Abstract
Podocytes play a key role in the maintenance of glomerular filtration barrier. Depletion or dysregulative mechanisms of podocytes can lead to the development of glomerulosclerosis. Signaling pathways that control these processes in podocytes are not fully understood. Recent studies from our and other laboratories found that genes that belong to the Notch pathway are regulated in patients and in animal models of renal disease. Genetic studies performed on mice with conditional expression of active Notch1 protein showed massive albuminuria, glomerulosclerosis ultimately renal failure and death of the animals. Gamma secretase inhibitors and genetic deletion of Notch transcriptional binding partner (Rbpj) protected animals from nephrotic syndrome. Further studies are needed to define whether the activation of Notch pathway in podocytes represents a common pathomechanism in glomerular injury and its potential to be a therapeutic target for the treatment of chronic kidney disease.
Keywords: Notch, Podocytes, Glomerulosclerosis, Albuminuria, Gamma secretase inhibitors
PODOCYTES PLAY A CRITICAL ROLE IN GLOMERULAR DISEASES
Glomerular visceral epithelial cells (podocytes) are located at the outer surface of the GBM and their foot processes (FP) are anchored to the GBM (1, 2). Neighboring foot processes are connected by a specialized cell-cell junction, the glomerular slit diaphragm (SD), which represent the main size selective filtering barrier. Alterations of the foot process and slit diaphragm configuration result in FP effacement and lead to the development proteinuria. Podocyte injury plays an important role in glomerular diseases and progressive nephron loss. Kriz et al. described three mechanisms of podocyte injury; the dysregulative, the inflammatory and the degenerative mechanisms(3).
The collapsing form of focal segmental glomerulosclerosis (FSGS) is a typical example of the dysregulative type of podocyte damage. In this case dedifferentiation of podocytes leads to regulatory defect. This is followed by cell proliferation in the Bowman’s space which leads to the collapse of the glomerulus(3). During various inflammatory glomerulonephritis abnormal activity of podocytes result in the fixation of podocytes to the parietal basement membrane followed by an establishment of tuft adhesion to the Bowman’s capsule leading to the formation of cellular crescents(3). In models of degenerative glomerular disease the progressive loss of glomerular podocytes is the culprit of the disease. Depletion of glomerular podocytes leads to the fixation of the parietal cells to the GBM followed by the establishment of tuft adhesions to the Bowman’s capsule. In the case of healing by fibrosis the lesion turns into segmental glomerulosclerosis. Toxic and metabolic glomerular injury models including the puromycin aminonucleotide (PAN), doxorubicin and diabetes induced nephrotic syndrome models lead to progressive renal damage via degenerative mechanisms(3).
PODOCYTE DEPLETION IN RENAL DISEASE
Detachment of glomerular epithelial cells has been documented in case of FSGS and membranous nephropathy of humans(4). A recent study using streptozotocin-treated rats suggests that podocytes may detach from GBM in this experimental diabetic nephropathy (DNP) model, leading to loss of podocytes into the urinary space(5). These investigators were able to culture cells from the urine expressing typical podocyte markers, indicating that some podocytes may remain viable after detachment(4, 6).
Apoptosis of resident glomerular podocytes has recently been proposed as a cellular mechanism that may underlie podocyte loss in non-diabetic glomerulopathies(7, 8) in case of the progressive glomerulosclerosis in TGFβ-1 transgenic mice(9), the CD2AP-/- mice (10) and the PAN nephropathy model(11). Interestingly, podocyte apoptosis precedes endocapillary and tubular epithelial apoptosis in these models. Since glomerular epithelial cells are unable to divide, therefore apoptosis or/and detachment of the cells will result in depletion of glomerular podocytes. However, it is not known whether podocyte apoptosis occurs in DNP and whether it contributes to the development of albuminuria and mesangial expansion, hallmarks of human and experimental DNP. It is also unclear whether there is a relationship between apoptosis and detachment as generally apoptotic cells might detach from the basement membrane and vice versa cells that detach from a GBM usually die (12, 13).
