Abstract
Endothelial nitric oxide synthase (eNOS) is an important regulator of vascular and cardiac function. Peroxynitrite (ONOO−) inactivates eNOS, but questions remain regarding the mechanisms of this process. It has been reported that inactivation is due to oxidation of the eNOS zinc-thiolate cluster, rather than the cofactor tetrahydrobiopterin (BH4); however, this remains highly controversial. Therefore, we investigated the mechanisms of ONOO−-induced eNOS dysfunction and their dose-dependence. Exposure of human eNOS to ONOO− resulted in a dose-dependent loss of activity with a marked destabilization of the eNOS dimer. HPLC analysis indicated that both free and eNOS-bound BH4 were oxidized during exposure to ONOO−; however, full oxidation of protein bound biopterin required higher ONOO− levels. Additionally, ONOO− triggered changes in UV/Visible spectrum and heme content of the enzyme. Pre-incubation of eNOS with BH4 decreased dimer destabilization and heme alteration. Addition of BH4 to the ONOO−-destabilized eNOS dimer only partially rescued enzyme function. In contrast to ONOO− treatment, incubation with the zinc chelator TPEN with removal of enzyme-bound zinc did not change the eNOS activity or stability of the SDS-resistant eNOS dimer, demonstrating that the dimer stabilization induced by BH4 does not require zinc occupancy of the zinc-thiolate cluster. While ONOO− treatment was observed to induce loss of Zn-binding this can not account for the loss of enzyme activity. Therefore, ONOO−-induced eNOS inactivation is primarily due to oxidation of BH4 and irreversible destruction of the heme/heme-center.
Endothelial nitric oxide synthase (eNOS) is a homodimeric enzyme found in many tissues, and catalyzes the NADPH-dependent conversion from L-arginine to L-citrulline and nitric oxide (NO). NO is involved in many physiological processes, including regulation of vascular tension, angiogenesis, and inflammation (1). Abnormalities in NO production by the vascular endothelium result in endothelial dysfunction, and underlie many disease pathologies that are associated with oxidant stress such as hypertension, diabetes, aging, post-ischemic injury, and atherosclerosis (2). As such, it is not surprising that multiple mechanisms and multiple steps regulate eNOS activity, including Ca2+/calmodulin binding, substrate and cofactor availability, post-translational modification, protein-protein interaction, gene expression, and protein localization (3-6).
Endothelial NOS, like all mammalian NOS isoforms, is composed of two domains. The C-terminal reductase domain contains FAD and FMN, along with the NADPH binding site. The N-terminal oxygenase domain contains the arginine binding site, heme group, and tetrahydrobiopterin (BH4). The reaction mechanism is composed of two sequential steps that involve electron transfer from NADPH through the flavins to the heme iron, followed by oxygen binding and activation, and subsequent substrate monooxygenation. BH4 is critical for the formation of the activated oxygen species necessary for substrate monooxygenation, and biochemical studies have demonstrated that electron transfer from the reductase domain to the oxygenase domain is an inter-monomer event, and as such NOS activity requires the association of two NOS monomers (7). While it is clear that the structure of the enzyme influences its activity, questions remain regarding the specific molecular mechanisms of the formation of an active NOS dimer (8, 9).
In addition to its involvement in the catalytic activity of the enzyme, the fully reduced biopterin is known to stabilize the eNOS dimer. Low-temperature SDS-PAGE (LT-PAGE) studies have shown that eNOS purified from a yeast expression system was mainly monomer, and that incubation with BH4 and L-arginine lead to a pronounced shift toward eNOS dimers (10). Although the enzyme can be purified as the BH4-free dimer from various expression systems, there is strong biochemical evidence indicating that the addition of BH4, to either monomeric eNOS or preformed dimer, greatly stabilizes the dimeric structure of the enzyme (11-14). The heme group of eNOS has also been reported as a necessary component for eNOS dimer formation. Deletion of the amino acid residues involved in the heme ligation inhibited the formation of dimeric eNOS (14). In support of this hypothesis, heme release was detected during eNOS dimer dissociation by temperature induced denaturation (13). However, the relationship between eNOS dimer formation and heme assembly remains uncertain.
X-ray crystallography has shown that the NOS isoforms contain a zinc-thiolate cluster located at the dimer interface of the oxygenase domain (15,16). It has been reported that alteration of the zinc-thiolate, either by oxidation or nitrosylation of the cysteine sulfhydryl groups or by the addition of TPEN (a zinc chelator), results in dimer dissociation and loss of activity (17-19). This loss of activity could be prevented by the addition of the thioredoxin/thioredoxin reductase system, or by the addition of exogenous zinc (17,19). Moreover, it has been postulated that oxidation of the zinc-thiolate cluster is the predominant mechanism involved in ONOO− -induced eNOS dysfunction (18). However, there have been contradictory reports indicating that neither chemical removal of zinc nor mutation of the cysteine residues involved in zinc binding produces an inactive enzyme (11, 20-22).
ONOO− is a highly reactive oxidant formed by the reaction of superoxide and NO (23). An increased formation of ONOO− in vivo has been detected in disease conditions such as ischemia-reperfusion injury, sepsis, inflammation, and atherosclerosis (24-27), and it is clear that eNOS is involved in the generation of ONOO− under pathological conditions (24,28). NOS enzymes are known to be susceptible to inactivation by oxidants, including ONOO− (18,29), but the specific mechanism of inactivation is controversial and uncertain. Therefore, we investigated the mechanisms of eNOS dimer destabilization and inactivation induced by ONOO−. We find that after free BH4 is consumed by ONOO− oxidation, further oxidation of protein-bound BH4 initiates eNOS dimer destabilization, and a ONOO−-induced disruption of the eNOS-heme. Furthermore, we observe that the dimer stabilization induced by BH4 is not dependent upon the presence of zinc in the zinc-thiolate cluster. While addition of fresh BH4 can reconstitute the ONOO−-destabilized eNOS dimer, the ONOO−-induced heme disruption is not reversed, and as such the SDS-resistant dimer is not fully active.
MATERIALS AND METHODS
Materials
Tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN) was obtained from Molecular Probes Inc. (Eugene, OR). Hemin was purchased from Frontier Scientific Company (Lagon, UT). CaM, NADPH, L-arginine, BH4, N-nitro-L-arginine methyl ester (L-NAME), and ONOO− was purchased from Upstate Cell Signaling Solutions (Lake Placid, NY); the ONOO− concentration was determined by absorbance at 302 nm (E302 = 1.67 mM-1cm-1). The ONOO− stock, in 0.3 M NaOH, was diluted with 10 mM NaOH to the appropriate concentrations just before addition to the protein samples. Other reagents were purchased from Sigma Chemical Co. (St. Louis, MO), unless otherwise indicated.
Protein Purification
Human eNOS cDNAs encoding the full protein sequence and the oxygenase domain (amino acids 1-491) were each over-expressed in yeast, and the recombinant protein was purified either in the presence of BH4 or in the absence of BH4 as described previously (30) with modifications (31). After purification, any excess BH4 was removed by ultrafiltration.
Detection of SDS-resistant eNOS dimers
The full-length eNOS SDS-resistant monomer:dimer equilibrium was assayed using low-temperature SDS-PAGE (LT-PAGE) under reducing conditions as described previously with modifications (32). After treatment, eNOS samples were subjected to low temperature SDS-PAGE in reducing conditions (with 2.5% 2-mercaptoethanol) to resolve the dimer and monomer. The protein bands were visualized by Coomassie blue staining and heme content detected as peroxidase activity of each protein band (see below). The signal intensity of each band was quantified using an Alphalmager™ high performance gel documentation and image analysis system, Model 3300 (Alpha Innotech Co. San Leandro, CA).
