Abstract
Nitric oxide (NO) is an important regulator of a variety of biological functions, and also has a role in the pathogenesis of cellular injury. It had been generally accepted that NO is solely generated in biological tissues by specific nitric oxide synthases (NOS) which metabolize arginine to citrulline with the formation of NO. However, over the last 15 years, nitrite-mediated NO production has been shown to be an important mechanism of NO formation in the heart and cardiovascular system. Now numerous studies have demonstrated that nitrite can be an important source rather than simply a product of NO in mammalian cells and tissues and can be a potential vasodilator drug for cardiovascular diseases. There are a variety of mechanisms of nitrite reduction to NO and it is now appreciated that this process, while enhanced under hypoxic conditions, also occurs under normoxia. Several methods, including electron paramagnetic resonance, chemiluminescence NO analyzer, and NO-electrode have been utilized to measure, quantitate, and image nitrite-mediated NO formation. Results reveal that nitrite-dependent NO generation plays critical physiological and pathological roles, and is controlled by oxygen tension, pH, reducing substrates and nitrite levels. In this manuscript, we review the mechanisms of nitrite–mediated NO formation and the effects of oxygen on this process with a focus on how this occurs in the heart and vessels.
Keywords: Nitrite, nitric oxide, heart and vessels, xanthine oxidase, aldehyde oxidase, electron paramagnetic resonance, oxygen tension
Introduction
Nitric oxide (NO) is a free radical endogenously produced in biological tissues and is an important regulator of numerous biological functions [1-4]. NO can also cause cellular injury via reaction with superoxide to form the potent oxidant peroxynitrite [5-7]. Although nitric oxide synthases (NOSs) have been generally considered to be the primary source of NO in biological systems, in 1995 we observed that prominent NO formation from nitrite occurs in heart tissues under conditions of intracellular acidosis [8]. A number of methods, including electron paramagnetic resonance, chemiluminescence NO analyzer, and NO-electrode were utilized to measure, quantitate, and image nitrite mediated NO formation. Now numerous studies have demonstrated that nitrite can be an important source rather than simply a product of NO in mammalian cells and tissues [8-24]. Results reveal that nitrite-dependent NO generation plays critical physiological and pathological roles, and is controlled by oxygen tension, pH, reducing substrates and nitrite levels. Nitrite is currently undergoing or planned for clinical trials as a vasodilator drug in patients with cardiovascular diseases such as ischemic stress, sickle cell disease, coronary artery disease, and pulmonary hypertension. Therefore fundamental understanding of the mechanisms of nitrite-dependent NO formation in the cardiovascular system is of great importance. In this manuscript, we review the mechanisms of nitrite–mediated NO formation and the effects of oxygen on this process with a focus on how this occurs in the heart and vessels.
1. Evidence for NO formation from nitrite in the heart
In the cardiovascular system, traditionally it was thought that NO is only produced in endothelial cells by endothelial nitric oxide synthase (eNOS) and then passively diffuses to either smooth muscle cells where it activates soluble guanylyl cyclase (sGC) or to the blood where it is mainly consumed by hemoglobin (Hb). However, in 1995, we first observed that nitrite can also be a prominent source of NO in heart tissues under conditions of ischemia with intracellular acidosis [8]. In the presence of isotopically labeled 15NO2−, NO production from 15N-nitrite in anoxic heart tissue was measured by EPR spectroscopy with the spin trap iron N-methyl-D-glucamine dithiocarbamate (Fe2+-MGD). 15NO gives rise to a characteristic doublet 15NO-Fe2+-MGD spectrum, rather than the triplet observed with natural abundance 14NO, enabling direct and selective detection of nitrite-derived NO formation. Hearts were labeled with 1 mM 15NO2− for 1 minute of normal perfusion or 1 minute of perfusion prior to the onset of ischemia. In the normally perfused control hearts, 15NO2− did not result in significant NO formation (Fig.1A, left). In hearts which were labeled and then subjected to 30 min of ischemia; however, marked NO formation was observed with the appearance of a very large doublet signal (Fig. 1B, left). In matched experiments performed with addition of natural abundance 14NO2−, large NO triplet signals were seen (Fig. 1C, left). While the observed signal was similar to that in the absence of added nitrite, the intensity of this signal was much higher. These experiments demonstrated that nitrite is reduced to NO in the ischemic heart.
Fig 1.
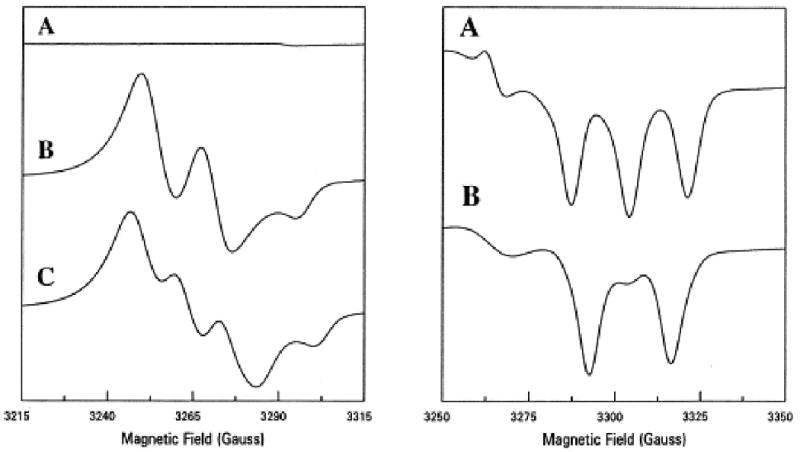
EPR spectra of NO formation in the heart from nitrite. Hearts were prelabeled with 1 mM 15N or 14N nitrite. The left panel shows spectra from hearts with Fe-MGD; A, before ischemia; B, 30 min of ischemia with 15N-nitrite; C, 30 min ischemia with 14N-nitrite. The right panel shows nitosyl-heme formation; A, with 14N-nitrite; B, with 15N-nitrite.
Experiments were also performed measuring nitrosyl-heme formation in ischemic heart tissue from hearts labeled with 1 mM 15NO2−. A prominent doublet nitrosyl-heme signal was seen due to the formation and binding of 15NO2− to these proteins, further confirming that NO is generated from nitrite in heart tissue subjected to long periods of ischemia (Fig. 1B, right). With 14NO2− a similar magnitude triplet NO signal was observed (Fig. 1A, right).
The rate of reduction of nitrite to NO is pH dependent. During myocardial ischemia, marked intracellular acidosis occurs, and pH values can fall to levels of 6.0 or below [21]. The low pH along with a highly reduced state under hypoxia could cause the rapid reduction of nitrite to NO. This process was shown to occur following myocardial ischemia as in the clinical setting of heart attack and it was extrapolated that nitrite disproportionation and reduction would be a generalized phenomena in any tissue under conditions of ischemia or shock, where poor perfusion and accompanying acidosis occur [8, 21].
