Abstract
Obtaining high quality fresh or frozen prostate tissue in gram amounts promotes the research of prostate cancer. Due to the inability to effectively recognize prostate cancer upon the cut section of a fresh prostate many pathology departments perform whole organ processing precluding the procurement of prostate tissue. Some sites randomly procure tissue, while others perform specialized procedures that can be difficult to duplicate. Neither of these types of procurement consistently results in gram quantities of tissue procured from each prostate with a high percentage of samples yielding tissue corresponding to cancer. In this study, we present a simplified model of prostate tissue procurement that has no impact on Gleason grade, tumor stage or margin status, requires minimal specialized processing, and obtains gram amounts of fresh tissue from each radical prostatectomy specimen with a high percentage yielding cancer tissue.
Keywords: prostate, procurement, radical prostatectomy, RNA
INTRODUCTION
Obtaining high quality fresh or frozen prostate tissue in gram amounts can be a difficult task. Needle core biopsies are too small and always processed in entirety for clinical pathological examination and diagnosis. Transurethral resection specimens often show extensive thermal degeneration and generally have cancer only in patients with extensive disease. Radical prostatectomy (RP) specimens are the only reasonable choice for such procurement, but there are many barriers to effective procurement of such specimens.
The single largest barrier to effective prostate cancer tissue procurement at most academic centers is the inability to effectively recognize prostate cancer upon the cut section of a fresh prostate. Consequently many pathology departments process the entire RP specimen for evaluation of Gleason grade, tumor stage and margin status. Whole-organ tissue processing for RP specimens with close-step sectioning by histology (0.5-cm intervals or less) versus less than complete examination has been examined in at least two large studies [1,2]. While these two studies showed some conflicting results for Gleason grade, tumor stage and margin status, both studies showed merit in complete examination of margins for RP specimens. One of these studies [1] demonstrated no significant differences between evaluations of patient specimens in the step-sectioned and non-step-sectioned groups with respect to pathologic tumor stage, prostatectomy Gleason score, or margin status. The other study [2] showed higher detection rates of extra-prostatic extension and seminal vesicle invasion in the close step-sectioned group, but did not show any difference in survival rates for this group of patients based upon method of specimen evaluation.