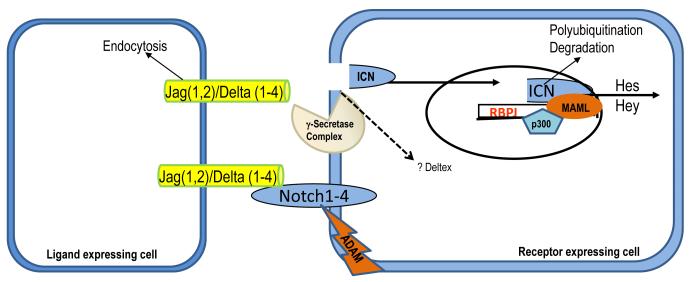
THE NOTCH SIGNALING PATHWAY was initially identified in Drosophila when a mutation in Notch was found to cause an increase in the number of neuroblasts at the expense of epidermal cells (14). This pathway is present in all metazoa and it functions as one of the major pathways that determine cell identity during development (15). Notch is a transmembrane protein that interacts with ligands of the Jagged and Delta family (16). There are four Notch members in mammals (Notch 1–4), two Jagged (Jag), and four Delta-like genes (17). Each of this protein shows a cell type- and tissue-specific expression during development. Notch is made in the endoplasmic reticulum as pre-Notch. O-fucose transferase (OFUT1) functions as a chaperone to transport Notch from the endoplasmic reticulum to the Golgi for glycosylation and fucosylation. A furin like convertase cleaves pre-Notch to intracellular and extracellular domain. This protein is then transported to the plasma membrane. Interaction of these ligands with Notch triggers a series of proteolytic cleavage, by ADAM proteases (S2) and finally by the gamma secretase complex. This final cleavage releases the Notch intracellular domain (ICN1), which is a transcription factor. Transport of ICN1 to the nucleus allows it to bind to other transcriptional activators, including RBP-Jk, MAML, p300 and the complex then mediates the transcription of various proteins including Hes and Hey family members, which are transcription factors by themselves to mediate the program of cell identity (Figure 1) (18), (19-21). In the absence of ICN1, RBP-Jk binds to a number of co-repressor molecules that repress transcription from the DNA bound to RBP-Jk (22). ICN1 later undergoes ubiquitination and proteosome mediated degradation.
Figure 1.
Schematic representation of the Notch signaling pathway. Upon Delta or Jagged activation Notch receptor undergoes proteolytic cleavage dependent on gamma secretase activity. Notch intracellular domain (NICD) translocates to the nucleus, and binding to Rbp (also known as CSL), activates the dissociation of co-repressor complex resulting in induction of Hes and other target genes. ICN1 later undergoes ubiquitination and proteosome mediated degradation.
The regulation of the Notch pathway also appears to be complex and it occurs at many different levels. The most important of all appears to be the ligand binding, followed by the gamma secretase mediated cleavage. In recent years the gamma secretase complex received significant attention as potential therapeutic target for Alzheimer disease and cancer. Multiple different compounds have been developed that target the gamma secretase complex. They have been extensively tested in animals and some of them are in Phase III clinical trials for Alzheimer’s disease and breast cancer (23).
THE NOTCH PATHWAY CONTROLS CELL DIFFERENTIATION, PROLIFERATION AND APOPTOSIS IN A CELL CONTEXT DEPENDENT MANNER
The Notch signaling pathway plays a critical role in cellular differentiation and organ development (including kidney, pancreas etc.). In diverse developmental context Notch signaling has been associated with amplification of some somatic stem cells, such as the neural and hemapoetic stem cells. The function of Notch is highly context dependent (24-27). For example, within the hemato-lymphoid compartment, constitutively overactive Notch signaling can be observed in large proportion of T-cell malignancies, and recent data identified Notch activating mutations as the most frequent event in human T-cell leukemia (28). In malignant T-cells, Notch signaling influences proliferation, differentiation and survival (29, 30). However, Notch receptor expressed in malignant B-cells resulting in constitutive Notch signaling leads to growth inhibition and apoptosis (31).
Experimental evidence supports the idea that signaling pathways essential for embryonic development also have a role in regulating self-renewing tissues (32, 33). Mutations in these pathways (such as TGFB, Wnt, and ErbB) often lead to tumorigenesis, as is also true for Notch. An interesting aspect of Notch is its apparently opposite functions in tumor development, because it can act as an oncogene or as a tumor suppressor. Several studies suggest that Notch activation plays and important oncogenic role in breast and intestinal cancer development (17, 33-35). Notch plays differential role in two types of skin cancer. Notch inhibits the development of keratinocyte-derived cancer(36), while it acts as oncogenic in melanomas(37). Very little is known about the role of Notch signaling in mature epithelial cells. For example in neuronal cells, Notch1 signaling inhibits growth through induction of cell cycle arrest and apoptosis via up-regulation of pro-apoptotic proteins including p53 and activation of JNK kinase (38).