2. Methods of measuring, quantitating and imaging nitrite mediated NO formation
NO is paramagnetic and binds with high affinity to the water-soluble spin trap, Fe2+-MGD, forming a mononitrosyl iron complex with characteristic triplet spectrum at g=2.04 with hyperfine splitting aN=12.8. From the intensity of the observed spectrum, quantitative measurement of NO generation can be performed [8, 21]. As noted above, this technique has been applied to measure nitrite-mediated NO generation and with 15N labeled nitrite it can be used to isotope trace the process of nitrite-mediated NO formation [16-18, 25-30].
The advantage of electrochemical NO sensors is their ability to directly detect NO concentration in solution or in biological samples with high sensitivity and a detection limit of a few nanomolar [31, 32]. This advantage allows NO electrodes to be an excellent tool for studying NO reaction kinetics, especially in biological samples. NO electrode studies were used to investigate enzyme catalyzed nitrite reduction as from XO and this method avoids any possible perturbation as might be caused by the presence of a trap [16-18]. The electrochemical detector continuously records the current through the working electrode, which is proportional to the NO concentration in the solution.
The real-time rate of the NO production can be measured using a chemiluminescence NO analyzer. In the analyzer, NO is reacted with ozone forming excited-state NO2, which emits light [33]. Mixing of reagents and separation of NO from the reaction mixture can be done at controlled temperature in a glass-purging vessel equipped with heating jacket. The release of NO is quantified by analysis of the digitally recorded signal from the photo-multiplier tube. Since the NO analyzer registers the flux of NO in the purging gas mixture it measures the rate of NO production from the system [33]. The rates of NO formation derived from Xanthine oxidoreductase (XOR), aldehyde oxidase (AO), cytochrome P450, rat tissues, and blood have been measured using the NO analyzer [16-18, 26, 34-36].
With the development of EPR imaging (EPRI) methods and low frequency EPR instrumentation, it has become possible to spatially map the presence, quantity, and location of free radicals and paramagnetic species in both in vivo and ex vivo biological tissues [37, 38]. With the use of 15N nitrite, spatial mapping of nitrite-derived NO was performed in the ischemic heart [39]. A series of rat hearts were loaded with Fe2+-MGD and 14N or 15N nitrite and subjected to global no flow ischemia. After 60 minutes, three-dimensional projection data were acquired and image reconstruction performed. Figure 2A shows 3D images obtained in these hearts. Both the 14N- and 15N-labeled hearts exhibited similar images of NO distribution. The 15NO image, however, was somewhat sharper due to enhanced sensitivity and better resolution of the hyperfine doublet structure. The images clearly show that NO is formed throughout the left ventricular (LV) myocardium. The NO image enabled visualization of the external shape of the epicardium, right ventricular (RV) myocardium and internal endocardial surface of the LV and LV chamber. The concentrations of NO in the RV, however, were much lower with levels only 20–25% of those seen in the LV wall. To understand the spatially resolved time course of NO generation in the ischemic heart, EPRI of NO formation was performed as a function of ischemic duration. A series of images were performed every 5 min during ischemia for a total period of 60 minutes. Representative slices of longitudinal and transverse cuts through the LV are shown in Figure 2B. It was observed that the NO concentration derived from nitrite gradually increases as a function of the duration of ischemia throughout the LV myocardium. Whole body EPR imaging of NO formation and trapping by heme proteins has also been reported [38].
Fig. 2.
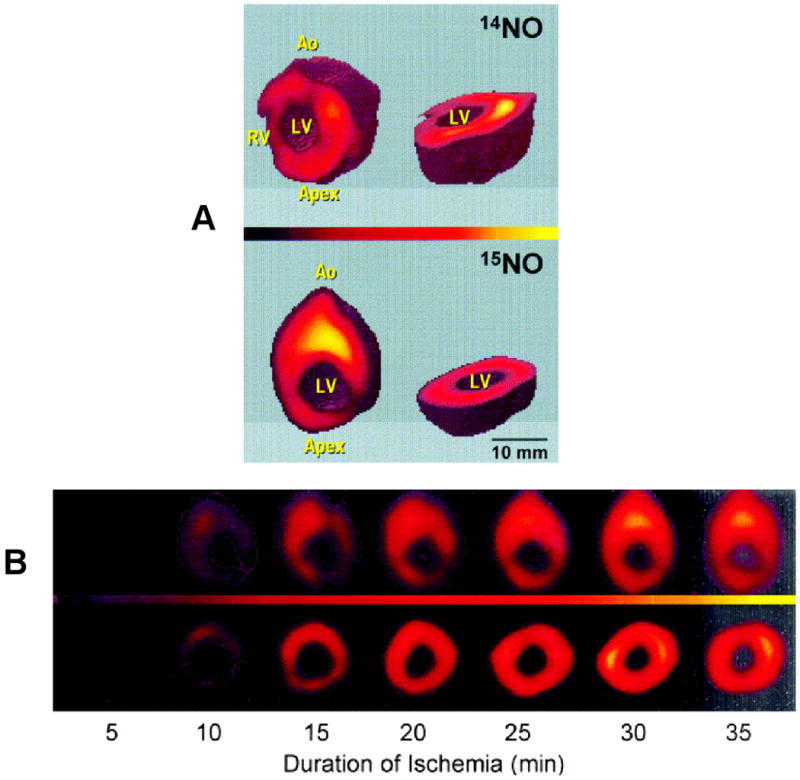
A. shows three-dimensional EPR images (25×25×25 mm3) of nitrite-derived NO formation in the isolated rat heart. The heart was loaded with Fe-MGD and either 14N or 15N nitrite and then subjected to no-flow ischemia. The images of NO bound to Fe-MGD are shown as sliced 3D views. B. Imaging of the time course of NO formation from nitrite in the ischemic heart. Rat hearts were loaded with 15N nitrite and imaging of the 15NO bound to Fe-MGD performed. A series of longitudinal (top panel) and transverse (bottom panel) slices are shown from the 3D spatial images as a function of ischemic duration.
3. Pathways involved: enzyme dependent or independent
Several mechanisms for the reduction of nitrite to NO have been identified by us and others including non-enzymatic disproportionation at low pH [40], enzyme-independent reduction [8, 21], enzymatic conversion by XOR [16-18, 36] and AO [26], reduction by cytochrome C [41, 42], P450 [35], and reduction by deoxyhemoglobin or myoglobin [9-14].
NO formation can occur by the simple process of nitrite disproportionation or enzyme-independent chemical reduction under acidic conditions. Benjamin and colleagues reported in 1994 that acidification of nitrite releases NO in the stomach [43]. Also, in 1994, Lundberg et al. reported measuring high levels of NO expelled in air from the stomach of humans [44]. We observed that nitrite is present in the tissue in micromolar concentrations and is converted to NO under the acidic and highly reduced conditions that occur during ischemia. Under ischemia, pH can go down to values as low as 5.5. NO formation rate from nitrite disproportionation is predicted to be 4–100 pM/s. This may result in 15–360 nM NO increase during 60 minutes of ischemia [21]. Thus, with long periods of tissue ischemia progressing to necrosis this mechanism of NO formation can be an important source of NO generation [8, 21].