Several specialized methods of prostate tissue procurement have been published [3–6]. All of these studies require either specialized processing or acquire minimal amounts of tissue using ex vivo biopsy techniques. None of these techniques have been accepted in general pathology practice. In this study, we present a simplified model of prostate tissue procurement that has no impact on Gleason grade, tumor stage or margin status, requires minimal specialized processing, and obtains gram amounts of fresh tissue from each RP specimen with a high percentage yielding cancer tissue.
MATERIALS ANDMETHODS
Patients
Fresh tissue was harvested from one hundred consecutive patients with a pre-operative diagnosis of prostatic adenocarcinoma and with at least 5% involvement of one needle core biopsy by cancer. All patients were consented for tissue procurement prior to a robotic RP.
Tissue Procurement Process
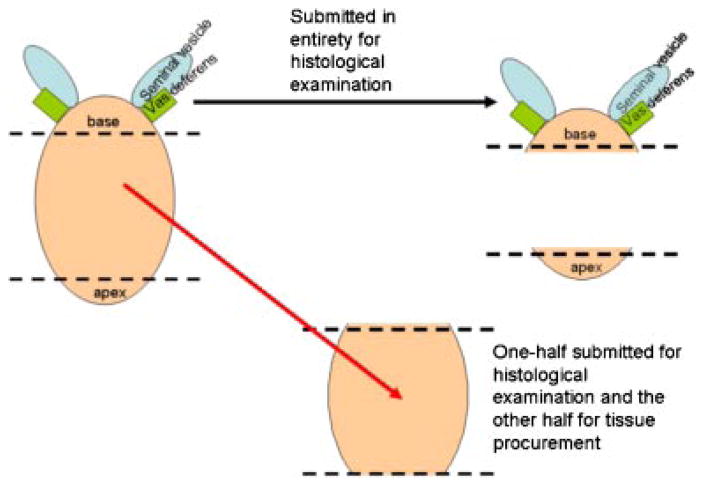
Immediately after arrival in the pathology grossing laboratory the prostate was weighed, oriented, described and inked according to protocol. One to 1.5 cm of the apex (inferior margin) was transected from the main specimen perpendicular to the prostatic urethra and was not eligible for tissue procurement. One to 1.5 cm of the base, including the bladder neck margin, seminal vesicles and vas deferens was transected from the specimen perpendicular to the prostatic urethra and was also not eligible for tissue procurement (Fig. 1). The remainder (central portion) of the prostate was sectioned at 4- to 5-mm intervals perpendicular to the prostatic urethra from the apex to the base, resulting in multiple sections of tissue from different levels of the prostate (Fig. 2). Every other level was submitted in entirety for clinical examination. The intervening levels were divided into quadrants, the capsule was removed and submitted for clinical examination, and the central portion was submitted for tissue procurement (Fig. 3). Each quadrant of each slice submitted to tissue procurement was halved parallel to the longest axis with one half mounted in OCT and the other half collected as fresh tissue in the appropriate media and immediately refrigerated. An H&E stained frozen section of the half mounted in OCT was immediately done and reviewed by a pathologist for evaluation of cancer versus benign. Areas of cancer were circled to guide macro-dissection of both fresh and frozen tissue by the procurement agent. The majority of prostates yield two-to-three slices (levels) for procurement for research, each with four quadrants of tissue resulting in eight to twelve frozen sections performed for each RP specimen. Macro-dissected tissue from each of the quadrants was organized and distributed as four categories: fresh benign, fresh malignant, frozen benign, or frozen malignant.
Fig. 1.
After the base and apex are removed the central portion of the prostate can be procured from excluding the capsule. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 2.
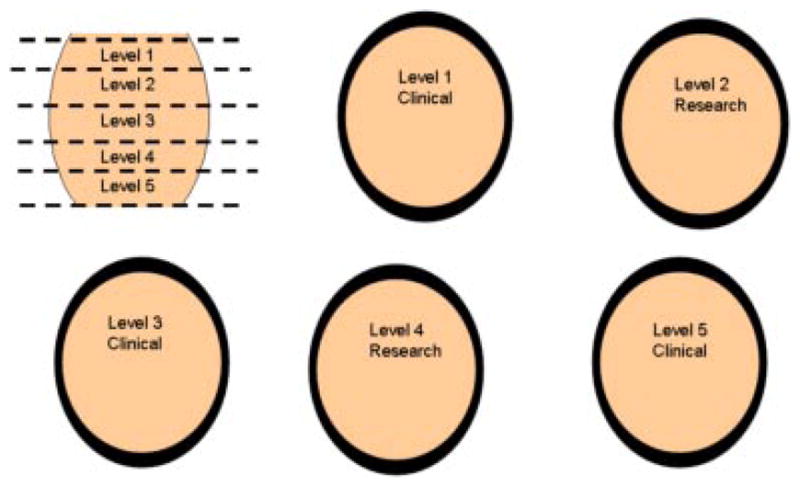
Every other level of the central portion of the prostate excluding the capsule is eligible for procurement.[Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 3.