ROLE OF NOTCH SIGNALING PATHWAY DURING RENAL DEVELOPMENT
Detailed analysis of the expression pattern of Notch and related genes during nephrogenesis have been performed (39-41). These studies have been excellently summarized recently by Kopan RT et al. (42). Notch1, Notch2, Dll1 and Jag1 mRNA are detected in the renal vesicles and its derivative. Expression of Notch1 partially overlaps with Notch2 in the S-shaped body (41). Notch2 and Jag1 are also expressed in the collecting duct. Notch3 expression has been reported in the distal portion of the S-shaped body (39). Notch 4 was mainly detected in endothelial cells.
Notch pathway proteins are not only expressed in the developing kidney, but they seem to play an important role in cellular differentiation. Humans haploinsufficient for jagged 1 (43) are prone to Alagille syndrome, one symptom of which can result in the development of renal abnormalities, whereas abnormal glomerulogenesis is also observed when Notch2 activity is reduced(44, 45). Mouse metanephroi cultured in the presence of a γ-secretase inhibitor (DAPT), to block Notch signaling, resulted in reduced ureteric bud branching but normal mesenchymal condensation and expression of markers indicating that mesenchyme induction had occurred. However, fewer renal epithelial structures were observed, with a severe deficiency in proximal tubules and glomerular podocytes, which are derived from cells in which activated Notch1 is normally present. Distal tubules were present but in reduced numbers, and this was accompanied by an increase in intervening, non-epithelial cells(46). Recently Cheng et al also showed that both Notch1 and Notch2 are detected in the early renal vesicle, however, Notch2 acts non-redundantly to control the processes of nephron segmentation through an Rbpj-dependent process. Genetic analysis reveals that only Notch2 is required for the differentiation of proximal nephron structures (podocytes and proximal convoluted tubules) despite the presence of activated Notch1 in the nuclei of putative proximal progenitors(47). Similar results were obtained when presenilin (an enzyme that is part of the gamma secretase complex) knock-out mice were studied (48). These observations suggest that the Notch pathway activation is required for the progression of renal vesicles to comma-and S-shaped bodies and determining the proximal tubule and podocyte fates.
ROLE OF NOTCH IN THE PATHOGENESIS OF GLOMERULOSCLEROSIS
In order to better understand the mechanism of glomerulosclerosis we performed gene expression studies (using microarrays) on kidney samples obtained from animal models of diabetes and DNP and also on kidney samples from patients with renal disease(49, 50). With a combined expression and pattern recognition analysis we found that genes that belong to the Notch pathway are regulated in glomeruli of mice with diabetic renal disease and in patients with DNP and FSGS (51). This observation led us to further study the potential role of Notch pathway in glomerular disease. We confirmed the increased Notch expression with immunohistochemistry studies as well. We found increased expression of Notch intracellular domain in podocytes of patients and mice with DNP and FSGS. Similar analysis performed by Walsh et al. also found increased Notch, Jagged and TGFβ expression in the tubulointerstitial compartment of human DNP samples, when they compared to control human kidney samples (52).
In order to study the in vivo function of increased Notch expression in glomerular podocytes, we used a unique in vivo podocyte specific titratable active Notch overexpression system. This was achieved by intercrossing of two already available and validated mouse models, the podocin rtTA (reverse tetracycline transactivator) (PodrtTA) mice that express the tetracycline inducible transactivator gene under the podocin promoter (53) with the tet-O-ICN1 mice (35). By intercrossing these two mouse strains NICD can be specifically induced in podocytes by tetracycline. Upon administration of tetracycline or doxycycline (dox), the rtTA (in podocytes) binds to the tetracycline responsive elements in the tet-O-ICN1 mice and regulates the expression ICN1. ICN1 can be turned on and off conveniently with the administration or withdrawal of doxycycline.
Renal development was normal in the PodrtTA/Tet-O-ICN1 mice; we did not observe albuminuria, glomerular or tubular abnormalities on PAS staining, even at 10 weeks of age without dox administration. Doxycycline containing food was administered starting at 3-4 weeks of age, after completion of kidney development. Immunohistochemistry analysis showed increased val1744 Notch1 staining (i.e. ICN1 expression) in glomerular podocytes of dox treated double transgenic animals compared to wild type animals(51).