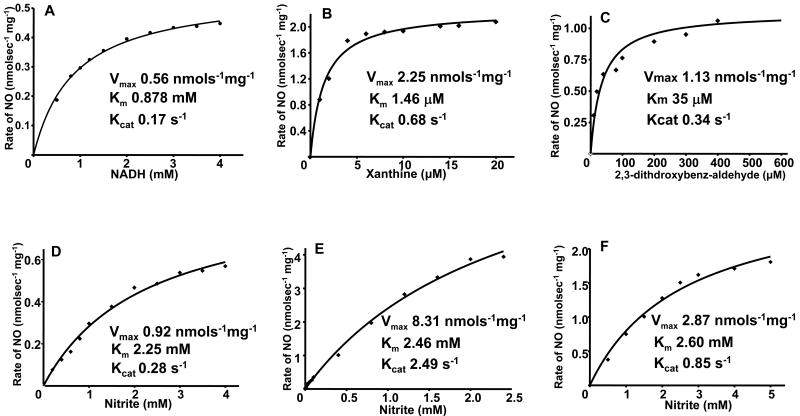
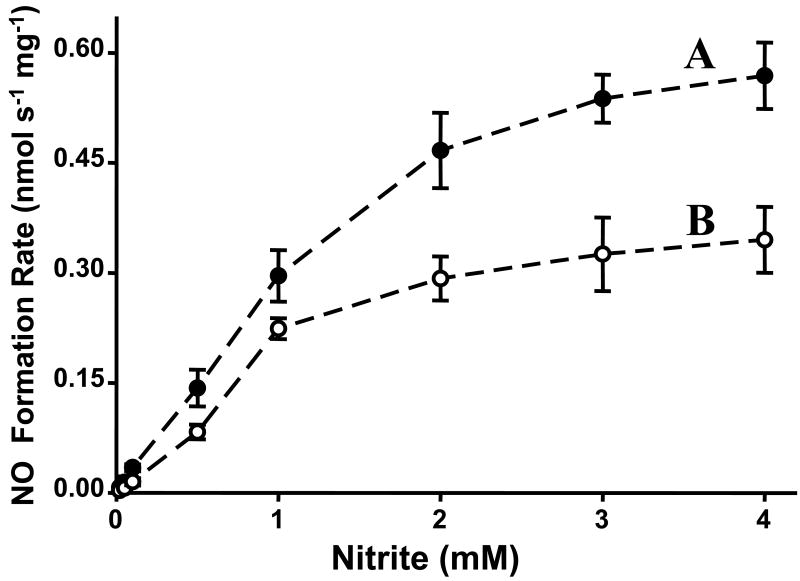
XOR is a molybdenum enzyme that plays a variety of important roles in normal physiology and disease. In mammals, XOR exists in two interconvertible forms, xanthine dehydrogenase (XDH) and XO, while XO prefers oxygen as electron acceptor, XDH prefers NAD+ that it converts to NADH. Both forms of XOR can serve as nitrite reductase to produce NO [16-18] [36]. In our previous investigation, the mechanism, magnitude, and physiological importance of XO-mediated nitrite generation was characterized [16-18]. In addition to its molybdenum, XO also has FAD binding site and iron sulfur centers. XO has different site-specific electron donors. Xanthine and 2,3-dihydroxybenz-aldehyde (DBA) serve as electron donor to XO by binding to the molybdenum site, the site at which XO-mediated nitrite reduction occurs [16]. However, NADH reduces XO at the FAD-site of the enzyme, which is the site that also reduces oxygen. XO reduces nitrite to NO at the molybdenum site of the enzyme with xanthine, NADH or aldehyde substrates serving to provide the necessary reducing equivalents [16-18]. To measure nitrite-mediated NO generation under anaerobic conditions, EPR studies were performed. The typical types of XO reducing substrates, including NADH, 2,3-dihydroxybenz-aldehyde (DBA), and xanthine, were found to function as electron donors for XO-catalyzed nitrite reduction and triggered large amounts of NO generation. Kinetic studies of XO-catalyzed nitrite reduction demonstrated that each of the typical reducing substrates support nitrite reduction to NO and each of these reactions followed Michaelis-Menten kinetics. For each of these reducing substrates, the rate of XO-mediated NO formation was also determined as a function of nitrite concentration. Again, typical Michaelis-Menten kinetics was observed. The Km, Kcat, and Vmax values were obtained by fitting the Michaelis-Menten equations (Fig. 3). From these kinetic data, and known enzyme and substrate levels in the tissues, the magnitude of XO-mediated NO formation rates could be predicted along with determination of the quantitative importance of this NO generation pathway in biological systems.
Fig. 3.
Kinetics of NO generation from XO as a function of reducing substrate or nitrite concentration. A shows the effect of [NADH] on the rate of NO generation from 0.04 mg/ml XO and 1.0 mM nitrite. B shows the effect of [xanthine] on the rate of NO generation from 0.02 mg/ml XO and 1.0 mM nitrite. C shows the effect of [2,3-dithdroxybenz-aldehyde] on the rate of NO generation from 0.02 mg/ml XO and 1.0 mM nitrite. D shows the rate of NO generation by 0.04 mg/ml XO and 1.0 mM NADH in the presence of 0.2-4 mM nitrite. E shows the rate of NO generation by 0.02 mg/ml XO, 5 μM xanthine in the presence of 5 μM-2.5 mM nitrite. F shows the rate of NO generation by 0.02 mg/ml XO, 40 μM 2,3-dithdroxybenz-aldehyde in the presence of 0.5 mM-5 mM nitrite. For each of these graphs, the corresponding fits (solid lines) Km, Vmax, and Kcat data were obtained using the Michaelis-Menten equation. Adopted from [35].
