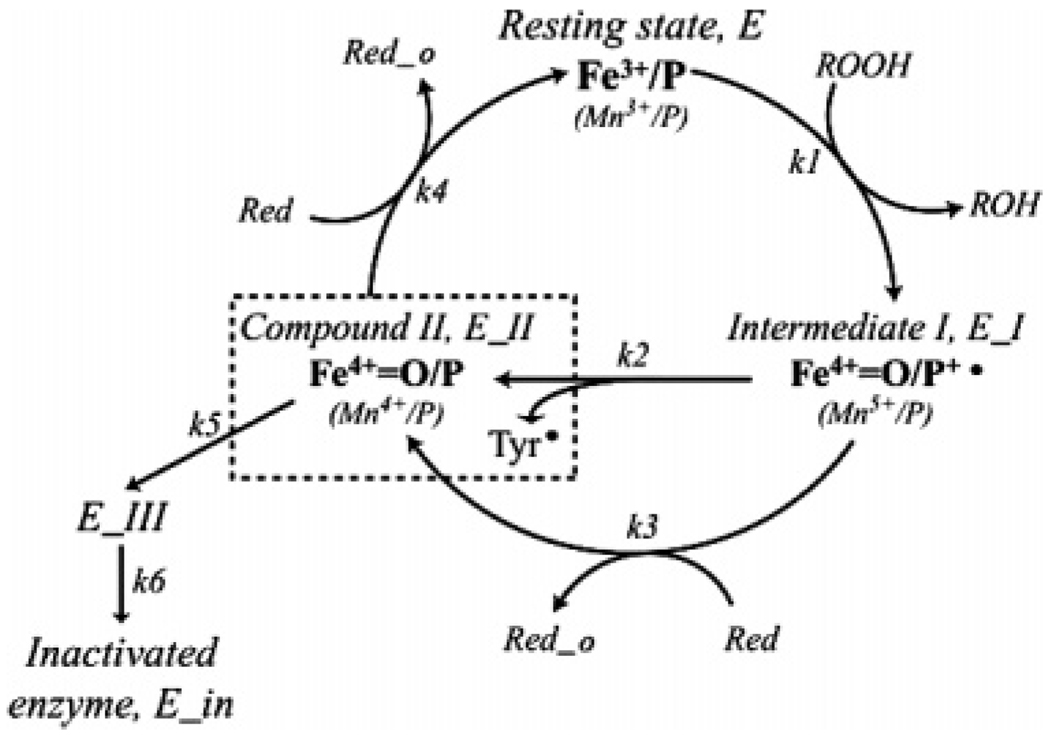
FIGURE 1.
Peroxidase mechanism of FePGHS-1 and MnPGHS-1 with hypothetical self-inactivation pathways, adapted from Dietz et al. (4) and Wu et al. (9). Fe4+=O/P+•, oxyferryl heme with a porphyrin cation radical, or Mn5+ (Intermediate I); Fe4+=O/P or Mn4+=O/P, Compound II; Fe4+=O/P or Mn4+=O/P + Tyr• (in dashed box), Intermediate II; ROOH, peroxide; ROH, alcohol; Red, reducing equivalent from cosubstrate; Red_o, reacted cosubstrate. Compound II and Tyr• are shown as discrete oxidized components of Intermediate II to emphasize their redox independence. The oxyferryl heme of Compound II is proposed to lead to inactive forms of enzyme (E_III and E_in).