Heme staining assay
After treatment, samples were subjected to LT-PAGE. The gels obtained from LT-PAGE were subjected to heme staining with 3,3’-dimethoxybenzidine/H2O2 according to the published method with modifications (13). Gels were washed with methanol/sodium acetate (0.25 M, pH 5.0; 3:7 v/v) for 10 min, and subsequently incubated in the dark for 20 min in a freshly prepared solution, containing 7 parts of 0.25 M sodium acetate, pH 5.0, and 3 parts of 6 mM 3,3’-dimethoxybenzidine dihydrochloride in methanol. Gels were developed for 60 min by adding 60 mM H2O2, and then washed with water/methanol/acetic acid (8:1:1, by vol.) for 30 min prior to densitometric analysis of heme staining.
Measurement of eNOS activity
The release of NO generated by eNOS was measured by the oxyhemoglobin to methemoglobin conversion assay following the procedures developed by Sheta et al. and Stuehr et al. with minor modification (33,34). The reaction system contained 5 μM oxyhemoglobin, 4 μg purified human eNOS, 0.2 mM DTT, 1 mM CaCl2, 1 μg/ml CaM, 5 μM BH4, 200 μM NADPH, in 50 mM HEPES buffer, pH 7.4, in a total volume of 100 μl. The reaction was initiated by the addition of 100 μM L-arginine. NO generation was measured by monitoring the change in absorbance A401-A410, and a millimolar absorbance coefficient of 38 mM-1cm-1. For activity measurements of the ONOO−-treated eNOS, the appropriate volume of diluted ONOO− was added and the samples were incubated on ice for 10 minutes. Since the half-life of ONOO− at neutral pH values is about 1 second, it is completely decomposed prior to addition to the assay mixture (35). For activity measurements with TPEN, a 1.25 mM TPEN solution was prepared in water, and this solution was used for the preparation of the reaction buffer above such that the final TPEN concentration was 1 mM.
Analysis of free and eNOS bound BH4
Total BH4 content in eNOS was measured using HPLC analysis. After eNOS or free BH4 was treated with ONOO−, eNOS-bound BH4 and free BH4 were extracted with buffer containing 6.5 mM dibasic sodium phosphate, 6 mM citric acid, 5 mM dithioerythritol, 2.5 mM DTPA, 3 mM 1-octanesulfonic acid, and 10% methanol. This buffer is able to extract and maintain the redox state of BH4. The HPLC system consisted of a CoulArray system from ESA Analytical, Ltd. (Chelmsford, MA) with automated gradient controller, refrigerated auto sampler, and ESA software for data collection and analysis. The chromatographic separation was carried out using a Toso Haas 5 μm ODS-80TM reverse phase column (4.6 mm × 25 cm) with flow rate of 1.3 mL/min. Tetrahydrobiopterin was electrochemically detected with the analytical electrodes set at 0.05 and 0.4 V. The guard cell is set at 0.8 V.
Heme and zinc quantitation
Heme content of eNOS was determined by the formation of pyridine hemochromogen (36). Briefly, 80 μg of purified eNOS was added to a solution of 0.15 M NaOH and 1.8 M pyridine, and the spectrum was recorded. The sample was then reduced with dithionite, and a second spectrum was recorded. The total heme content was calculated from the difference spectrum (A 556-538nm), using a millimolar absorbance coefficient of 24 mM-1cm-1. Free zinc was measured using the 4-(2-pyridylazo) resorcinol (PAR) assay as described previously (18). Zinc, iron, and copper content were also measured by ICP-MS. For ICP-MS 50 μg of eNOS was added to 50 mM KPO4, 150 mM NaCl, 0.6 mM dithiothreitol, and 2 mM DTPA (to chelate adventitial metals) at pH 7.4, in a total volume of 150 μl. Three sets of samples were analyzed, an untreated sample, a sample treated with 100 μM ONOO−, and a sample treated with 1 mM TPEN. Each sample was incubated on ice for two hours, and then filtered through 3,000 MW-cutoff Microcon spin columns and washed twice with the treatment buffer. The filtrates and washed protein samples were then assayed for Zn, Fe, and Cu by ICP-MS as described previously (37).
UV/Vis spectroscopy
Optical absorbance was measured in a Cary 300 double beam spectrophotometer from Varian, Inc (Palo Alto, CA), using 1-cm path length cuvettes at room temperature (23 °C).
Statistical Analysis
Data are expressed as mean ±SE experiments with given number or at least three independent experiments. Differences in mean value were analyzed by Student's T-test and a value of P < 0.05 was considered as statistically significant.
RESULTS
ONOO− inactivates eNOS and this is inhibited by exogenous BH4
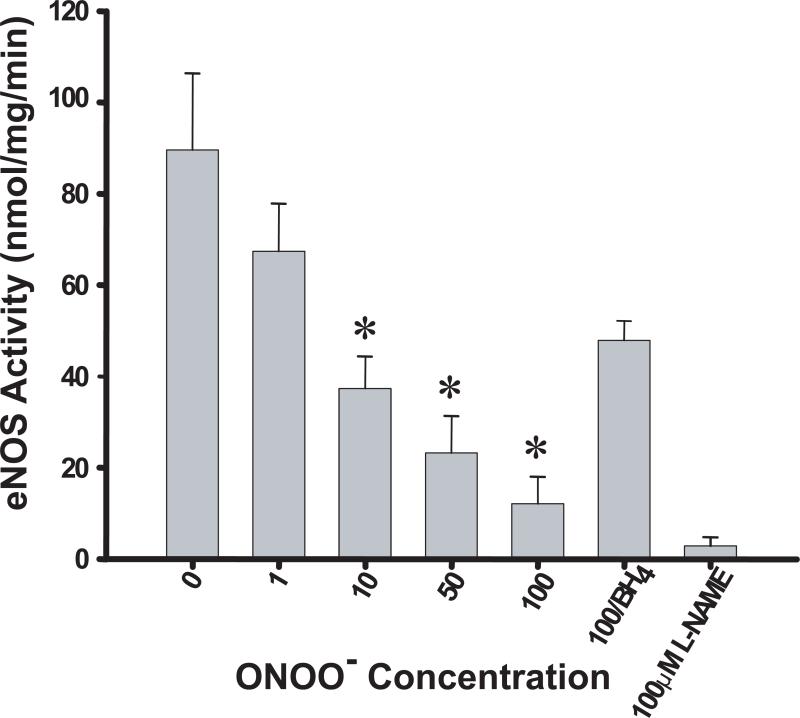
To investigate the effect of ONOO− on the activity of eNOS, we treated recombinant human eNOS with increasing concentrations of ONOO− and subsequently measured the rate of NO production. The activity of untreated eNOS purified from yeast expression system was 92 ± 18.4 nmol/mg/min (Figure 1), which is consistent with previously reported activities for the endothelial isoform (10, 12). Incubating eNOS with ONOO− resulted in a dose-dependent loss of eNOS activity, in agreement with previously published results (18). The NO generation rate was decreased to 16.5 ± 6.5 nmol/mg/min when eNOS was treated with 100 μM ONOO−. Although preincubation of eNOS with 100 μM BH4 did not fully protect the enzyme from oxidative damage, it did provide some protection, decreasing the ONOO− inactivation by ~70%.
Figure 1.