As early as 7 days following the initiation of doxycycline treatment the podrtTA/Tet-O-ICN1 mice developed proteinuria. After 2 weeks treatment with doxycycline containing chow, animals developed severe albuminuria reaching 5,000 μg/mg albumin/creatinine. Albuminuria was around 50 μg/mg albumin/creatinine in doxycycline treated wild type, tet-O-ICN1 or podrtTA mice or podrtTA/tet-O-ICN1 mice that were not treated with doxycycline. Histological examination of the kidneys showed severe glomerular abnormalities. The early lesions were characterized by diffuse mesangial matrix accumulation in the glomerulus. More advanced lesion showed segmental sclerosis of some of the glomeruli most which was most similar to FSGS of humans. Control animals showed no histological abnormalities. Immunohistochemistry showed severe reduction (almost absence) of podocyte specific markers i.e. nephrin and WT-1, indicating severe decrease in podocyte number or loss of specific markers on existing podocytes. Therefore electron microscopy (EM) analysis was performed to evaluate the podocytes. EM analysis showed severe reduction in podocyte number and almost complete effacement of podocyte foot processes. Endothelial cells appeared normal and fenestrated. No immundeposits were observed in the glomerulus. Interestingly we also observed remnants of dead cells occasionally. These cell remnants showed nuclear condensation, with a perinuclear rim, which could be consistent with apoptotic cell bodies. In order to further evaluate whether podocyte apoptosis occurs in this animal model we performed TUNEL staining in control and double transgenic animals. We found an increase in TUNEL positive staining in nuclei of glomerular podocytes of doxycycline treated double transgenic animals compared to wild type mice. We also found the pro-apoptotic genes, including trp53, Bax and Apaf expression were significantly increased in double transgenic animals. In separate cell culture experiment we confirmed the pro-apoptotic role of active Notch1 in podocytes. This proof of principle study establishes that Notch activation in podocytes is “pathogenic“, it is alone sufficient to induce podocyte foot process effacement, podocyte depletion and subsequently albuminuria and glomerulosclerosis.
Waters et al performed a similar experiment (54), using different mouse strains, when the expression of active Notch1 domain was turned on during the capillary loop stage of development. They used the nephrincre and STOP-NotchIC mice to achieve their goal. Histologic and molecular analyses revealed normal glomerular morphology and expression of podocyte markers in newborn podocyte specific NOTCH-IC-expressing mice. At 2 weeks of age animals developed profound albuminuria, glomerulosclerosis and early mortality due to end stage renal failure in these animals. Upon histological analysis of this model they found severe foot processes effacement, loss of expression of Wt1, nephrin and podocin and the expression of Pax2 was increased. The renal histology was more consistent with diffuse mesangial sclerosis-like disease. In addition, in contrast to our studies they found that podocytes expressing active Notch1 undergo massive proliferation. The damaging effects of NOTCH-IC expression were prevented in transgenic mice after simultaneous conditional inactivation of Rbpj in murine podocytes using the Rbpjflox mice.
There are many similarities in the two studies described above, including the rapid development of albuminuria, foot process effacement, loss of Wt1, nephrin and podocin expression and the development of glomerulosclerosis in both models. However, it appears that while active Notch1 expression induced apoptosis in mature podocytes it lead to their proliferation, if it was expressed prior to their full differentiation. It is not clear whether this differential response was induced by the different degree of Notch1 expression or by the different timing of Notch1 expression. Nevertheless it appears that Notch1 can act on different target genes in developing and in mature (fully differentiated) podocytes and emphasize the critical spatial and temporal role of this pathway.
At present there is no specific cure for most forms of acquired chronic kidney disease. Therefore we explored whether inhibition of the Notch pathway can influence glomerular disease. The critical and regulated step in Notch activation is the gamma secretase complex mediated cleavage of the receptor. During the last few decades many different inhibitors have been developed that block this enzyme complex. Many of these compounds are tested in early PhaseI and II trials and appear to be relatively safe in humans.
Our next question was whether or not Notch activation contributes to the development of albuminuria or glomerular disease, by performing a loss of function experiments. Our experiments showed that gamma secretase inhibitor DBZ, inhibits increased cleaved Notch1 accumulation, Hes and Hey mRNA increase and podocyte apoptosis. Chronic DBZ treatment of mice is shown to be safe and well tolerated (55). In this initial experiment we used the puromycin aminonucletide (PAN) induced albuminuria model of rats. PAN treatment of rats leads to the development of nephrotic syndrome and it is a well established model of podocyte apoptosis, detachment and podocyte depletion (11).
