent cation is related to surface binding and the subsequent conformational change leading to its activation [19].
EGF-like domains
Two regions of FXII are homologous to an epidermal-growth-factor-like sequence that has been found in many proteins including transforming growth factor type 1, tissue plasminogen activator (tPA), single chain urokinase plasminogen activator and several clotting factors. In each of these proteins, there is a highly conserved region of 50 amino acids with nine invariant cysteine and glycine residues [20]. The carboxyl-terminal growth factor domain also contains the invariant glycine residues; in the amino-terminal domain, however, one invariant glycine residue has been replaced with a histidine. Epidermal growth factor (EGF) is a known mitogen for a variety of cells and stimulates a pleiotropic response in target cells, including increased DNA and protein synthesis [21]. The mechanism of EGF action is characterized by EGF receptor binding and autophosphorylation followed by phosphorylation of tyrosine residues in intracellular proteins like the mitogen-activated/extracellular signal-regulated protein kinase (MAPK/ERK) pathways. FXIIa has been recognized to regulate the expression of the monocyte FcγII receptor [22]. FXII and α-FXIIa have also been shown to enhance cell proliferation, [3H]thymidine incorporation, and [3H]leucine incorporation in HepG2 cells [23, 24]. Furthermore, FXII induces MAP kinase in Hep G2 cells and smooth muscle fibroblasts [23, 25]. However, currently it is not known if its EGF-like domains mediate these activities.
Fibronectin type I region
Separating the two growth factor-like regions of FXII is a 43 amino acid peptide that shares limited sequence homology with the type I regions of fibronectin, each of which are characterized by two disulfide bonds giving a two-loop structure that has been named a “finger” domain [6]. Its precise function is not known, but it has been characterized to participate in artificial surface binding by Citarella et al. [26].
Kringle domain
Another type of homology found in FXII is the kringle domain (Figure 1). Kringle domains are typically 80 amino acids in length and form three characteristic disulfide bonds. The kringles in FXII and tPA, share approximately 41% sequence homology. Its function in FXII is also unclear but it has been proposed as a putative artificial surface binding site as well [11].
Proline-rich region
The kringle structure is followed by a region in which 33% of the residues are proline. This region does not share any sequence homology with other proline-rich proteins. The significance of this region in FXII remains undetermined.
Catalytic domain
The catalytic domain of FXII is the single largest region and the region of the protein that is best known (Figure 1). The active site of FXIIa consists of 3 amino acids, H394, D442, and S544, indicating that in vivo the catalytic domain is globular bringing these 3 residues in close apposition. Proteolytic cleavage of its R353-V354 site converts single chain zymogen FXII (80 kDa) to α-FXIIa. In vivo, this cleaved protein circulates as a two chain protein, a heavy chain of ~50 kDa (353 residues) held together by a disulfide bond between two cysteines with a ~30 kDa light chain (243 residues). Reduction of the disulfide bond liberates the ~30 kDa light chain as β-FXIIa (Hageman factor fragment, HFf, β-HFa) [27, 28]. Although retaining its proteolytic activity towards protein substrates, β-FXIIa is unable to bind to negatively charged surfaces and hence to promote clotting [29].
The critical issue that is still unsettled is how does FXII become activated in vivo? FXII is activated by proteolytic cleavage by plasma proteinases, such as plasma kallikrein and plasmin (fluid-phase activation). We have shown that on cultured endothelial cells, the serine protease prolylcarboxypeptidase activates prekallikrein (Km=9 nM) and its formed plasma kallikrein leads to kinetically favorable FXII activation (Km=11µM) [30]. This mechanism has not been demonstrated in vivo yet. In addition to this proteolytic pathway, FXII upon contact with negatively charged surfaces autoactivates (solid-phase activation) into α-FXIIa [29, 31]. In vitro, formed α-FXIIa results in PK activation to plasma kallikrein (Km=2.4 µM) with reciprocal activation of FXII (Km=11 µM) and activation amplification of the system. The mechanism for FXII autoactivation is not completely known. Upon adhering to surfaces there is a change in the structure of FXII as indicated by circular dichroism and sum frequency generation studies [32, 33]. However, the molecular basis for FXII autoactivation will only be solved by crystallographic studies.