Inactivation of eNOS activity by ONOO− and the protective effect of BH4. Purified human eNOS (0.1 μg/μL) in 50 mM HEPES, pH 7.4, was treated with the indicated concentrations of ONOO−. After incubation (45 min on ice) eNOS activity was measured as described in the Materials and Methods. The values of eNOS activity presented are obtained from the average of six independent assays (*, P<0.05).
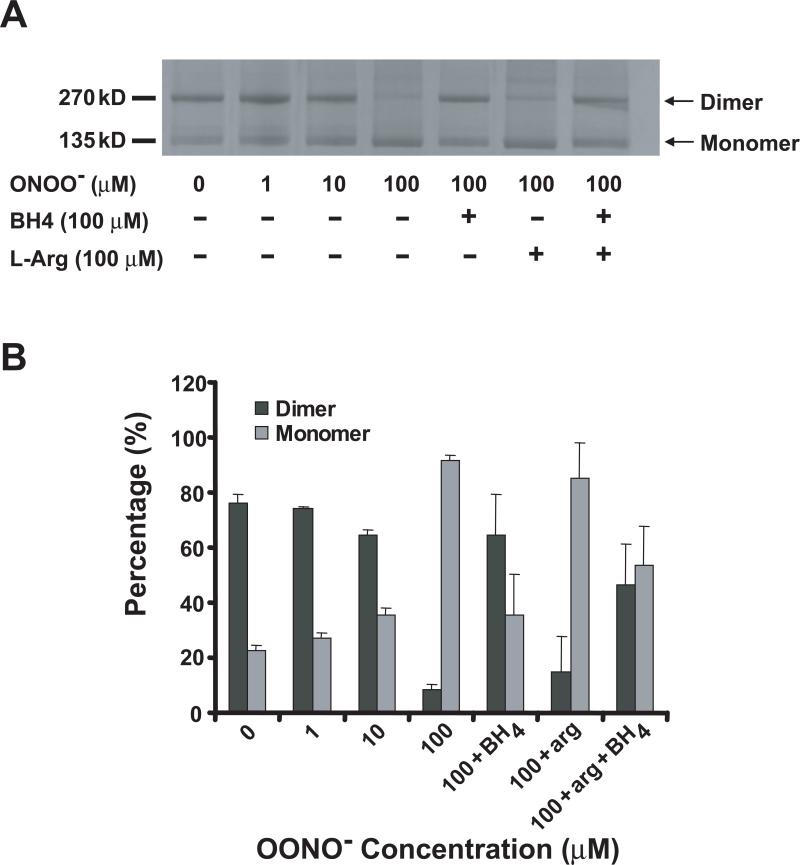
To begin to elucidate the molecular mechanism underlying the ONOO−-induced eNOS inactivation, we sought to determine if exposure to ONOO− induced destabilization of the eNOS dimer. As shown in Figure 2A, low-temperature SDS-PAGE can be used to detect changes in dimer stability, detecting both the eNOS monomer with a molecular weight of 135 kDa, and the homodimer with a molecular weight of 270 kDa. When saturated with BH4, eNOS demonstrated approximately 80% SDS-resistant dimer. Incubation of eNOS with ONOO− destabilized the eNOS dimer in a concentration-dependent manner. The percentage of SDS-resistant eNOS dimer decreased to less than 10% when eNOS was incubated with 100 μM ONOO− for 45 minutes at room temperature, with a concomitant increase of apparent monomer. A similar pattern of ONOO−-induced dimer destabilization was also observed using recombinant bovine eNOS expressed in E. coli (data not shown). Pre-incubation of eNOS with 100 μM BH4 significantly protected the eNOS dimer from the destabilization induced by 100 μM ONOO−. The SDS-resistant dimer content of ONOO−-treated eNOS with or without BH4-preincubation was 64% and <10%, respectively. However, we did not observe a protective effect from 100 μM L-arginine. Only 14% SDS-resistant dimer was observed when eNOS was pretreated with L-arginine and then treated with ONOO−. Furthermore, there was no synergistic protective effect of BH4 and L-arginine (Figures 2A and 2B).
Figure 2.
ONOO−-induced eNOS dimer destabilization and the protective effects of BH4. (A) Purified human eNOS (0.1 μg/μL) in 50 mM HEPES, pH 7.4, was preincubated with L-arginine (100 μM) or BH4 (100 μM) at 0 °C for 30 min. The enzyme was treated with indicated concentration of ONOO− for 45 min at 0 °C. The entire mixture was then subjected to LT-PAGE, and the protein bands were visualized by Coomassie Blue staining. (B) The ratio of eNOS dimer/monomer in (A) was quantitated by digital densitometry as described in the Materials and Methods. The data analyses were obtained from the average of six independent experiments.
BH4 bound to eNOS is a target of oxidation by ONOO−
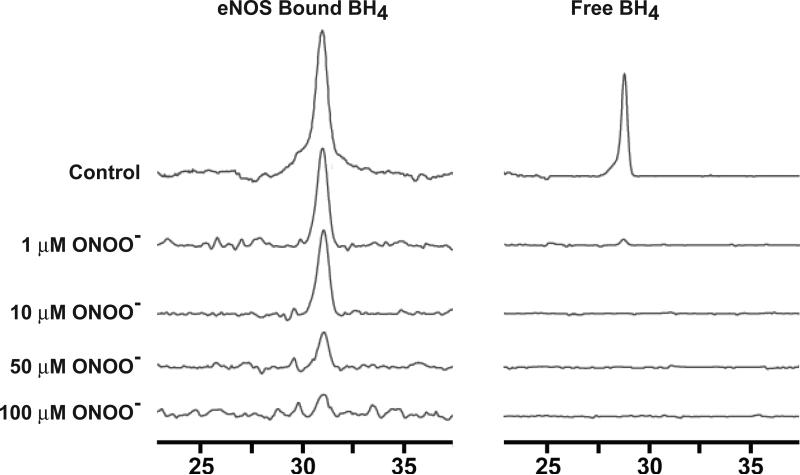
Previously reported results indicated that BH4 inhibited eNOS dimer dissociation induced by ONOO− (19). Pure BH4 can react with ONOO− to form an intermediate, the BH3 radical, and subsequently BH2 (38), and studies have shown that auto-oxidation of BH4 in eNOS resulted in the loss of enzymatic activity (31). To directly address whether or not BH4 bound to eNOS is a target for ONOO− oxidation, 15 μg of eNOS was incubated with various concentrations of ONOO− for 45 min at room temperature, and BH4 was then extracted from the reaction mixtures followed by HPLC analysis. A chromatogram representing typical results is shown in Figure 3; BH4 had a retention time of ~30 min. As shown in Figure 3, the amount of BH4 extracted from ONOO− treated eNOS gradually decreased as the dosage of ONOO− was elevated. Only trace amounts of BH4 (~ 8 %) were still bound to eNOS at 100 μM ONOO−. We also investigated the ONOO− induced oxidation of free BH4. Pure BH4 (1 μM) was incubated with various concentration of ONOO−, and the results detected by HPLC. As shown in Figure 3, addition of 1 μM ONOO− oxidized more than 95% of free BH4. Thus, free BH4 is oxidized in essentially a 1:1 stoichiometric ratio by addition of ONOO− (38).
Figure 3.
ONOO−-induced oxidation of BH4 in eNOS or free BH4 as measured by HPLC with electrochemical detection. Purified eNOS (0.15 μg/μL) or free BH4 (1 μM) was incubated in 100 μL of 50 mM HEPES, pH 7.4, with different concentrations of ONOO− as indicated for 45 min. 80 μl of BH4 extraction buffer was added into the solution and centrifuged for 15 min at 16,000 × g. The supernatant was filtered and aliquots (25 μl) of the filtrate were subjected to HPLC analysis.