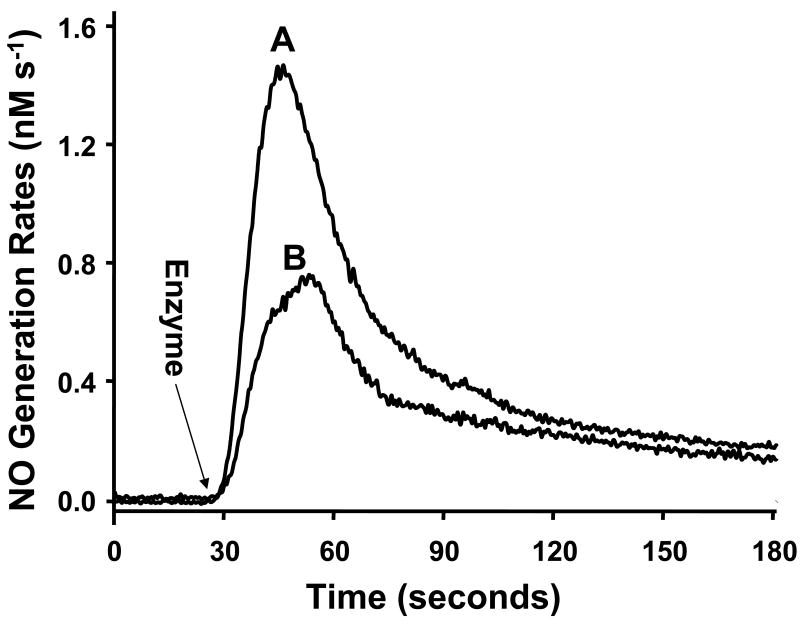
In addition to XO, our recent studies indicate that another molybdenum enzyme AO can also be a source of NO in the tissues [26]. AO is a cytosolic enzyme that plays an important role in the biotransformation of drugs and xenobiotics (21). Both XO and AO are present in the liver but they are also broadly distributed in other tissues, such as lung, blood vessels, heart and kidney [45-49]. The amino acid sequence of AO and XO are remarkably similar, with ∼ 86% homology, and they belong to the same family of molybdenum-containing proteins with two-iron-sulfur clusters, a flavin cofactor, and a molybdopterin cofactor (22, 23). As investigated under anaerobic conditions using the NO analyzer, prior to the addition of AO, no detectable NO generation was seen from nitrite (100 μM) in the presence of NADH (500 μM). However, after addition of AO (0.01 mg/ml) or XO (0.04 mg/ml), prominent NO generation was triggered (Fig 4A, B). These results further confirm that the molybdenum enzymes AO and XO function as potent nitrite reductases.
Fig. 4.
Measurement of NO generation from isolated enzyme-mediated nitrite reduction. Measurements were performed using a chemiluminescence NO analyzer under anaerobic conditions at 37 °C in 5 ml of PBS. The arrow shows the time at which AO, XO, or ferrous hemoglobin was added. NO generations are shown from nitrite (100 μM), NADH (500 μM), and AO (0.01 mg/ml) (trace A); nitrite (100 μM), NADH (500 μM), and XO (0.04 mg/ml) (trace B). Reproduced from [26].
4. Modulation by oxygen
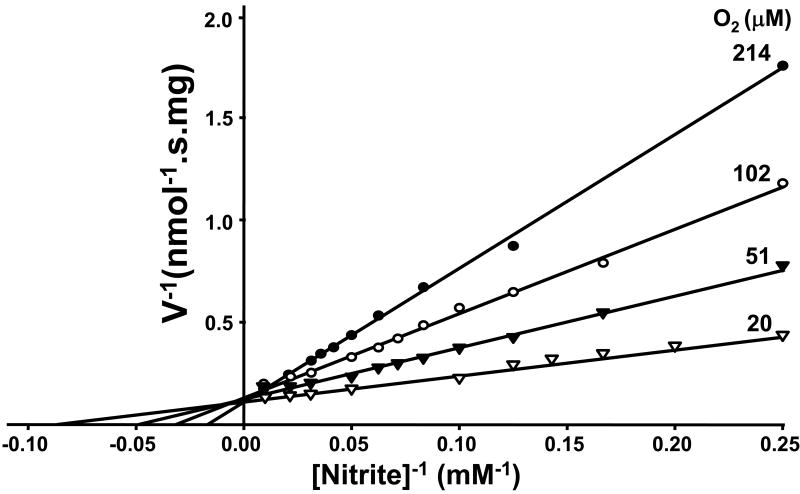
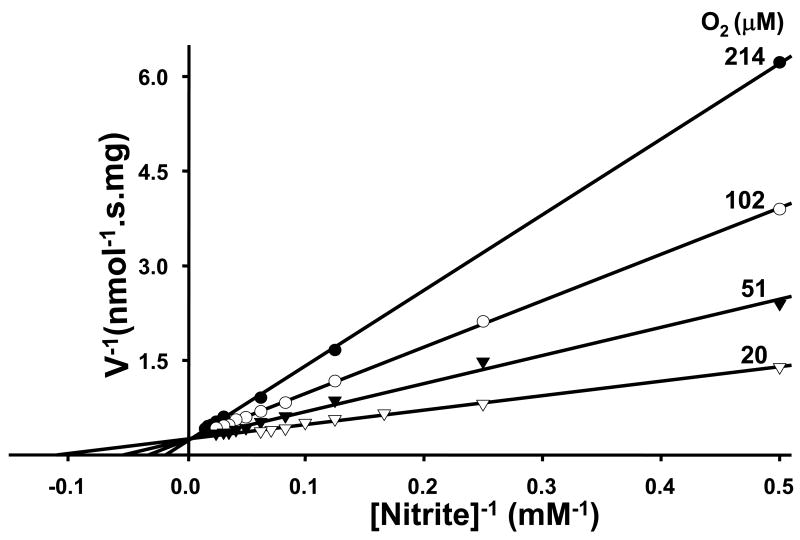
Our previous investigation reveals that XO-mediated NO formation from nitrite is sensitively controlled by oxygen tension [18]. For XO there are different site-specific electron donors. Xanthine and DBA bind and donate electrons at the molybdenum site, where XO-mediated nitrite reduction also occurs [16]. NADH, however, reduces XO at its FAD-site, where oxygen reduction occurs. Xanthine and DBA were found to be more effective electron donors than NADH for anaerobic XO-mediated nitrite reduction [16]. However, substrate preference and control under aerobic conditions is quite different. With xanthine or DBA that provide electrons to the molybdenum site of XO, the NO production rate follows Michaelis-Menten kinetics and oxygen serves as a competitive inhibitor. Lineweaver-Burk plots characterized the shift of Km value by oxygen and demonstrate that oxygen is a competitive inhibitor of nitrite reduction (Fig. 5A,B). However, with FAD site-binding substrate NADH as electron donor, aerobic NO generation rates from nitrite do not follow Michaelis-Menten kinetics (Fig. 6). Aerobic NO production was maintained at more than 70% of anaerobic levels and binding of NADH to the FAD site appeared to prevent oxygen binding.
Fig. 5.
Lineweaver-Burk analysis of NO generation by XO as a function of nitrite concentration at different oxygen tensions. Reciprocal plot, 1/velocity versus 1/[nitrite]. A, 0.02 mg/ml XO, 0.02 mM xanthine in the presence of 400 units/ml SOD and 4–60 mM nitrite. B, 0.02 mg/ml XO and 0.5 mM DBA in the presence of 400 units/ml SOD and 2–64 mM nitrite. Oxygen concentrations of 214, 102, 51, and 20 μM, respectively, were achieved with purging of the sample solutions at 37 °C with 21, 10, 5, or 2% oxygen. Reproduced from [18].
Fig. 6.