Negatively charged surfaces such as glass, kaolin, ellagic acid, sulfatide micelles, high molecular weight dextran sulfate, bismuth subgallate, dacron, polyethylene, silicone rubber, and various polymers support FXII autoactivation. Biologic substances that support FXII autoactivation include articular cartilage, skin, fatty acids, endotoxin, sodium urate crystals, calcium pyrophosphate, l-homocysteine, hematin, protoporphyrins, heparins, chondroitin sulfate, and phosphatidylserine, phosphatidylglycerol, phosphatidic acid, and phosphatidylinositol. The ability of excess negatively charged material in vivo to produce disease was recently driven home by the clinical outcomes of patients who received lots of porcine heparin sulfate adulterated with chondroitin sulfate [34]. Patients who received the chondroitin sulfate-adulterated heparin had allergic reactions and hypotension due to FXII autoactivation with secondary formation of plasma kallikrein leading to bradykinin formation and C3 activation [34].
Although a number of biologic substances had been shown to support FXII activation, it has always been questioned as to whether FXII autoactivation occurs during endogenous physiologic or pathophysiologic activities in vivo. New interest has arose recently on FXII autoactivation occurring in vivo by the recognition that FXII contributes to the extent of induced thrombus formation. This observation will be discussed below in the section on thrombosis. Recently, several biologic substances elaborated about developing thrombus have been shown to support FXII autoactivation. These substances include extracellular RNA [35], polyphosphates with chain lengths greater than 75 subunits released from platelet granules upon platelet activation [36], aggregated proteins [37], and collagen-exposed in arterial tissue [38]. Last, FXII activation also occurs under conditions of sepsis, by the negatively charged surface provided by bacteria [39]. Alternatively, microbial enzymes can activate FXII by direct proteolysis [40].
The major plasma protease inhibitor of α-FXIIa and β-FXIIa is C1 esterase inhibitor (C1 inhibitor, C1INH), accounting for greater than 90% of the inhibition of these proteases in plasma [41]. C1 inhibitor binds both of these enzymes and irreversibly inactivates them. When associated with a kaolin surface, FXIIa is protected from C1 inhibitor inactivation [42]. Antithrombin III has some inhibitory effect on FXIIa [43]. Heparin, even at therapeutic levels, does not, however, significantly enhance the ability of ATIII to inhibit FXIIa [43]. A deficiency (type 1) or defect (type 2) in C1 esterase inhibitor results in the inflammatory condition called hereditary angioedema, a disorder associated with tissue swelling due to local increased bradykinin formation [44]. A type of hereditary angioedema has also been recognized to arise out of a constitutively active form of FXIIa with normal C1 esterase inhibitor [45].
Regulation of FXII expression
Little is known about FXII expression. The FXII gene has a consensus estrogen-response element and estrogen therapy is known to increase liver production of FXII [46]. The hepatocyte nuclear factor-4 (HNF-4) transcription factor inhibits estrogen induction of the FXII promoter in fibroblasts but not in HepG2 cells where it potentiates estrogen-induced FXII expression [47]. HNF-4 null mice also show reduced FXII expression [48]. This latter mechanism may be important to understand the mechanism(s) for deficiency seen in patients with apparent FXII decrease.
Role of Factor XII in Hemostasis and Thrombosis
Role in hemostasis
FXII deficiency as already stated is not associated with a bleeding state both in man and mouse. Recently, Spronk et al. demonstrated that tissue factor (TF) is the physiologic initiator of blood coagulation leading to hemostasis [49]. Mice with total deficiency of FXII and low TF are viable and phenotypically similar to low TF mice with normal FXII expression. In contrast, superimposed FXI deficiency on the low TF background results in death in utero[49]. Thus, FXII is not essential for hemostasis, even though it is essential for a normal result of surface-activated blood coagulation tests used for diagnosis of potential bleeding disorders [50].
Role in thrombosis
New interest in FXII has arisen, however, by the observation that FXII deficient mice have delayed times for induced arterial thrombosis using various techniques [51, 52]. These observations suggest that FXII in the developing clot contribute to the extent of thrombosis seen. The mechanism by which this may be occurring has not been precisely described, but is believed to be related to FXII autoactivation on substances like platelet polyphosphates, extracellular RNA, and or exposed collagen in the arterial wall [35–38]. To date, no investigation has shown formed FXIIa in developing or developed thrombus. Collagen exposure leads to FXII autoactivation which in flowing blood contributes to the extent of thrombus [38]. Inhibition of platelet activation or FXIIa activity in developing thrombus in flowing blood reduces thrombus formation [38]. These combined studies suggest that FXIIa inhibitors may reduce the extent of arterial thrombus in flowing blood. If so, FXIIa inhibitors may be clinically useful for thrombus reduction arising in coronary artery disease, thrombotic stroke, and peripheral vascular disease surgery.