The effect of ONOO− on the heme center of eNOS and Hemin (Fe- protoporphyrin IX)
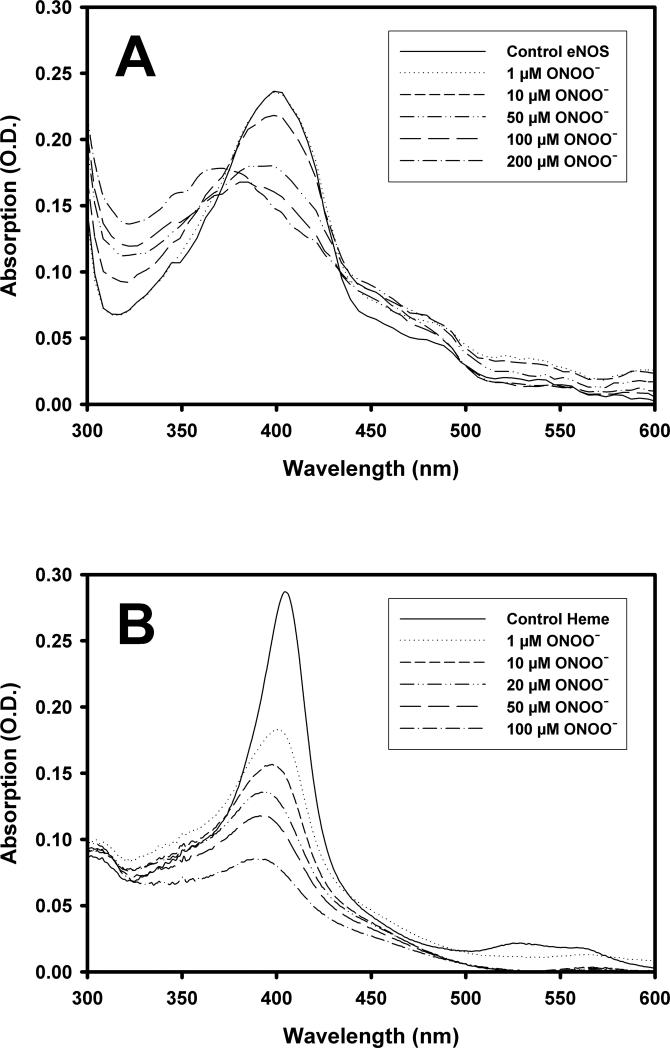
The NOS heme center has been implicated as critical to eNOS dimer formation, and deletion of the cysteine involved in heme binding resulted in the inhibition of eNOS dimer association (14). To investigate how ONOO− affects the heme center of eNOS, we followed the absorption changes of the heme as eNOS was exposed to ONOO−. As shown in Figure 4A, recombinant eNOS demonstrated a typical NOS high-spin heme absorption with a Soret band at 398 nm. As the ONOO− concentration was increased, the heme absorption at 398 nm decreased. Approximately 70% of eNOS-bound heme absorption was bleached by the addition of 100 μM ONOO− (Figure 4A). Similar spectral changes were observed using eNOS and eNOS oxygenase domain purified from E. coli. Moreover, when pure iron protoporphyrin IX was incubated with increasing concentrations of ONOO−, a dose dependent decrease in heme absorption at the Soret γ band (λmax = 405 nm) was also observed (Figure 4B). More than 85% of the iron protoporphyrin IX absorption was bleached when exposed to 100 μM ONOO−, indicating that free iron protoporphyrin IX is more sensitive to ONOO− treatment than eNOS-bound heme (Figure 4B)
Figure 4.
ONOO−-induced changes in the UV/VIS spectra of eNOS and pure heme. (A) eNOS (0.24 μg/μL) in 50 mM HEPES, pH 7.4, was treated with various concentrations of ONOO−, incubated for 10 min at RT, and the UV/VIS spectra recorded. (B) same as (A) except that the buffer included 5% pyridine and the eNOS was replaced with pure iron protoporphyrin (2 μM).
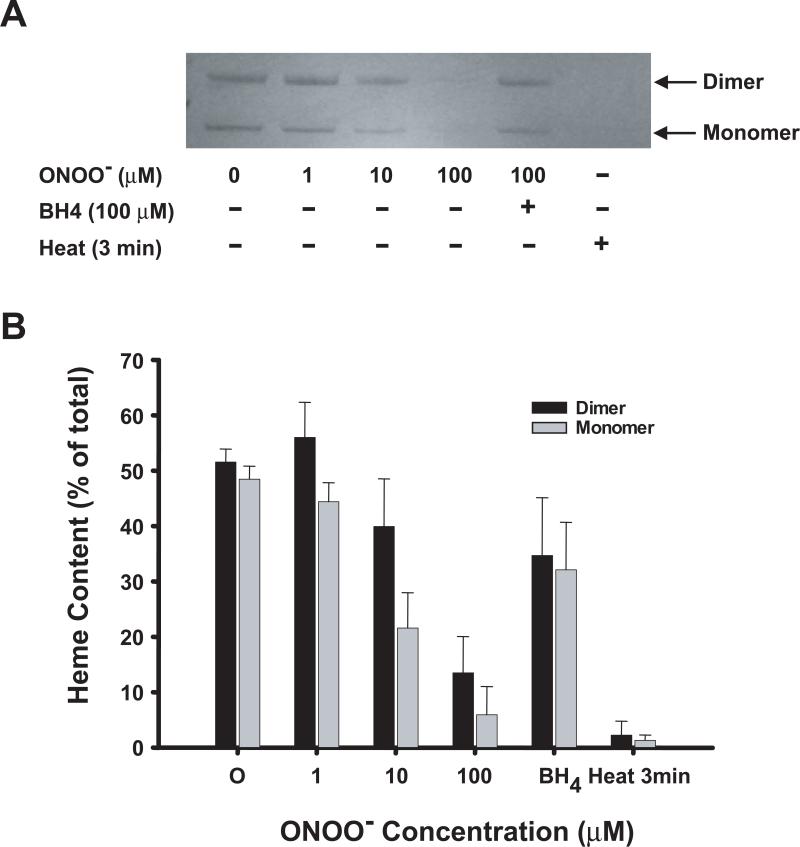
To investigate the role of the observed ONOO−-induced heme bleaching in the eNOS dimer formation/dissociation, ONOO−-treated enzyme was subjected to LT-PAGE and heme content was determined. For native recombinant eNOS, heme can be detected in both SDS-resistant dimer and SDS-induced monomer (Figure 5). Incubating eNOS with various concentrations of ONOO− at room temperature decreased the detectible heme content of both monomer and dimer (as detected on low temperature SDS-PAGE) in a dose-dependent manner. Heme was almost completely undetectable after treatment with 100 μM ONOO−. Pre-incubation of eNOS with 100 μM BH4 for 30 minutes significantly inhibited ONOO−-induced heme loss. Heating eNOS (95 °C) for 3 minutes in SDS-PAGE buffer resulted in complete loss of detectible heme from eNOS (Figure 5A). Notably, we detected a greater ONOO−-induced heme loss from the monomer compared to SDS-resistant dimer of eNOS at all ONOO− concentrations tested. It should be noted that our heme staining method detects the inherent peroxidase activity of the heme, and as such any detectable loss of activity that migrates with the full length eNOS on SDS-PAGE can be interpreted as release of the heme from the enzyme or disruption of the bound heme.