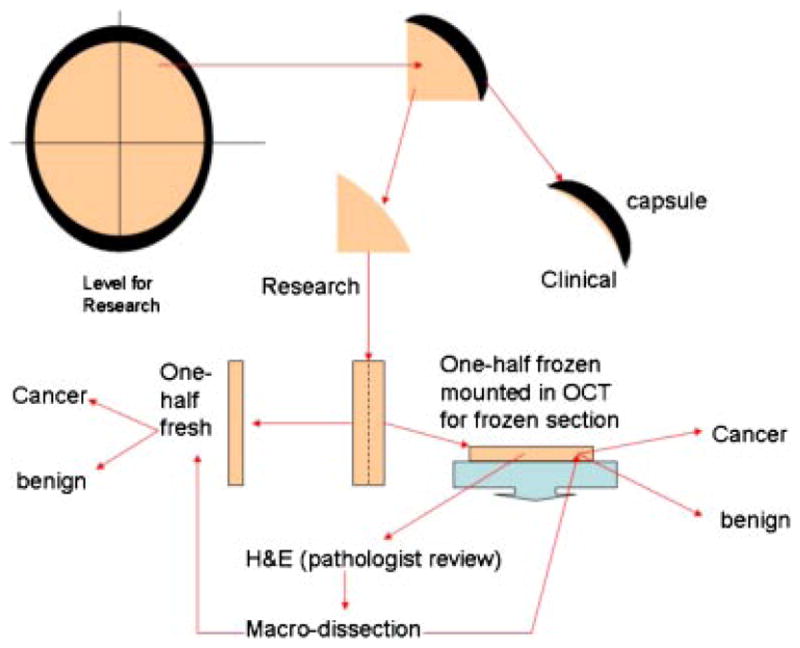
Division of levels into quadrants that are subsequently divided into fresh and frozen specimens. Quality control that directs macro-dissection is performed from review of a stained section cut from the frozen tissue.[Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
RESULTS
One-hundred seventeen consecutive patients that underwent an RP at Roswell Park Cancer Institute (RPCI) were evaluated to allow procurement of tissue from 100 prostatectomy specimens since tissue was not procured from 17 patients with a pre-operative diagnosis of prostatic adenocarcinoma but had less than or equal to 5% cancer present in only one diagnostic needle core biopsy. For 100 consecutive RP specimens with tissue procured, the average prostate weight was 53.2 g (median 49.0) (Table I). After removal of the apex, base, seminal vesicles and vas deferens, the average weight of the central portion of the prostate was 28.5 g (median 25.5). An average of 11.1 g (median 9.3) of tissue was procured from each prostate that consisted of an average of 42% (median 39%) of the central prostate that was eligible for procurement. Cancer was identified in 72 of 100 procured research specimens and an average of 0.9 g (median 0.5) of macro-dissected cancer was procured for each of these specimens. Cancer was present in 99 of 100 of the clinical cases upon surgical pathological examination with the percent gland involvement ranging from 1% to 70% (average 19; median 15). The one case in which no cancer was identified in the clinical specimen was also negative for cancer in the matching procured tissue. Importantly, there were no discrepancies between Gleason grade of procured cancer tissue compared to that reported in the surgical pathology specimen for any patient. Furthermore, since there was no procurement of tissue from the apex, base, seminal vesicles or capsule there was no impact on assessment of margin status or extra-capsular extension.
TABLE I.
Summary of Procurement from 100 Consecutive RP Specimens
| Total prostate weight (g) | Weight of prostate excluding apex, base, seminal vesicles and vas deferens | Total tissue procured (g) | Percent of central prostate procured (%) | Procured benign tissue (g) | Procured tumor (g) | Percent gland involvement of clinical specimen (%) | |
|---|---|---|---|---|---|---|---|
| Average | 53.2 | 28.5 | 11.1 | 42 | 10.1 | 0.9 | 19 |
| Median | 49.0 | 25.5 | 9.3 | 39 | 8.5 | 0.5 | 15 |
| Range | 25–196 | 11–79 | 2.72–72.2 | 17–72 | 2.15–72.2 | 0.3–8.2 | 1–70 |
Of the 17 patients not eligible for tissue procurement, cancer was present upon clinical surgical pathological examination in all patients and the percent gland involvement ranged from 1% to 25% (average 12; median 15).
Forty-four of 72 frozen cancer specimens were chosen randomly for RNA isolation with a standard Trizol protocol Using 100–200 mg of tissue the average yield of RNA per specimen was 122 μg (median 117; range 33–214), and all but one specimen yielded good quality RNA using the Agilent Bioanalyzer 2100 platform as a reference standard. The average RNA Integrity Number (RIN) was 7.4 (median 7.5; range 6.1–8.6). RIN values above 7 are acceptable for gene expression studies [7].
DISCUSSION
Our model of prostate procurement is simple, yields gram amounts of fresh and frozen tissue for research, and has minimal impact on the clinical surgical pathological examination. The successful procurement of prostate cancer tissue for research from 72% of RP specimens was remarkably better than expected. Furthermore, the success in providing macro-dissected benign and near 100% pure cancer tissue to researchers facilitates basic research in prostate cancer as well as enabling preparation of RNA and DNA from areas of pure cancer from small specimens.