XO-mediated NO generation from nitrite with NADH as reducing substrate under anaerobic or aerobic conditions. Initial rates of NO generation were measured by chemiluminescence NO analyzer. A, the effects of nitrite concentration on the rate of NO generation from 0.02 mg/ml XO and 1 mM NADH in the presence of 0.02–4 mM nitrite and 400 units/ml SOD purged with argon. B, as in A, but purged with air. Reproduced from [18].
With NADH as reducing substrate, the possible XO-mediated NO generation may occur through two possible processes as shown below:
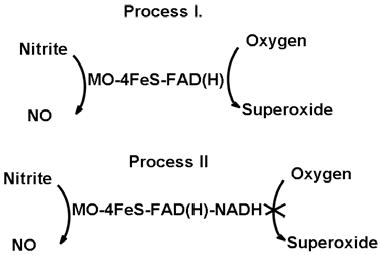
In process I, with FAD site free, oxygen and nitrite both are able to accept electrons from reduced XO. In process II, oxygen reduction is totally blocked by the binding of NADH at the FAD site; meanwhile at the molybdenum site, XO-mediated nitrite reduction is unaffected. Under aerobic conditions with NADH as reducing substrate, less than 30% of nitrite reductase activity of XO is inhibited, which suggests that most nitrite reduction happens while the FAD-site is occupied with NADH. NADH, which is the least effective reducing substrate under anaerobic conditions, was the most potent reducing substrate in the presence of oxygen.
Of note, XO reduces oxygen to superoxide and hydrogen peroxide and it is thought to be one of the key enzymes responsible for reactive oxygen species in the cardiovascular system [45, 50-53]. XO can act as a powerful nitrite reductase under ischemic conditions, but also a source of the NO scavenger superoxide under aerobic conditions. With administration of nitrite, XO can be a source of both NO and superoxide, and the products and functions of XO are sensitively controlled by oxygen tension, reducing substrates, and pH. This may explain the observation of lack of inhibition by oxypurinol to nitrite induced intravascular vasodilation [58], while other studies reveal that XO is the major enzyme-reducing nitrite to NO [59-61]. From the reported enzyme concentrations, kinetic data, reducing substrates levels, in the heart, molybdenum enzyme XO and AO-mediated NO generation from physiological amounts of nitrite can approach or excess that of the maximal NO generation from constitutive NOS, estimated to be 1.5 nM/s [8, 54], This nitrite-derived NO production from XO and AO in tissues could serve as an alternative source of NO under ischemic conditions in which NO production from NOS is impaired.
5. Conversion of nitrite to NO in the vessel wall
Nitrite and nitrate in the body can come from the NO synthase (NOS) pathway endogenously and from diet exogenously. Nitrite in the blood is converted to nitrate by reacting with oxy-hemoglobin. Nitrite level detected in blood is normally 1 μM or less. However, the nitrite levels in the tissues are much higher. It has been shown that in the heart nitrite levels can reach above 10 μM [8]. It was shown that under hypoxic condition, 10 μM nitrite can cause a large vasodilation (>40%) in rabbit aortic rings [55]. Infusion or dietary supplementation with nitrite or nitrate at physiological levels to healthy volunteers reveal significant vasodilation of the forearm circulation [56] and a significant decrease in blood pressure [57], indicating that nitrite and nitrate reduction in the body is physiologically relevant and is involved in regulation of blood pressure. Under pathological or pharmacological conditions, nitrite concentrations in the tissues can increase to much higher levels. Since NOS pathway requires oxygen as substrate and the non-enzyme pathway converting nitrite/nitrate into NO is much more effective as oxygen concentration decreases, this NOS-independent pathway can be used as a back-up system for NOS-dependent NO formation to ensure sufficient NO formation when oxygen supply is limited [24, 58, 59]. All these research results suggest the potential physiological, pathological, and pharmacological importance of nitrite-dependent NO generation. However, the mechanism of nitrite biotransformation in the tissues remains unclear.
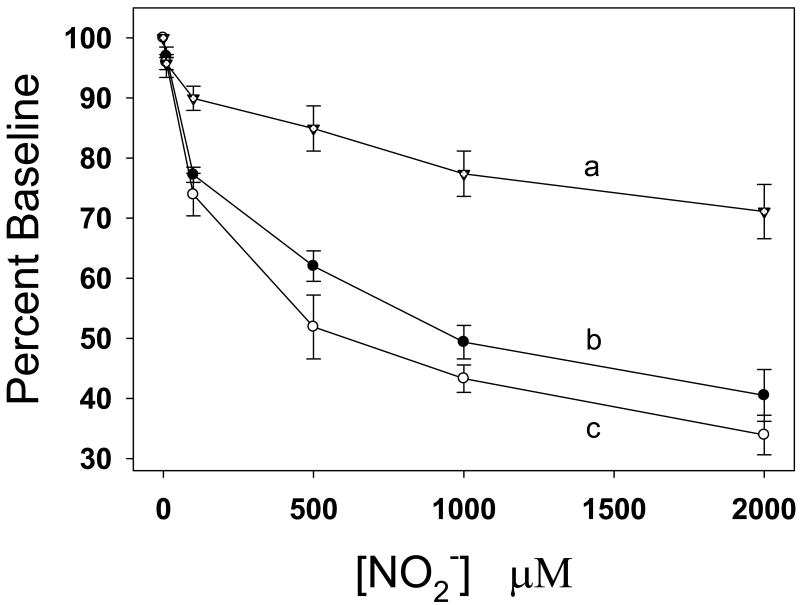
To determine the mechanisms by which nitrite is converted to NO, studies were performed in the rat model measuring the effects of nitrite on in vivo hemodynamics and in vitro vasorelaxation in isolated aorta under aerobic conditions. Nitrite was infused slowly over 1–3 min as a concentrated 200 mM stock dissolved in PBS, pH 7.4, to the left jugular vein of rats achieve the final concentration from 10 μM to 2 mM calculated according to the body weight and circulating blood volume [60]. With administration of nitrite to anesthetized rats, it was demonstrated that 10 μM levels of nitrite in the blood stream appreciably reduced systolic and diastolic blood pressure. With increase of nitrite concentration from 10 μM to 2 mM, blood pressure decreased in a dose-dependent manner [60], implying that nitrite or a nitrite-derived species is the vasodilator (Fig. 7).
Fig. 7.
Concentration-dependent effects of nitrite on the blood pressure (BP) and heart rate of anesthetized rats with normal body temperature (37°C). Curve a: heart rate; curve b: systolic BP; curve c: diastolic BP. Ordinate BP and heart rate are expressed as percentage baseline that was assigned as 100%.