Figure 5.
Detection of heme release from ONOO−-treated eNOS by heme staining. (A) Purified human eNOS (0.1 μg/μL) was incubated in HEPES, pH 7.4, with the indicated concentration of ONOO− for 45 min. The samples were subjected to LT-PAGE, and heme staining as described in Materials and Methods. (B) The heme content was quantitated by digital densitometry as described in Materials and Methods, and represented as the percent of the total eNOS heme detected in either the monomer or dimer.
BH4 does not require zinc binding for eNOS dimer stabilization
Previous studies have indicated that the zinc-thiolate (ZnS4) cluster, identified by examination of the x-ray crystal structure of the oxygenase domain dimer, plays an important role in eNOS structure and function (9,15) and the 1:2 zinc to iron stoichiometry of eNOS zinc binding has been demonstrated (39). Our studies have indicated that BH4 contributes to eNOS dimer stabilization and inhibits the dimer destabilization induced by ONOO−. It is important to clarify whether the zinc-thiolate cluster is the predominant factor for eNOS dimer stabilization, and whether both BH4 and the zinc-thiolate cluster can affect eNOS dimer formation in a synergistic way.
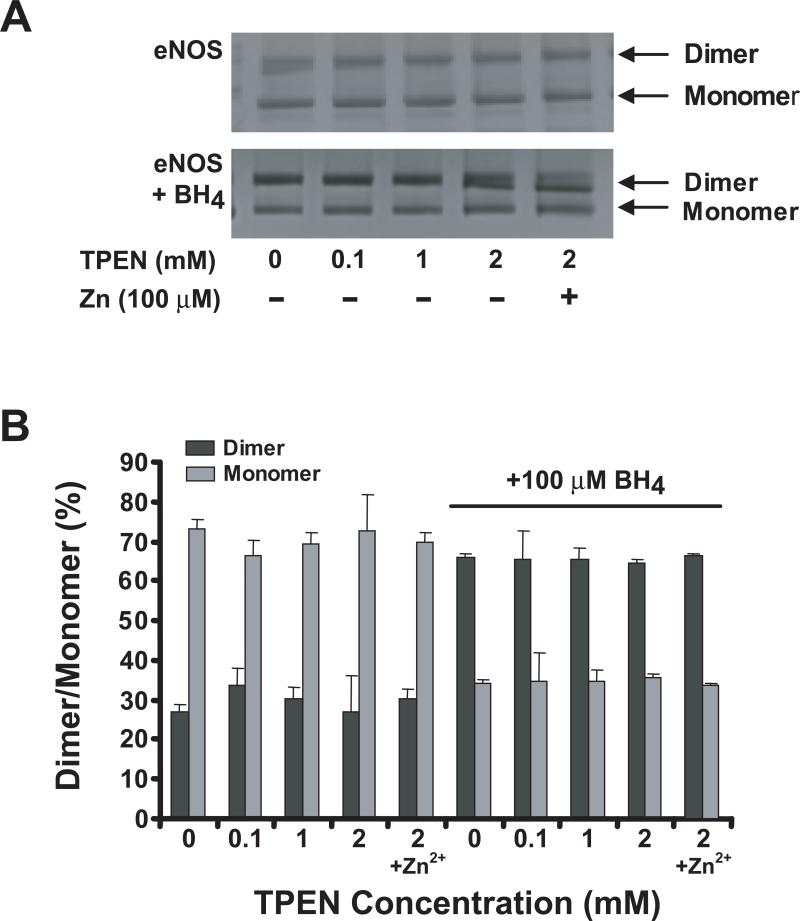
In order to confirm ONOO−-induced zinc release, we exposed our purified eNOS to 100 μM ONOO−, and measured free zinc with the PAR assay (18). For our recombinant eNOS, this treatment resulted in the release of ~0.35 nmoles of zinc for each nmole of eNOS dimer, thus it is clear that ONOO−-treatment does induce zinc loss. Next we exposed eNOS to the selective zinc-chelating agent TPEN, in the absence or presence of BH4. Incubation of eNOS with 1 mM TPEN for two hours on ice did not alter the activity of the enzyme. We measured the amount of zinc removed by TPEN treatment using ICP-MS, and found that ~80% of the eNOS-bound zinc was removed by the TPEN treatment. To determine if incubation with TPEN altered the stability of the dimeric structure of eNOS, we used low temperature SDS-PAGE. As shown in Figure 6A (upper panel), BH4-free eNOS was only 27% dimer, and incubation of eNOS with TPEN did not cause a detectable change in dimer stabilization. Moreover, incubation with 100 μM Zn2+ did not alter the SDS-resistant eNOS dimer structure. These results are not consistent with those previously reported (18). However, 100 μM BH4 did significantly enhance eNOS dimer stabilization (Figure 6A, lower panel), consistent with previously published reports (10,12-14). Incubation of eNOS with 100 μM BH4 increased the dimer percentage from ~27 % to ~65 %, irrespective of the presence or absence of TPEN, and like in the BH4 free enzyme, exogenous Zn2+ had no effect on eNOS dimer stabilization in the absence or presence of 100 μM BH4 (Figure 6B).
Figure 6.
Effect of TPEN, Zn2+, and BH4 on eNOS dimerization. (A) Purified human eNOS (0.1 μg/μL) in 50 mM HEPES, pH 7.4, was treated with various concentrations of TPEN for 45 min in the presence or absence of 100 μM Zn2+. The mixture was subjected to LT-PAGE and the protein bands were visualized by coomassie blue staining. (B) Same as (A), except the eNOS was preincubated with or without BH4 (100 μM) prior to addition of TPEN and Zn2+. The ratios of eNOS dimer/monomer were quantitated by digital densitometry as described in Materials and Methods.