Next we examined mRNA levels of Notch pathway genes in glomerular extracts of PAN treated rats. Similar to the diabetic animal model, we found a significant increase in Notch1, 2 and Notch target genes Hes1,5, Hey1 levels in glomeruli, 6 days following single 20mg/kg PAN injection of Sprague Dawley rats (n=10/group). The mRNA increase was more pronounced in this model and we observed an approximately 10-15 fold increase in Notch1 levels compared to control rats. Val1744Notch1 and Hes1 immunostaining was also increased in glomeruli of PAN treated rats. Most of the staining appeared to be in glomerular podocytes, however some endocapillary staining can not be excluded.
PAN treated rats developed albuminuria already starting from day4. Chronic DBZ treatment blocked the increase in Notch pathway gene expression in isolated glomeruli, indicating the effectiveness of the treatment. Rats treated with DBZ had significantly less albuminuria than control animals. DBZ treatment also effectively protected rats from the development of podocyte foot process effacement as shown by EM analysis(51). We also performed TUNEL staining to examine podocyte apoptosis. Our results indicate that PAN+DBZ treated rats had less podocyte apoptosis compared to PAN treated animals. DBZ treatment was not only effective when it was initiated before the PAN injection but it effectively reduced albuminuria after the development of albuminuria.
While our studies show a strong correlation between DBZ treatment and Notch activation, from the current studies we can not exclude the possibilities that other gamma secretase dependent mechanisms are responsible for the effect of DBZ. The specificity of the gamma secretase inhibitors including potential effects on CD44, erbB4 and N-cadherin cleveage are currently being addressed in our laboratory(56-58). However it should be noted that currently no information is available regarding the role of any these pathways in podocytes and in nephrotic syndrome.
In addition, we also performed studies on mice with podocyte specific genetic deletion of Rbpj (thereby Notch signaling). Diabetic mice with podocyte specific deletion of Rbpj showed lower proteinuria and less podocyte damage, when compared to wild type diabetic mice. These studies again confirm that podocyte specific Notch activation plays a critical role in the development proteinuria and glomerular damage.
In summary, we can conclude that gamma secretase and Rbpj dependent mechanisms plays a critical role in the development of podocyte damage and proteinuria, suggesting that Notch activation and the development of proteinuria and podocyte damage are causally linked. Therefore the gamma secretase (Notch) pathway appears to be a useful novel therapeutic target for the cure of podocyte damage, proteinuria.
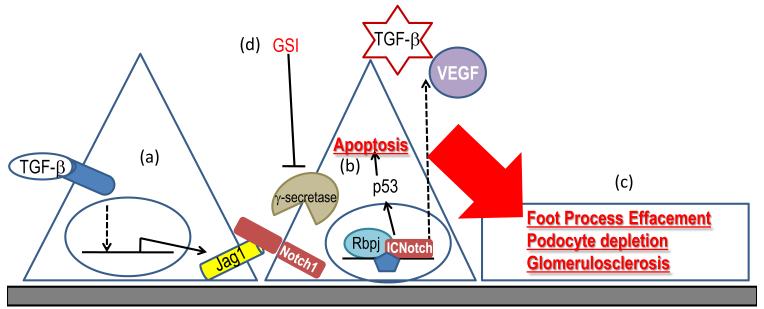
Figure2.