The above investigations in murine models do not completely support, however, interpretations of patient studies. FXII deficiency was once thought to represent a risk for venous thrombus [53]. However a careful investigation in 350 Dutch patients with idiopathic deep venous thrombosis did not result in an increase in heterozygous factor XII deficiency over controls [54]. More recent investigations suggest that FXII may be related to coronary artery thrombosis [55, 56]. A polymorphism in which 46C to T substitution in the 5’-untranslated region of FXII gene resulting in reduced plasma FXII levels was recognized to confer protection from acute coronary syndrome and to lower thrombosis risk [57, 58]. Conflicting data were reported, however, suggesting that the TT genotype of this polymorphism is associated with higher risk of coronary disease [57, 59]. Results from the Study of Myocardial Infarction-Leiden (SMILE) project demonstrate an inverse relation between FXII levels and risk of myocardial infarction [60]. However, mortality for patients with severely low FXII levels (1–10% of normal) is similar to mortality for the median of the population [61]. These clinical data do not completely follow from the observations with FXII deleted mice. Low FXII does not protect from arterial thrombosis as would be predicted from the murine knockout investigations. This finding is not surprising because only 15–20% normal levels of FXII are sufficient to support all its surface-associated proteolytic activity. Recently Sabeter Lleal et al. described two new mutations, a C/G substitution at position-8 and a C/T substitution at position -17 of the FXII gene. Both mutations are located in the promoter region of the FXII gene in a putative binding site for the hepatocyte nuclear factor 4-α transcription factor (HNF4α) [62]. HNF4-a is a liver-enriched transcription factor that influences expression of FXII [63]. Each mutation summates with the 46C/T polymorphism to lower plasma FXII levels.
Summary
It remains essential to recognize and understand the role of FXII in order to interpret the mechanism for normal values of surface-activated blood coagulation assays. FXII may also be a contributor to the extent of arterial thrombosis. Inhibitors to FXII may have a role in reducing arterial thrombus formation in, e.g. coronary artery disease and arterial bypass surgery without increasing patient’s risk to bleed. Last, FXII is a growth factor and may have a role in tissue repair and post-natal angiogenesis after injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955 Apr;34(4):602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davie EW, Ratnoff OD. Waterfall Sequence for Intrinsic Blood Clotting. Science. 1964 Sep;18(145):1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 3.Ghebrehiwet B, Silverberg M, Kaplan AP. Activation of the classical pathway of complement by Hageman factor fragment. J Exp Med. 1981 Mar 1;153(3):665–676. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citarella F, Tripodi M, Fantoni A, Bernardi F, Romeo G, Rocchi M. Assignment of human coagulation factor XII (fXII) to chromosome 5 by cDNA hybridization to DNA from somatic cell hybrids. Hum Genet. 1988 Dec;80(4):397–398. doi: 10.1007/BF00273661. [DOI] [PubMed] [Google Scholar]
- 5.Cool DE, MacGillivray RT. Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region. J Biol Chem. 1987 Oct 5;262(28):13662–13673. [PubMed] [Google Scholar]
- 6.Petersen TE, Thogersen HC, Skorstengaard K, Vibe-Pedersen K, Sahl P, Sottrup-Jensen L, et al. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada KM. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- 8.Clarke BJ, Cote HC, Cool DE, Clark-Lewis I, Saito H, Pixley RA, et al. Mapping of a putative surface-binding site of human coagulation factor XII. J Biol Chem. 1989 Jul 5;264(19):11497–11502. [PubMed] [Google Scholar]
- 9.Samuel M, Samuel E, Villanueva GB. Histidine residues are essential for the surface binding and autoactivation of human coagulation factor XII. Biochem Biophys Res Commun. 1993 Feb 26;191(1):110–117. doi: 10.1006/bbrc.1993.1191. [DOI] [PubMed] [Google Scholar]