BH4 rescues ONOO−-destabilized eNOS (SDS-resistant dimer) but not eNOS function
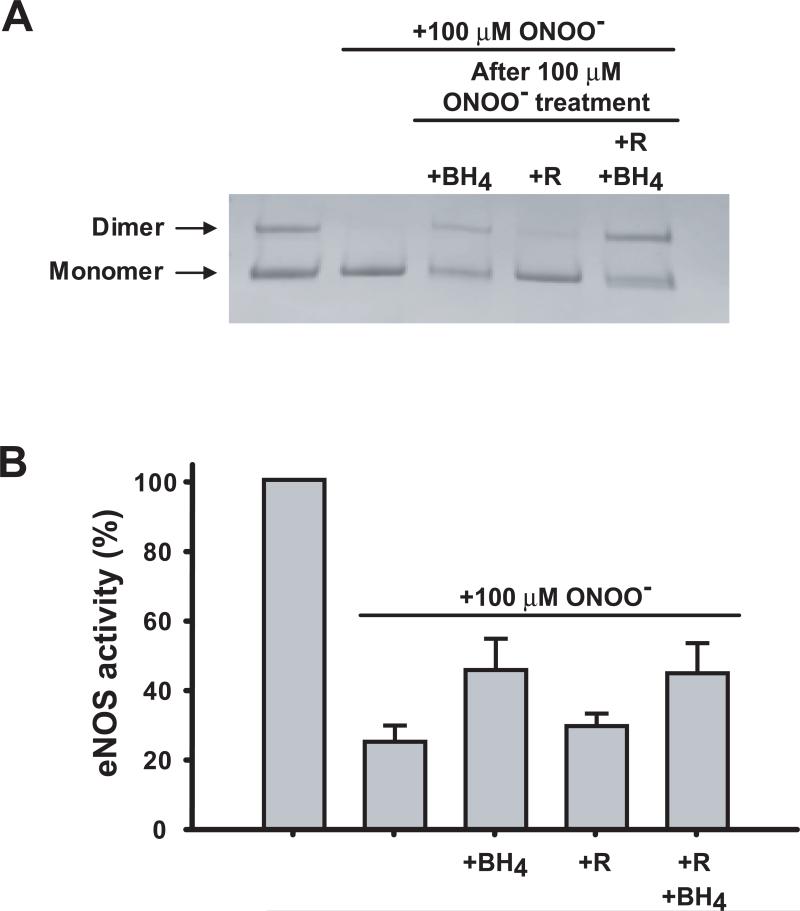
Our current and previously published work (29), demonstrate that BH4 is oxidized by ONOO− leading to inhibition of NOS-dependent NO formation. Additionally, preincubation of eNOS with excess BH4 can significantly protect eNOS structure and function against ONOO− damage. It is of interest to determine whether BH4 functions just as an ONOO− scavenger or if it can restore the stability of the eNOS dimer and rescue ONOO−- induced decreases in enzyme activity. To address this issue, the eNOS dimer stability and enzymatic activity were measured before and after subsequent incubation with BH4, L-arginine, or both. As shown in Figure 7A, incubation of 100 μM BH4 with ONOO−-treated eNOS resulted in recovery of the SDS-resistant eNOS dimer structure, and this recovery was mildly synergistic with arginine; however, incubation with L-arginine (100 μM) alone did not recover the eNOS dimer. Although BH4 addition did rescue some ONOO−-induced loss of eNOS activity, unlike the dimer stabilization this recovery of activity was not complete. The activity of ONOO−-treated eNOS was increased from 25%, to 46% (+BH4), to 29% (+Arg), and to 45% (+BH4; +Arg) (Figure 7B), as such BH4 is only able to restore about one third of the ONOO−-induced loss of eNOS activity.
Figure 7.
Effects of BH4 post-addition on the restoration of eNOS dimer stability and the enzymatic activity of eNOS. (A) eNOS (0.2 μg/μL) in HEPES, pH 7.4, was treated with 100 μM ONOO−. BH4, L-arginine, or both were then added to the ONOO−-treated eNOS. The mixtures were incubated for 30 min, and then subjected to LT-PAGE and staining with coomassie blue. (B) Samples were the same as (A), except that aliquots of each mixture were withdrawn for measurement of eNOS activity as described in Materials and Methods.
DISCUSSION
In the current studies, we have demonstrated that ONOO− causes eNOS dimer destabilization via the oxidation of BH4. Exogenous BH4 can inhibit the ONOO−-mediated eNOS dimer destabilization, mainly by acting as a scavenger of the oxidant. Furthermore, our studies indicate that BH4 is the predominant factor for SDS-resistant eNOS dimer stabilization when compared with the effects induced by L-arginine binding and/or by influences of the presence of zinc in the zinc-thiolate cluster. We have also demonstrated the novel finding that ONOO− treatment causes a major alteration in heme structure rendering the NOS heme negative for peroxidase-activity based staining. Finally, although BH4 can reconstitute the ONOO−-destabilized SDS-resistant eNOS dimer, the reformed dimer is not fully functional.
ONOO− is a highly reactive oxidant formed in biological systems when superoxide and NO are produced simultaneously, and the physiological flux of ONOO− has been estimated to be approximately 0.2 – 0.4 uM/sec (23,40). The majority of NO in biological systems is generated from the various NOS isoforms, and it is clear that the eNOS produces superoxide under certain conditions (41,42). Indeed, under conditions of limited L-arginine or BH4 depletion the NOS isozymes generate both NO and superoxide simultaneously (41,43-45). Additionally, superoxide is produced from a number of different sources under various physiologic and pathophysiologic conditions (46). The ONOO− formed in vivo has a wide array of tissue damaging effects ranging from lipid peroxidation, inactivation of enzymes and ion channels via protein oxidation and nitration, and inhibition of mitochondrial respiration (23). It has been reported that ONOO− inactivates NO generation from all the NOS isoforms (18,29,47,48); however, the mechanisms by which this inactivation occurs have been controversial.
Peroxynitrite destabilizes the eNOS dimer via oxidation of BH4, not removal of zinc from the zinc-thiolate cluster
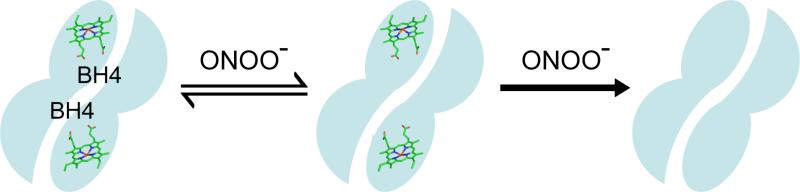
It has been reported that ONOO− caused eNOS dimer dissociation by breaking the putative zinc-thiolate cluster that is located at the dimer interface, and that this dimer dissociation effectively uncouples NADPH oxidation from NO production leading to loss of NOS activity (18). Additionally, these authors postulate that the oxidation of the zinc-thiolate cluster is the predominant factor in the observed ONOO−-induced uncoupling of eNOS rather than oxidation of BH4. However, there is also structural and biochemical evidence demonstrating that BH4 is involved in the stabilization of the NOS dimer. X-ray crystal structures of the oxygenase domains from many NOS isoforms demonstrate that the BH4 binding site is located at the dimer interface, situated in a pocket composed of several conserved residues (9, 15). Although BH4 is not strictly required for dimerization, there is a marked stabilization of the active dimer with BH4 binding (11-14,49,50). From our HPLC analysis, and from previously published data (38, 51-53), it is clear that BH4, both when it is free in solution and when it is protein bound, is a target for oxidation. It is well established that the fully reduced biopterin is required for the coupling of NADPH oxidation to the production of NO and for suppression of superoxide generation from eNOS (41,42). Therefore, it is clear that oxidation of BH4 is instrumental in the uncoupling of NOS irrespective of the stability of the NOS dimer, and our results suggest that oxidation of BH4 in eNOS is a predominant driving force in ONOO−-induced SDS-resistant dimer dissociation.
Although we could detect ONOO−-induced release of zinc from eNOS, in contrast to previously published work we could not detect any change in the SDS-resistant eNOS monomer: dimer ratio with either the addition of exogenous zinc or the addition of the zinc chelator TPEN. While we cannot completely rule out the involvement of the zinc-thiolate cluster in the ONOO− - induced destabilization of the eNOS dimer, our data and previously published work on other NOS isoforms indicate that the direct contribution of the presence of zinc in the cluster to dimer stabilization is small relative to that provided by bound BH4 and the heme. Indeed, studies have been performed on nNOS, which indicated that removal of zinc from nNOS by reversible chemical modification of the cysteine residues destabilized the SDS-resistant dimer, but that this effect was blocked by the addition of BH4 (11). Additionally, upon reversal of the cysteine modification, the zinc-free nNOS retained almost 100% activity.