a. TGFβ1 leads to Notch pathway activation in podocytes via directly inducing Notch ligand Jag1
b. Active Notch1 expression in podocytes leads to podocyte apoptosis via inducing p53
c. Conditional expression Notch intracellular domain in vivo in podocytes is sufficient to induce foot process effacement albuminuria and glomerulosclerosis
d. Blocking Notch activation with γ-secretase inhibitors (GSI) ameliorates albuminuria and renal damage in animal models
REFERENCES
- 1.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 2.Mundel P, Shankland SJ. Glomerular podocytes and adhesive interaction with glomerular basement membrane. Exp Nephrol. 1999;7:160–166. doi: 10.1159/000020596. [DOI] [PubMed] [Google Scholar]
- 3.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 4.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64:1222–1231. doi: 10.1046/j.1523-1755.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Petermann AT, Pippin J, Krofft R, Blonski M, Griffin S, Durvasula R, Shankland SJ. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98:e114–123. doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 7.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 8.Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol. 2005;16:2257–2262. doi: 10.1681/ASN.2004121134. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffer M, Mundel P, Shaw AS, Bottinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem. 2004;279:37004–37012. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 12.Cybulsky AV, McTavish AJ, Papillon J, Takano T. Role of extracellular matrix and Ras in regulation of glomerular epithelial cell proliferation. Am J Pathol. 1999;154:899–908. doi: 10.1016/S0002-9440(10)65337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cybulsky AV. Extracellular matrix as a determinant of signaling responses in glomerular epithelial cells. Kidney Int. 1999;56:1242–1246. doi: 10.1046/j.1523-1755.1999.00699.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe PE, Greenspan RJ. Local function of the Notch gene for embryonic ectodermal pathway choice in Drosophila. Cell. 1986;46:773–783. doi: 10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- 15.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 16.Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–138. [PubMed] [Google Scholar]
- 17.Callahan R, Egan SE. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia. 2004;9:145–163. doi: 10.1023/B:JOMG.0000037159.63644.81. [DOI] [PubMed] [Google Scholar]
- 18.Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 19.Leimeister C, Bach A, Woolf AS, Gessler M. Screen for genes regulated during early kidney morphogenesis. Dev Genet. 1999;24:273–283. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<273::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- 22.Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe MS. Therapeutic strategies for Alzheimer’s disease. Nat Rev Drug Discov. 2002;1:859–866. doi: 10.1038/nrd938. [DOI] [PubMed] [Google Scholar]
- 24.Kopan R, Turner DL. The Notch pathway: democracy and aristocracy in the selection of cell fate. Curr Opin Neurobiol. 1996;6:594–601. doi: 10.1016/s0959-4388(96)80090-0. [DOI] [PubMed] [Google Scholar]
- 25.Berset T, Hoier EF, Battu G, Canevascini S, Hajnal A. Notch inhibition of RAS signaling through MAP kinase phosphatase LIP-1 during C. elegans vulval development. Science. 2001;291:1055–1058. doi: 10.1126/science.1055642. [DOI] [PubMed] [Google Scholar]
- 26.Newman AP, Sternberg PW. Coordinated morphogenesis of epithelia during development of the Caenorhabditis elegans uterine-vulval connection. Proc Natl Acad Sci U S A. 1996;93:9329–9333. doi: 10.1073/pnas.93.18.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Sternberg PW. Pattern formation during C. elegans vulval induction. Curr Top Dev Biol. 2001;51:189–220. doi: 10.1016/s0070-2153(01)51006-6. [DOI] [PubMed] [Google Scholar]
- 28.Grabher C, von Boehmer H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6:347–359. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- 29.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 30.von Boehmer H. Notch in lymphopoiesis and T cell polarization. Nat Immunol. 2005;6:641–642. doi: 10.1038/ni0705-641. [DOI] [PubMed] [Google Scholar]
- 31.Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, Aster JC, Allman D, Pear WS. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B cell malignancies. Blood. 2005 doi: 10.1182/blood-2005-01-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 33.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580:2860–2868. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Zecchini V, Domaschenz R, Winton D, Jones P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691. doi: 10.1101/gad.341705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. Embo J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns. 2004;4:707–711. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, Fopma A, Brantley JG, Vanden Heuvel GB. Coexpression of Cux-1 and Notch signaling pathway components during kidney development. Dev Dyn. 2004;231:828–838. doi: 10.1002/dvdy.20175. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- 42.Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol. 2007;18:2014–2020. doi: 10.1681/ASN.2007040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 44.Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 45.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. [DOI] [PubMed] [Google Scholar]
- 46.Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- 47.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development. 2003;130:5019–5029. doi: 10.1242/dev.00682. [DOI] [PubMed] [Google Scholar]
- 49.Susztak K, Sharma K, Schiffer M, McCue P, Ciccone E, Bottinger EP. Genomic strategies for diabetic nephropathy. J Am Soc Nephrol. 2003;14:S271–278. doi: 10.1097/01.asn.0000078035.81397.8a. [DOI] [PubMed] [Google Scholar]
- 50.Susztak K, Bottinger E, Novetsky A, Liang D, Zhu Y, Ciccone E, Wu D, Dunn S, McCue P, Sharma K. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- 51.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 52.Walsh DW, Roxburgh SA, McGettigan P, Berthier CC, Higgins DG, Kretzler M, Cohen CD, Mezzano S, Brazil DP, Martin F. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbadis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003. doi: 10.1681/ASN.V1481998. [DOI] [PubMed] [Google Scholar]
- 54.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 56.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 57.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 58.Tsai JY, Wolfe MS, Xia W. The search for gamma-secretase and development of inhibitors. Curr Med Chem. 2002;9:1087–1106. doi: 10.2174/0929867023370185. [DOI] [PubMed] [Google Scholar]