- 10.Pixley RA, Stumpo LG, Birkmeyer K, Silver L, Colman RW. A monoclonal antibody recognizing an icosapeptide sequence in the heavy chain of human factor XII inhibits surface-catalyzed activation. J Biol Chem. 1987 Jul 25;262(21):10140–10145. [PubMed] [Google Scholar]
- 11.Citarella F, Ravon DM, Pascucci B, Felici A, Fantoni A, Hack CE. Structure/function analysis of human factor XII using recombinant deletion mutants. Evidence for an additional region involved in the binding to negatively charged surfaces. Eur J Biochem. 1996 May 15;238(1):240–249. doi: 10.1111/j.1432-1033.1996.0240q.x. [DOI] [PubMed] [Google Scholar]
- 12.Citarella F, Fedele G, Roem D, Fantoni A, Hack CE. The second exon-encoded factor XII region is involved in the interaction of factor XII with factor XI and does not contribute to the binding site for negatively charged surfaces. Blood. 1998 Dec 1;92(11):4198–4206. [PubMed] [Google Scholar]
- 13.Baglia FA, Jameson BA, Walsh PN. Identification and characterization of a binding site for factor XIIa in the Apple 4 domain of coagulation factor XI. J Biol Chem. 1993 Feb 25;268(6):3838–3844. [PubMed] [Google Scholar]
- 14.Mahdi F, Madar ZS, Figueroa CD, Schmaier AH. Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood. 2002 May 15;99(10):3585–3596. doi: 10.1182/blood.v99.10.3585. [DOI] [PubMed] [Google Scholar]
- 15.Henderson LM, Figueroa CD, Muller-Esterl W, Bhoola KD. Assembly of contact-phase factors on the surface of the human neutrophil membrane. Blood. 1994 Jul 15;84(2):474–482. [PubMed] [Google Scholar]
- 16.Bradford HN, Pixley RA, Colman RW. Human factor XII binding to the glycoprotein Ib-IX-V complex inhibits thrombin-induced platelet aggregation. J Biol Chem. 2000 Jul 28;275(30):22756–22763. doi: 10.1074/jbc.M002591200. [DOI] [PubMed] [Google Scholar]
- 17.Hasan AA, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3615–3620. doi: 10.1073/pnas.95.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahdi F, Shariat-Madar Z, Todd RF, 3rd, Figueroa CD, Schmaier AH. Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001 Apr 15;97(8):2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 19.Rojkaer R, Schousboe I. Partial identification of the Zn2+binding sites in factor XII and its activation derivatives. Eur J Biochem. 1997 Jul 15;247(2):491–496. doi: 10.1111/j.1432-1033.1997.00491.x. [DOI] [PubMed] [Google Scholar]
- 20.Cool DE, Edgell CJ, Louie GV, Zoller MJ, Brayer GD, MacGillivray RT. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985 Nov 5;260(25):13666–13676. [PubMed] [Google Scholar]
- 21.Cohen S. Epidermal growth factor. In Vitro Cell Dev Biol. 1987 Apr;23(4):239–246. doi: 10.1007/BF02623704. [DOI] [PubMed] [Google Scholar]
- 22.Chien P, Pixley RA, Stumpo LG, Colman RW, Schreiber AD. Modulation of the human monocyte binding site for monomeric immunoglobulin G by activated Hageman factor. J Clin Invest. 1988 Nov;82(5):1554–1559. doi: 10.1172/JCI113765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon EM, Venkatesan N, Salazar R, Tang H, Schmeidler-Sapiro K, Buckley S, et al. Factor XII-induced mitogenesis is mediated via a distinct signal transduction pathway that activates a mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2174–2179. doi: 10.1073/pnas.93.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeidler-Sapiro KT, Ratnoff OD, Gordon EM. Mitogenic effects of coagulation factor XII and factor XIIa on HepG2 cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4382–4385. doi: 10.1073/pnas.88.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernando AN, Fernando LP, Fukuda Y, Kaplan AP. Assembly, activation, and signaling by kinin-forming proteins on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005 Jul;289(1):H251–H257. doi: 10.1152/ajpheart.00206.2004. [DOI] [PubMed] [Google Scholar]
- 26.Citarella F, te Velthuis H, Helmer-Citterich M, Hack CE. Identification of a putative binding site for negatively charged surfaces in the fibronectin type II domain of human factor XII--an immunochemical and homology modeling approach. Thromb Haemost. 2000 Dec;84(6):1057–1065. [PubMed] [Google Scholar]