Mutational analyses in nNOS (C331A) and eNOS (C99A) have demonstrated that although the presence of zinc in the Zn-S4 cluster is not strictly required for catalysis, the structural integrity of the cluster which is altered by the mutations, is important for binding BH4 and/or arginine (21,22). The C331A nNOS mutation produced an inactive enzyme, but overnight incubation with excess arginine restored activity (21), and likewise, the inactive C99A eNOS mutant was restored by overnight incubation with BH4 (22). This is not surprising given the structural contiguity identified between the Zn-S4 cluster and the cofactor/substrate binding sites (15). Additionally, mutation of the specific cysteine residues involved in the zinc binding of iNOS to alanine resulted in the loss of zinc but did not inhibit dimer formation or enzyme activity under normal assay conditions (20). These authors did demonstrate an inhibition of enzyme activity in the cysteine to alanine mutants when the enzyme was preincubated at 37°C, and demonstrated an inactivation of the wild type enzyme via an NO driven nitrosylation of the zinc-thiolate cysteines. Additionally, the zinc content of both nNOS and eNOS has been shown to decrease in the absence of BH4 (39.). Therefore, although BH4 provides a much more significant stabilization of the eNOS dimer relative to the zinc occupancy of the Zn-S4 cluster, it is clear that the structure of this cluster is involved in the formation of the fully active conformation of the enzyme. As such, modification of this cluster, either by oxidation or nitrosylation could provide a means by which enzyme activity is regulated by changing BH4 and/or arginine binding affinity.
Peroxynitrite-induced heme destruction
We have observed the novel finding that the BH4 mediated ONOO−-induced dimer destabilization and enzyme inactivation was accompanied by an alteration in the bound heme cofactor or its environment, as indicated by marked changes in the optical absorbance spectrum. Similar ONOO−-induced changes in visible absorption spectra have been observed for both iNOS and nNOS (47,48). We have demonstrated that these spectral changes are accompanied by heme loss or destruction from both the SDS-resistant eNOS dimer and monomer. Furthermore, addition of ONOO− to free hemin resulted in significant quenching. Therefore, we hypothesize that in addition to oxidizing BH4, ONOO− treatment also disrupts the heme center via either an indirect mechanism involving oxidation of amino acids required for heme binding, producing heme release and subsequent destruction of the free heme, or a direct mechanism by modification of the NOS bound heme.
The cysteine residue proximal to the heme is a good candidate target for the destruction mechanism. Indeed, the ONOO− -induced heme spectral changes we observed are similar to those observed with formation of an inactive NOS P420 species generated by NO-induced dissociation of the proximal cysteine (54). However, our observed spectral changes are of the ferric enzyme and are not reversible by the addition of reducing agents or BH4. Additionally, conversion to the P420 form has not been demonstrated to produce heme loss (54,55), and the P420 form would still be expected to retain peroxidase activity (56). Thus, we conclude that ONOO− -induced dissociation of the proximal cysteine is not the only mechanism involved in the observed heme destruction.
The observed ONOO− -induced change in the heme optical absorption (bleaching) occurs irrespective of the expression system used for the recombinant enzyme and is not inhibited by the presence of bound BH4 (although exogenously added BH4 will act as a ONOO− scavenger and inhibit the bleaching). The extent of the observed spectral change is dependent upon the ONOO−/eNOS molar ratio, with higher ratios giving more bleaching. Our data are consistent with a model similar to that proposed for the reaction of peracids with cytochromes P450 (57, 58), wherein destruction of the porphyrin ring (which leads to the observed bleaching of the Soret transition) is due to the reaction of excess peracid with a higher oxidation state heme. Peracids can react with P450 hemes to form the oxenoid intermediate (FeIV=O:cation radical), and subsequent reaction with another equivalent of peracid leading to heme bleaching. It has been demonstrated that heme-thiolate groups, including the iNOS heme, can react with ONOO−, potentially via a ferryl-heme intermediate (59, 60). Thus, we hypothesize that ONOO− first reacts with the eNOS heme forming the ferryl intermediate (or other higher oxidation state heme) which then reacts with a second equivalent of ONOO−, leading to the observed heme bleaching and the loss of eNOS peroxidase activity. As expected for any P450-type enzyme, heme destruction results in eNOS inactivation (Figure 7A.).
In conclusion, ONOO− oxidizes the eNOS-bound-BH4, triggering eNOS dimer dissociation and loss of activity. With increasing concentrations ONOO− also induces the destruction of the heme group and further irreversible loss of activity. Oxidation of the zinc-thiolate cluster with loss of zinc binding does occur but the zinc loss does not appear to cause enzyme inactivation. Preincubation of eNOS with BH4 can protect the structural integrity and enzymatic activity of eNOS from ONOO− damage via an antioxidant mechanism. Post-addition of BH4 can re-stabilize the ONOO−-destabilized eNOS dimer, but it cannot completely restore the enzymatic activity due to damage to the eNOS heme. Therefore, we propose a model of ONOO−-induced inactivation of eNOS wherein at low oxidant concentrations, BH4 is the primary target for oxidation, leading to reversible enzyme inactivation; while at higher ONOO− concentrations, the heme or the heme site becomes susceptible to damage, leading to heme destruction and irreversible enzyme inactivation.
ACKNOWLEDGMENTS
We would like to thank Dr. John W. Olesik and Anthony Lutton of the Trace Element Research Laboratory, School of Earth Sciences, The Ohio State University, for excellent technical assistance with the ICP-MS.
ABBREVIATIONS
- eNOS
endothelial nitric oxide synthase
- TPEN
Tetrakis-(2-pyridylmethyl) ethylenediamine
- BH4
(6R)-5,6,7,8-tetrahydrobiopterin
- ONOO−
peroxynitrite
- DTPA
diethylene triamine pentaacetic acid
- PAR
4-(2-pyridylazo) resorcinol
Footnotes
This work was supported by National Institute of Health Grants HL38324, HL63744, HL 65608 (JLZ), and NIGM 56818 (ALT).