The disadvantages of this procurement model are the time required, and the need for dedicated pathology faculty and staff. At Roswell Park Cancer Institute, the procurement process is initiated by a fully trained and licensed pathologist assistant that performs the initial prostate assessment and divides the prostate into clinical and research levels, a process that requires several additional minutes beyond the standard grossing procedure. A procurement agent subsequently divides the research levels into quadrants, embed and freeze one-half of each quadrant in OCT, and transport the specimen to a central research histology facility for cutting of frozen sections. Finally, a trained histologist cuts 8–12 frozen sections from each specimen. This entire process often takes 25–30 min to complete. A pathologist immediately reviews these frozen section slides and marks them for macro-dissection into benign and cancer for the procurement agent. In summary, the entire process requires 30–45 min and a dedicated pathology staff.
The high yield of good quality RNA from the majority of these specimens was remarkable considering the delay due to the tissue procurement process and that all of these RP were robotically performed. The impact on downstream processing of the fresh component of the procured tissue was minimized by placing the matching fresh tissue is in transport media during the processing of the frozen component.
The exclusion for procurement of patients for whom there was less than, or equal to, 5% cancer present in only one diagnostic biopsy may not be valid. There was no statistical difference between the average and median percent gland involvement by cancer in these patients versus those from whom tissue was procured. All of the 17 patients with less than or equal to 5% cancer in only a single diagnostic biopsy had cancer in the final surgical pathological evaluation. Similarly, patients for whom tissue was procured had cancer in 99 of 100 cases in the final surgical pathological evaluation. The lack of cancer in at least 1% of the final surgical pathology specimens is not an unexpected finding [8].
In summary, gram amounts of prostate tissue can be procured successfully from RP specimen, with greater than 70% of procured specimens having sufficient cancer present that it can be macro-dissected for research. Importantly, no negative impact on Gleason grading, pathologic stage or margin status was identified. Our protocol requires a dedicated effort by the department of pathology, and a team effort spanning the entire process from surgeon to researcher to be effective. While the protocol for the tissue procurement process is relatively simple, the hurdles to organization of the overall process should not be underestimated.
References
- 1.Grossfeld GD, Chang JJ, Broering JM, Miller DP, Yu J, Flanders SC, Carroll PR. Does the completeness of prostate sampling predict outcome for patients undergoing radical prostatectomy?: Data from the CAPSURE database. Urology. 2000;56:430–435. doi: 10.1016/s0090-4295(00)00705-6. [DOI] [PubMed] [Google Scholar]
- 2.Desai A, Wu H, Sun L, Sesterhenn IA, Mostofi FK, McLeod D, Amling C, Kusuda L, Lance R, Herring J, Foley J, Baldwin D, Bishoff JT, Soderdahl D, Moul JW. Complete embedding and close step-sectioning of radical prostatectomy specimens both increase detection of extra-prostatic extension, and correlate with increased disease-free survival by stage of prostate cancer patients. Prostate Cancer Prostatic Dis. 2002;5:212–218. doi: 10.1038/sj.pcan.4500600. [DOI] [PubMed] [Google Scholar]
- 3.Furman J, Murphy WM, Rice L, Drew PA, Narayan P. Prostatectomy tissue for research: Balancing patient care and discovery. Am J Clin Pathol. 1998;110:4–9. doi: 10.1093/ajcp/110.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Bova GS, Fox WM, Epstein JI. Methods of radical prostatectomy specimen processing: A novel technique for harvesting fresh prostate cancer tissue and review of processing techniques. Mod Pathol. 1993;6:201–207. [PubMed] [Google Scholar]
- 5.Wheeler TM, Lebovitz RM. Fresh tissue harvest for research from prostatectomy specimens. Prostate. 1994;25:274–279. doi: 10.1002/pros.2990250507. [DOI] [PubMed] [Google Scholar]
- 6.Walton TJ, McCulloch TA, Rees RC, Bishop MC. Obtaining fresh prostate cancer tissue for research: A novel biopsy needle and sampling technique for radical prostatectomy specimens. Prostate. 2005;64:382–386. doi: 10.1002/pros.20264. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiGieuseppe JA, Sauvageot J, Epstein JI. Increasing incidence of minimal residual cancer in radical prostatectomy specimens. Am J Surg Pathol. 1997;21:174–178. doi: 10.1097/00000478-199702000-00006. [DOI] [PubMed] [Google Scholar]