- 27.Revak SD, Cochrane CG, Griffin JH. The binding and cleavage characteristics of human Hageman factor during contact activation. A comparison of normal plasma with plasmas deficient in factor XI, prekallikrein, or high molecular weight kininogen. J Clin Invest. 1977 Jun;59(6):1167–1175. doi: 10.1172/JCI108741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujikawa K, McMullen BA. Amino acid sequence of human beta-factor XIIa. J Biol Chem. 1983 Sep 25;258(18):10924–10933. [PubMed] [Google Scholar]
- 29.Dunn JT, Silverberg M, Kaplan AP. The cleavage and formation of activated human Hageman factor by autodigestion and by kallikrein. J Biol Chem. 1982 Feb 25;257(4):1779–1784. [PubMed] [Google Scholar]
- 30.Rojkjaer R, Hasan AA, Motta G, Schousboe I, Schmaier AH. Factor XII does not initiate prekallikrein activation on endothelial cells. Thromb Haemost. 1998 Jul;80(1):74–81. [PubMed] [Google Scholar]
- 31.Wiggins RC, Cochrane CC. The autoactivation of rabbit Hageman factor. J Exp Med. 1979 Nov 1;150(5):1122–1133. doi: 10.1084/jem.150.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate. Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J Biol Chem. 1992 Sep 25;267(27):19691–19697. [PubMed] [Google Scholar]
- 33.Chen X, Wang J, Paszti Z, Wang F, Schrauben JN, Tarabara VV, et al. Ordered adsorption of coagulation factor XII on negatively charged polymer surfaces probed by sum frequency generation vibrational spectroscopy. Anal Bioanal Chem. 2007 May;388(1):65–72. doi: 10.1007/s00216-006-0999-8. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008 Jun 5;358(23):2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006 Jan 24;103(4):903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maas C, Govers-Riemslag JW, Bouma B, Schiks B, Hazenberg BP, Lokhorst HM, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008 Sep;118(9):3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, Kuijpers MJ, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009 Jul 23;114(4):881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Yamamoto T, Kamata R, Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- 40.Kaminishi H, Hamatake H, Cho T, Tamaki T, Suenaga N, Fujii T, et al. Activation of blood clotting factors by microbial proteinases. FEMS Microbiol Lett. 1994 Sep 1;121(3):327–332. doi: 10.1111/j.1574-6968.1994.tb07121.x. [DOI] [PubMed] [Google Scholar]
- 41.Forbes CD, Pensky J, Ratnoff OD. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- 42.Pixley RA, Schmaier A, Colman RW. Effect of negatively charged activating compounds on inactivation of factor XIIa by Cl inhibitor. Arch Biochem Biophys. 1987 Aug 1;256(2):490–498. doi: 10.1016/0003-9861(87)90606-0. [DOI] [PubMed] [Google Scholar]
- 43.Stead N, Kaplan AP, Rosenberg RD. Inhibition of activated factor XII by antithrombin-heparin cofactor. J Biol Chem. 1976 Nov 10;251(21):6481–6488. [PubMed] [Google Scholar]
- 44.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., 3rd Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest. 2002 Apr;109(8):1057–1063. doi: 10.1172/JCI14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cichon S, Martin L, Hennies HC, Muller F, Van Driessche K, Karpushova A, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006 Dec;79(6):1098–1104. doi: 10.1086/509899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farsetti A, Misiti S, Citarella F, Felici A, Andreoli M, Fantoni A, et al. Molecular basis of estrogen regulation of Hageman factor XII gene expression. Endocrinology. 1995 Nov;136(11):5076–5083. doi: 10.1210/endo.136.11.7588244. [DOI] [PubMed] [Google Scholar]
- 47.Farsetti A, Moretti F, Narducci M, Misiti S, Nanni S, Andreoli M, et al. Orphan receptor hepatocyte nuclear factor-4 antagonizes estrogen receptor alpha-mediated induction of human coagulation factor XII gene. Endocrinology. 1998 Nov;139(11):4581–4589. doi: 10.1210/endo.139.11.6299. [DOI] [PubMed] [Google Scholar]