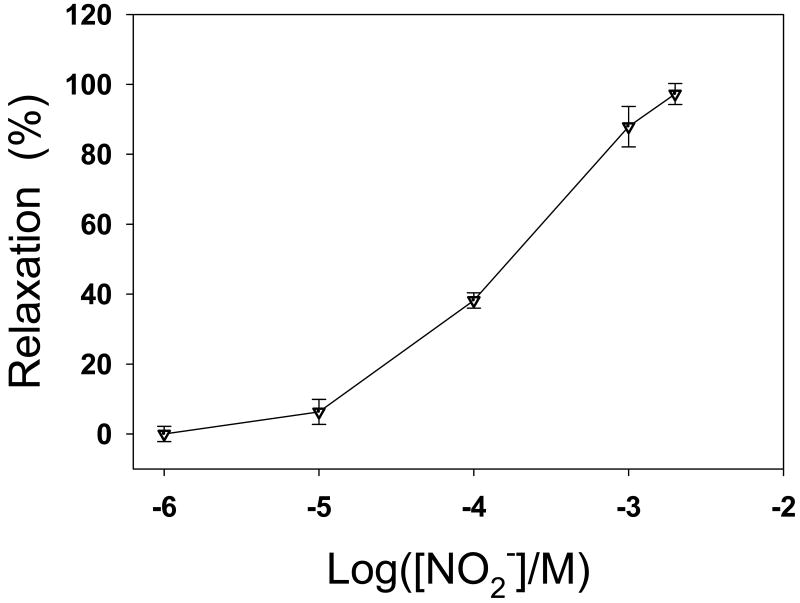
To determine if the vasodilator is nitrite or a nitrite-derived species, the effect of nitrite on the tension of rat aortic rings was measured in organ chamber experiments. It was observed that nitrite induced aortic dilation in a dose-dependent manner when nitrite concentration is greater than 10 μM and reached full relaxation (∼100%) with a nitrite concentration of 2 mM (Fig. 8). The nitrite induced vasodilation was inhibited by 20 μM oxyHb, a NO scavenger. This indicates that the nitrite-derived NO, rather than nitrite itself, is the species responsible for the nitrite induced vasodilation. It has been demonstrated that Hb in RBCs can convert nitrite to NO and this conversion is more efficient under hypoxic conditions [61]. However, in our experiments, the nitrite-induced vasodilation occurs in the absence of RBCs, suggesting that RBCs are not involved in the vasodilation under our experimental conditions. Since the nitrite-induced aortic dilation occurs in the absence of RBCs, the conversion of nitrite to NO must be carried out in the aortic wall. Several lines of evidence have shown that nitrite can be also converted to NO by XOR, mitochondrial electron transport chain, cytochrome P450 or NOS in tissues [16, 35, 36, 62, 63]. Thus, it was unclear what proteins are responsible for the conversion of nitrite into NO in the vascular wall. Therefore further experiments were designed to study the nitrite-induced vasodilation and determine if measurable NO is generated from nitrite in the vascular wall, and what proteins are involved in the vascular conversion of nitrite to NO.
Fig. 8.
Effects of nitrite on the relaxation of isolated rat aorta precontracted with phenylephrine (10 μM) under aerobic conditions (pH 7.4, 37°C). Relaxant effects expressed as the percentage relaxation from the maximal phenylephrine contraction.
6. Measurement of nitric oxide generation from nitrite in the vessel wall
NO electrodes and EPR techniques were used to further confirm NO generation from nitrite in the vascular wall [60]. To detect the nitrite-derived NO in the vascular wall, a segment of aortic wall was placed on the bottom of a chamber containing tissue buffer at 37 °C with the endothelial side of the aortic wall facing upward. When NO is generated from nitrite in the aortic wall, the generated NO can diffuse out of the aortic wall and into the solution. Thus, in the solution phase, NO concentration is highest at the endothelial surface and decreases to zero in the bulk solution away from the endothelial surface. Therefore, the NO electrode was placed near the endothelial surface. After 500 μM nitrite was added into the chamber, it was observed that NO concentration at the endothelial surface slowly increased and reached a plateau (Fig. 9). To test if the detected NO was generated from nitrite, EPR spectroscopy study was performed. Since 14N and 15N NO will cause triplet and doublet EPR signals respectively [8], we added 14N or 15N nitrite to the aorta. If the generated NO is derived from nitrite, we would observe triplet or doublet EPR signals, respectively. Our experimental results were consistent with this prediction (Fig. 10), indicating that the detected NO was converted from nitrite in the vascular wall.
Fig. 9.
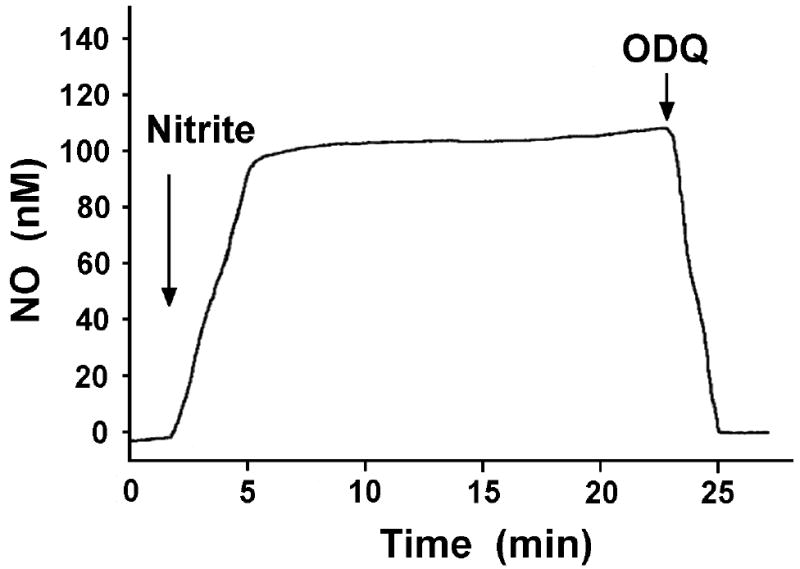
Effects of ODQ on nitrite-converted NO from aortic rings under aerobic conditions (pH 7.4, 37°C). A piece of aortic wall was placed on the bottom of a chamber. A Clark-type NO electrode was put near the surface of the aortic wall. NO generation was detected by the NO electrode after 500 μM nitrite was added to the chamber. The detected NO was abolished by 10 μM ODQ. Arrows show the time at which different drugs were added.
Fig. 10.