REFERENCES
- 1.Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep. 2003;5:473–480. doi: 10.1007/s11906-003-0055-4. [DOI] [PubMed] [Google Scholar]
- 3.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 5.García-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 6.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 7.Siddhanta U, Presta A, Fan B, Wolan D, Rousseau DL, Stuehr DJ. Domain swapping in inducible nitric-oxide synthase. Electron transfer occurs between flavin and heme groups located on adjacent subunits in the dimer. J Biol Chem. 1998;273:18950–18958. doi: 10.1074/jbc.273.30.18950. [DOI] [PubMed] [Google Scholar]
- 8.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 9.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK, Weber PC. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 10.Leber A, Hemmens B, Klösch B, Goessler W, Raber G, Mayer B, Schmidt K. Characterization of recombinant human endothelial nitric-oxide synthase purified from the yeast Pichia pastoris. J Biol Chem. 1999;274:37658–37664. doi: 10.1074/jbc.274.53.37658. [DOI] [PubMed] [Google Scholar]
- 11.Hemmens B, Goessler W, Schmidt K, Mayer B. Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric-oxide synthase. J Biol Chem. 2000;275:35786–3578691. doi: 10.1074/jbc.M005976200. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Crespo I, Gerber NC, Ortiz de Montellano PR. Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem. 1996;271:11462–11467. doi: 10.1074/jbc.271.19.11462. [DOI] [PubMed] [Google Scholar]
- 13.List BM, Klösch B, Völker C, Gorren AC, Sessa WC, Werner ER, Kukovetz WR, Schmidt K, Mayer B. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J. 1997;323:159–165. doi: 10.1042/bj3230159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venema RC, Ju H, Zou R, Ryan JW, Venema VJ. Subunit interactions of endothelial nitric-oxide synthase. Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem. 1997;272:1276–1282. doi: 10.1074/jbc.272.2.1276. [DOI] [PubMed] [Google Scholar]
- 15.Raman CS, Li H, Martásek P, Král V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 16.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK, Weber PC. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 17.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell DA, Erwin PA, Michel T, Marletta MA. S-Nitrosation and regulation of inducible nitric oxide synthase. Biochemistry. 2005;44:4636–4647. doi: 10.1021/bi0474463. [DOI] [PubMed] [Google Scholar]
- 21.Martásek P, Miller RT, Liu Q, Roman LJ, Salerno JC, Migita CT, Raman CS, Gross SS, Ikeda-Saito M, Masters BS. The C331A mutant of neuronal nitric-oxide synthase is defective in arginine binding. J Biol Chem. 1998;273:34799–34805. doi: 10.1074/jbc.273.52.34799. [DOI] [PubMed] [Google Scholar]
- 22.Chen PF, Tsai AL, Wu KK. Cysteine 99 of endothelial nitric oxide synthase (NOS-III) is critical for tetrahydrobiopterin-dependent NOS-III stability and activity. Biochem Biophys Res Commun. 1995;215:1119–1129. doi: 10.1006/bbrc.1995.2579. [DOI] [PubMed] [Google Scholar]
- 23.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 25.Torres-Dueñas D, Celes MR, Freitas A, Alves-Filho JC, Spiller F, Dal-Secco D, Dalto VF, Rossi MA, Ferreira SH, Cunha FQ. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br J Pharmacol. 2007;152:341–352. doi: 10.1038/sj.bjp.0707393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 27.White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–2972. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Druhan LJ, Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–133. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du M, Yeh HC, Berka V, Wang LH, Tsai AL. Redox properties of human endothelial nitric-oxide synthase oxygenase and reductase domains purified from yeast expression system. J Biol Chem. 2003;278:6002–6011. doi: 10.1074/jbc.M209606200. [DOI] [PubMed] [Google Scholar]
- 31.Berka V, Yeh HC, Gao D, Kiran F, Tsai AL. Redox function of tetrahydrobiopterin and effect of L-arginine on oxygen binding in endothelial nitric oxide synthase. Biochemistry. 2004;43:13137–13148. doi: 10.1021/bi049026j. [DOI] [PubMed] [Google Scholar]
- 32.Klatt P, Schmidt K, Lehner D, Glatter O, Bächinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheta EA, McMillan K, Masters BS. Evidence for a bidomain structure of constitutive cerebellar nitric oxide synthase. J Biol Chem. 1994;269:15147–15153. [PubMed] [Google Scholar]
- 34.Stuehr DJ, Kwon NS, Nathan CF, Griffith OW, Feldman PL, Wiseman J. Nomega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991;266:6259–6263. [PubMed] [Google Scholar]
- 35.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1998;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berka V, Palmer G, Chen PF, Tsai AL. Effects of various imidazole ligands on heme conformation in endothelial nitric oxide synthase. Biochemistry. 1998;37:6136–6144. doi: 10.1021/bi980133l. [DOI] [PubMed] [Google Scholar]
- 37.Raman SV, Winner MW, 3rd, Tran T, Velayutham M, Simonetti OP, Baker PB, Olesik J, McCarthy B, Ferketich AK, Zweier JL. In vivo atherosclerotic plaque characterization using magnetic susceptibility distinguishes symptom-producing plaques. JACC Cardiovasc Imaging. 2008;1:49–57. doi: 10.1016/j.jcmg.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 39.Miller RT, Martásek P, Raman CS, Masters BS. Zinc content of Escherichia coli-expressed constitutive isoforms of nitric-oxide synthase. Enzymatic activity and effect of pterin. J Biol Chem. 1999;274:14537–14540. doi: 10.1074/jbc.274.21.14537. [DOI] [PubMed] [Google Scholar]
- 40.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 41.Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berka V, Wang LH, Tsai AL. Oxygen-induced radical intermediates in the nNOS oxygenase domain regulated by L-arginine, tetrahydrobiopterin, and thiol. Biochemistry. 2008;47:405–420. doi: 10.1021/bi701677r. [DOI] [PubMed] [Google Scholar]
- 46.Elmarakby AA, Williams JM, Pollock DM. Targeting sources of superoxide and increasing nitric oxide bioavailability in hypertension. Curr Opin Investig Drugs. 2003;4:282–290. [PubMed] [Google Scholar]
- 47.Hühmer AF, Nishida CR, Ortiz de Montellano PR, Schöneich C. Inactivation of the inducible nitric oxide synthase by peroxynitrite. Chem Res Toxicol. 1997;10:618–626. doi: 10.1021/tx960188t. [DOI] [PubMed] [Google Scholar]
- 48.Pasquet JP, Zou MH, Ullrich V. Peroxynitrite inhibition of nitric oxide synthases. Biochimie. 1996;78:785–791. doi: 10.1016/s0300-9084(97)82537-0. [DOI] [PubMed] [Google Scholar]
- 49.Panda K, Rosenfeld RJ, Ghosh S, Meade AL, Getzoff ED, Stuehr DJ. Distinct dimer interaction and regulation in nitric-oxide synthase types I, II, and III. J Biol Chem. 2002;277:31020–31030. doi: 10.1074/jbc.M203749200. [DOI] [PubMed] [Google Scholar]
- 50.Pant K, Crane BR. Structure of a loose dimer: an intermediate in nitric oxide synthase assembly. J Mol Biol. 2005;352:932–940. doi: 10.1016/j.jmb.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 51.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 52.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 54.Huang L, Abu-Soud HM, Hille R, Stuehr DJ. Nitric oxide-generated P420 nitric oxide synthase: characterization and roles for tetrahydrobiopterin and substrate in protecting against or reversing the P420 conversion. Biochemistry. 1999;38:1912–1920. doi: 10.1021/bi981954t. [DOI] [PubMed] [Google Scholar]
- 55.Voegtle HL, Sono M, Adak S, Pond AE, Tomita T, Perera R, Goodin DB, Ikeda-Saito M, Stuehr DJ, Dawson JH. Spectroscopic characterization of five- and six-coordinate ferrous-NO heme complexes. Evidence for heme Fe-proximal cysteinate bond cleavage in the ferrous-NO adducts of the Trp-409Tyr/Phe proximal environment mutants of neuronal nitric oxide synthase. Biochemistry. 2003;42:2475–2484. doi: 10.1021/bi0271502. [DOI] [PubMed] [Google Scholar]
- 56.Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 57.Spolitak T, Dawson JH, Ballou DP. Reaction of ferric cytochrome P450cam with peracids: kinetic characterization of intermediates on the reaction pathway. J Biol Chem. 2005;280:20300–20309. doi: 10.1074/jbc.M501761200. [DOI] [PubMed] [Google Scholar]
- 58.Yeh HC, Gerfen GJ, Wang JS, Tsai AL, Wang LH. Characterization of the peroxidase mechanism upon reaction of prostacyclin synthase with peracetic acid. Identification of a tyrosyl radical intermediate. Biochemistry. 2009;48:917–28. doi: 10.1021/bi801382v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehl M, Daiber A, Herold S, Shoun H, Ullrich V. Peroxynitrite reaction with heme proteins. Nitric Oxide. 1999;3:142–152. doi: 10.1006/niox.1999.0217. [DOI] [PubMed] [Google Scholar]
- 60.Maréchal A, Mattioli TA, Stuehr DJ, Santolini J. Activation of peroxynitrite by inducible nitric-oxide synthase: a direct source of nitrative stress. J Biol Chem. 2007;282:14101–14112. doi: 10.1074/jbc.M609237200. [DOI] [PubMed] [Google Scholar]