- 48.Inoue Y, Peters LL, Yim SH, Inoue J, Gonzalez FJ. Role of hepatocyte nuclear factor 4alpha in control of blood coagulation factor gene expression. J Mol Med. 2006 Apr;84(4):334–344. doi: 10.1007/s00109-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 49.Spronk H, Heemskerk JW, Ten Cate H, Knetch ML, Gailani D, Pawlinski R, et al. Feedback activation of factor XI by thombin is essential for haemostasis in vivo. Journal of Thrombosis and Haemostasis. 2009;7(s2):1–316. [Google Scholar]
- 50.Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- 51.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005 Jul 18;202(2):271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006 Mar 20;203(3):513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halbmayer WM, Mannhalter C, Feichtinger C, Rubi K, Fischer M. The prevalence of factor XII deficiency in 103 orally anticoagulated outpatients suffering from recurrent venous and/or arterial thromboembolism. Thromb Haemost. 1992 Sep 7;68(3):285–290. [PubMed] [Google Scholar]
- 54.Koster T, Rosendaal FR, Briet E, Vandenbroucke JP. John Hageman's factor and deep-vein thrombosis: Leiden thrombophilia Study. Br J Haematol. 1994 Jun;87(2):422–424. doi: 10.1111/j.1365-2141.1994.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 55.Santamaria A, Mateo J, Tirado I, Oliver A, Belvis R, Marti-Fabregas J, et al. Homozygosity of the T allele of the 46 C->T polymorphism in the F12 gene is a risk factor for ischemic stroke in the Spanish population. Stroke. 2004 Aug;35(8):1795–1799. doi: 10.1161/01.STR.0000133127.68041.a3. [DOI] [PubMed] [Google Scholar]
- 56.Soria JM, Almasy L, Souto JC, Bacq D, Buil A, Faure A, et al. A quantitative-trait locus in the human factor XII gene influences both plasma factor XII levels and susceptibility to thrombotic disease. Am J Hum Genet. 2002 Mar;70(3):567–574. doi: 10.1086/339259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zito F, Lowe GD, Rumley A, McMahon AD, Humphries SE. Association of the factor XII 46C>T polymorphism with risk of coronary heart disease (CHD) in the WOSCOPS study. Atherosclerosis. 2002 Nov;165(1):153–158. doi: 10.1016/s0021-9150(02)00196-x. [DOI] [PubMed] [Google Scholar]
- 58.Endler G, Mannhalter C, Sunder-Plassmann H, Lalouschek W, Kapiotis S, Exner M, et al. Homozygosity for the C-->T polymorphism at nucleotide 46 in the 5' untranslated region of the factor XII gene protects from development of acute coronary syndrome. Br J Haematol. 2001 Dec;115(4):1007–1009. doi: 10.1046/j.1365-2141.2001.03201.x. [DOI] [PubMed] [Google Scholar]
- 59.Colhoun HM, Zito F, Norman Chan N, Rubens MB, Fuller JH, Humphries SE. Activated factor XII levels and factor XII 46C>T genotype in relation to coronary artery calcification in patients with type 1 diabetes and healthy subjects. Atherosclerosis. 2002 Aug;163(2):363–369. doi: 10.1016/s0021-9150(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 60.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006 Dec 15;108(13):4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 61.Gailani D, Renne T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007 Jun;5(6):1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 62.Sabater-Lleal M, Chillon M, Mordillo C, Martinez A, Gil E, Mateo J, et al. Combined cis-regulator elements as important mechanism affecting FXII plasma levels. Thromb Res. 2009 Sep 26; doi: 10.1016/j.thromres.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sladek FM, Ruse MD, Jr., Nepomuceno L, Huang SM, Stallcup MR. Modulation of transcriptional activation and coactivator interaction by a splicing variation in the F domain of nuclear receptor hepatocyte nuclear factor 4alpha1. Mol Cell Biol. 1999 Oct;19(10):6509–6522. doi: 10.1128/mcb.19.10.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMullen BA, Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor) J Biol Chem. 1985 May 10;260(9):5328–5341. [PubMed] [Google Scholar]
- 65.Schousboe I. Contact activation in human plasma is triggered by zinc ion modulation of factor XII (Hageman factor) Blood Coagul Fibrinolysis. 1993 Oct;4(5):671–678. [PubMed] [Google Scholar]
- 66.Ravon DM, Citarella F, Lubbers YT, Pascucci B, Hack CE. Monoclonal antibody F1 binds to the kringle domain of factor XII and induces enhanced susceptibility for cleavage by kallikrein. Blood. 1995 Dec 1;86(11):4134–4143. [PubMed] [Google Scholar]