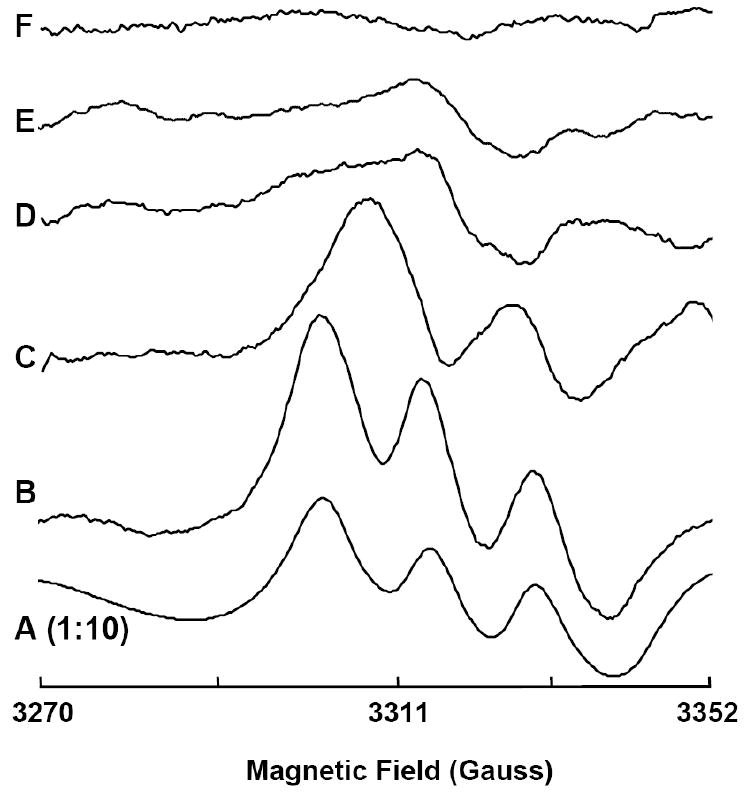
Representative electron paramagnetic resonance (EPR) spectra for NO-Fe2+-DETC complex formed from isolated rat aorta in the presence of added nitrite or NO at 77 K. EPR measurements were performed using a Bruker ER 300 spectrometer operating at X-band, 9.77 GHz. Aorta were incubated in PBS with 0.5% BSA, nitrite (500 μM), ammonium-iron(II) sulfate(1 mM), and diethyldithiocarbamate (DETC) (2 mM), pH 7.4 at 37°C with the following: A: NO (15 μM); B: 14N nitrite (500 μM); C: 15N nitrite (500 μM); D: high temperature (100°C, 8 min) and 14N nitrite (500 μM); and E: ODQ (20 μM) and 14N nitrite (500 μM). F: background effects for 14N nitrite (500 μM) without aorta. Each spectrum is representative of at least 5 independent experiments. Please note that the A is presented at 1:10 magnitude of all others. Reproduced from [60].
7. Heme proteins are responsible for the conversion of nitrite to NO in the vessel wall
Different inhibitors have been used to determine the possible pathways converting nitrite to NO [60]. It was observed that oxypurinol, rotenone, myxothiazol, 17-Octadecynoic acid (17-ODYA) and L-Nitro-Arginine Methyl Ester (L-NAME) did not have any significant effect on the aortic dilation response to nitrite, suggesting respectively that XOR, mitochondrial electron transport chain, cytochrome P450 or NOS are not the main mechanism for the conversion of nitrite to NO. In contrast, the nitrite-induced aortic dilation was abolished by 10 μM 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ). It is known that ODQ is a potent inhibitor of sGC [64, 65]. ODQ is also an oxidant to heme proteins [60, 66]. Thus, inhibition of vasodilation by ODQ may be caused by blocking the NO-sGC-cGMP pathway and/or blocking the conversion of nitrite to NO through vascular heme proteins.
To test if the rate of NO generation from nitrite was changed in the presence of ODQ, we placed a NO electrode near the endothelial surface to detect NO generation from nitrite by the aortic wall in the presence of 500 μM nitrite. After 50 μM ODQ was added into the solution, we observed that NO concentration at the endothelial surface rapidly fell to zero (Fig. 9), suggesting that NO generation from nitrite is inhibited. After the inhibition, the relatively high NO concentration at the endothelial surface rapidly diffuses into the surrounding solution so that NO concentration at the endothelial surface rapidly decreases and approaches to zero, the NO concentration in the bulk solution. The above experimental results strongly suggest that vascular heme proteins convert nitrite to NO in the vascular wall. Our experimental results show that nitrite evokes dose-dependent vasorelaxation of the rat aorta and causes a dose-dependent decrease in blood pressure under aerobic conditions. Both functional and analytical studies show that the vasorelaxant effect of nitrite is mediated by NO formation through sGC or other heme proteins inhibited by ODQ. These findings provide strong evidence that a ferrous heme protein in aortic smooth muscle is involved in nitrite induced NO production under aerobic conditions. From this work it remains unclear as to the identity of this heme protein. Both hemoglobin (Hb) and myoglobin (Mb) have been proposed to function as nitrite reductases but are not present in significant levels in the vessel wall. Cytoglobin, however, is ubiquitous in many tissues including vessels and remains a likely candidate for this heme-protein based nitrite reduction. Of note we have observed that Cytoglobin is oxidized by ODQ [60].
Similar to Hb, Mb acts either as an NO-scavenger under oxygenated conditions or as a nitrite-reductase under conditions of hypoxia and ischemia [58, 67]. Mb must be at least partially deoxygenated to act as nitrite-reductase, and when the oxygen level falls below the P50 of Mb this would be potentiated. However, it has been previously proposed that the regulatory role of Mb is based on the ability of oxygenated Mb to react with and scavenge NO [68]. Experiments demonstrated that NO is more effective in the regulation of coronary tone and myocardial contractility in hearts from Mb-/- mice. Ongoing work continues to evaluate the role of sGC, Cytoglobin, P450, Cytochrome C or other heme proteins in this process.
8. Conclusions
Over the last 15 years, nitrite-mediated NO production has been shown to be an important mechanism of NO formation in the heart and cardiovascular system. It occurs via a variety of mechanisms the relative importance of which are influenced by the oxygen tension, redox state, pH, and tissue type or location. Several techniques including chemiluminescence NO analyzer, NO electrode, and EPR spectroscopy and spin trapping along with isotope tracer studies have been used to specifically measure and image nitrite-mediated NO formation. These techniques along with functional correlation and the use of specific inhibitors has enabled mapping the pathways of nitrite-mediated NO formation in normoxic and hypoxic tissues as well as the effects of nitrite therapy. Questions remain regarding the specific pathways of nitrite reduction under aerobic conditions and their relative importance in cardiovascular physiology and disease. In view of the emerging role of nitrite based therapeutics, further investigation will be needed to better understand these processes and how to optimize the therapeutic benefits of nitrite therapy while minimizing any potential adverse effects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3(9):2007–18. [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42. [PubMed] [Google Scholar]
- 5.Beckman JS, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman JS. Ischaemic injury mediator. Nature. 1990;345(6270):27–8. doi: 10.1038/345027b0. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271(46):29223–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 8.Zweier JL, et al. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1(8):804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 9.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 10.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107(2):566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladwin MT. Hemoglobin as a nitrite reductase regulating red cell-dependent hypoxic vasodilation. Am J Respir Cell Mol Biol. 2005;32(5):363–6. doi: 10.1165/rcmb.f294. [DOI] [PubMed] [Google Scholar]
- 12.Gladwin MT, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1(6):308–14. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 13.Huang KT, et al. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280(35):31126–31. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115(8):2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454(12):127–30. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 16.Li H, et al. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276(27):24482–9. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 17.Li H, et al. Characterization of the Magnitude and Kinetics of Xanthine Oxidase-Catalyzed Nitrate Reduction: Evaluation of Its Role in Nitrite and Nitric Oxide Generation in Anoxic Tissues. Biochemistry. 2003;42(4):1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 18.Li H, et al. Characterization of the Effects of Oxygen on Xanthine Oxidase-mediated Nitric Oxide Formation. J Biol Chem. 2004;279(17):16939–46. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 19.Millar TM, et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427(2):225–8. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 20.Nohl H, et al. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim Pol. 2000;47(4):913–21. [PubMed] [Google Scholar]
- 21.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411(23):250–62. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, et al. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: a potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem Biophys Res Commun. 1998;249(3):767–72. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- 23.Wink DA. Ion implicated in blood pact. Nat Med. 2003;9(12):1460–1. doi: 10.1038/nm1203-1460. [DOI] [PubMed] [Google Scholar]
- 24.van Faassen EE, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29(5):683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y, Zweier JL. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc Natl Acad Sci U S A. 1997;94(23):12705–10. doi: 10.1073/pnas.94.23.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, et al. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 2008;283(26):17855–63. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988;85(11):4046–50. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995;270(1):304–7. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- 29.Vanin AF, et al. Redox properties of iron-dithiocarbamates and their nitrosyl derivatives: implications for their use as traps of nitric oxide in biological systems. Biochim Biophys Acta. 2000;1474(3):365–77. doi: 10.1016/s0304-4165(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 30.Samouilov A, Zweier JL. Analytical implications of iron dithiocarbamates for measurement of nitric oxide. Methods Enzymol. 2002;352:506–22. doi: 10.1016/s0076-6879(02)52044-9. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, et al. Quantitative measurements of NO reaction kinetics with a Clark-type electrode. Nitric Oxide. 2005;13(1):68–77. doi: 10.1016/j.niox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, et al. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277(29):26194–9. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 33.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258(2):322–30. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 34.Li H, et al. Xanthine oxidase catalyzes anaerobic transformation of organic nitrates to nitric oxide and nitrosothiols: characterization of this mechanism and the link between organic nitrate and guanylyl cyclase activation. J Biol Chem. 2005;280(17):16594–600. doi: 10.1074/jbc.M411905200. [DOI] [PubMed] [Google Scholar]
- 35.Li H, et al. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem. 2006;281(18):12546–54. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 36.Godber BL, et al. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275(11):7757–63. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 37.Kuppusamy P, et al. Three-dimensional spectral-spatial EPR imaging of free radicals in the heart: a technique for imaging tissue metabolism and oxygenation. Proc Natl Acad Sci U S A. 1994;91(8):3388–92. doi: 10.1073/pnas.91.8.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuppusamy P, et al. Whole body detection and imaging of nitric oxide generation in mice following cardiopulmonary arrest: detection of intrinsic nitrosoheme complexes. Magn Reson Med. 2001;45(4):700–7. doi: 10.1002/mrm.1093. [DOI] [PubMed] [Google Scholar]
- 39.Kuppusamy P, et al. Spatial mapping of nitric oxide generation in the ischemic heart using electron paramagnetic resonance imaging. Magn Reson Med. 1996;36(2):212–8. doi: 10.1002/mrm.1910360207. [DOI] [PubMed] [Google Scholar]
- 40.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys. 1998;357(1):1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 41.Chen YR, et al. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radic Biol Med. 2004;37(10):1591–603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Basu S, et al. Nitrite reductase activity of cytochrome c. J Biol Chem. 2008;283(47):32590–7. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamin N, et al. Stomach NO synthesis. Nature. 1994;368(6471):502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JO, et al. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35(11):1543–6. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Y, Zweier JL. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem. 1995;270(32):18797–803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 46.Beedham C. Molybdenum hydroxylases: biological distribution and substrate-inhibitor specificity. Prog Med Chem. 1987;24:85–127. doi: 10.1016/s0079-6468(08)70420-x. [DOI] [PubMed] [Google Scholar]
- 47.Beedham C, Bruce SE, Rance DJ. Tissue distribution of the molybdenum hydroxylases, aldehyde oxidase and xanthine oxidase, in male and female guinea pigs. Eur J Drug Metab Pharmacokinet. 1987;12(4):303–6. doi: 10.1007/BF03189918. [DOI] [PubMed] [Google Scholar]
- 48.Moriwaki Y, et al. Immunohistochemical localization of aldehyde and xanthine oxidase in rat tissues using polyclonal antibodies. Histochem Cell Biol. 1996;105(1):71–9. doi: 10.1007/BF01450880. [DOI] [PubMed] [Google Scholar]
- 49.Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest. 1996;74(1):48–56. [PubMed] [Google Scholar]
- 50.McCord JM, Roy RS, Schaffer SW. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv Myocardiol. 1985;5:183–9. [PubMed] [Google Scholar]
- 51.McCord JM. Free radicals and myocardial ischemia: overview and outlook. Free Radic Biol Med. 1988;4(1):9–14. doi: 10.1016/0891-5849(88)90005-6. [DOI] [PubMed] [Google Scholar]
- 52.Chambers DE, et al. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985;17(2):145–52. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- 53.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 54.Giraldez RR, Zweier JL. An improved assay for measurement of nitric oxide synthase activity in biological tissues. Anal Biochem. 1998;261(1):29–35. doi: 10.1006/abio.1998.2721. [DOI] [PubMed] [Google Scholar]
- 55.Ingram TE, et al. Blood vessel specific vaso-activity to nitrite under normoxic and hypoxic conditions. Adv Exp Med Biol. 2009;645:21–5. doi: 10.1007/978-0-387-85998-9_4. [DOI] [PubMed] [Google Scholar]
- 56.Dejam A, Hunter CJ, Gladwin MT. Effects of dietary nitrate on blood pressure. N Engl J Med. 2007;356(15):1590. doi: 10.1056/NEJMc070163. author reply 1590. [DOI] [PubMed] [Google Scholar]
- 57.Larsen FJ, et al. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355(26):2792–3. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 58.Shiva S, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100(5):654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 59.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 60.Alzawahra WF, et al. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295(2):H499–508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112(7):2636–47. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanin AF, et al. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64(1):96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nohl H, Staniek K, Kozlov AV. The existence and significance of a mitochondrial nitrite reductase. Redox Rep. 2005;10(6):281–6. doi: 10.1179/135100005X83707. [DOI] [PubMed] [Google Scholar]
- 64.Garthwaite J, et al. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4-]oxadiazolo-[4,3-a] quinoxalin-1-one. Mol Pharmacol. 1995;48(2):184–8. [PubMed] [Google Scholar]
- 65.Schrammel A, et al. Characterization of 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol. 1996;50(1):1–5. [PubMed] [Google Scholar]
- 66.Moro MA, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93(4):1480–5. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rassaf T, et al. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100(12):1749–54. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 68.Flogel U, et al. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98(2):735–40. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]