Abstract
Physiological states of insulin resistance such as obesity and diabetes have been linked to abnormalities in female reproductive function. However, it is difficult to distinguish the direct effects of impaired insulin signaling from those of adiposity or hyperglycemia because these conditions often coexist in human syndromes and animal models of insulin resistance. In this study, we used lean, normoglycemic mouse lines with differing degrees of hyperinsulinemia and insulin receptor (Insr) expression to dissect the effects of altered insulin signaling on female reproduction. All three mouse lines [Ttr-Insr−/−, Insr+/−, and Insr+/+ (wild type)] are able to maintain fertility. However, the insulin-resistant and hyperinsulinemic mice demonstrate altered duration of estrous cycles as well as aberrant distribution and morphology of ovarian follicles. These effects appear to be independent of hyperandrogenism in the mice. Pregnancy studies indicate decreased success in early progression of gestation. In successful pregnancies, decreased embryo weights and increased placental calcification also implicate altered insulin signaling in later gestational effects. Thus, abnormal insulin signaling, independent of adipose tissue mass, adipokine expression levels, and hyperglycemia, can affect parameters of the female hypothalamic-pituitary-gonadal axis and pregnancy outcomes.
The effects of altered insulin action on female reproductive function, independent of hyperglycemia or adiposity in mice, are discussed.
Altered insulin function has long been associated with abnormalities in female reproduction (1,2,3). Conditions associated with insulin resistance, such as obesity and diabetes mellitus, are often accompanied by increased adiposity or hyperglycemia (4). Obesity and diabetes are independently associated with altered female reproductive function (5,6,7,8). Therefore, it is difficult to distinguish the effects of insulin signaling from those of adiposity or glucotoxicity in these conditions.
Studies in animal models suggest a direct role for insulin action in female reproduction. In Caenorhabditis elegans and Drosophila melanogaster, mutations in components of the insulin/IGF-I signaling pathway result in reduced fertility (9,10). Disruption of the Igf1 gene or the insulin receptor substrate-2 gene in female mice leads to infertility with abnormalities at multiple levels of the hypothalamic-pituitary-gonadal (HPG) axis (11,12). Neuron-specific Insr knockout mice implicate a role for insulin signaling in the central nervous system in maintaining female reproduction (13).
Despite these phenotypes, the exact mechanism by which insulin signaling regulates reproduction remains to be elucidated. The complex nature of the relationship is suggested by the fact that increased insulin sensitivity as seen with caloric restriction is also associated with impaired reproductive function (14,15). In the current study, we attempted to isolate the role of insulin action by comparing reproductive phenotypes of two mouse lines representing varying degrees of insulin resistance without accompanying adiposity or hyperglycemia: Ttr-Insr−/− (L1) and Insr+/− (IR+/−), with Insr+/+ [wild type, (WT)].
In L1 mice, Insr knockouts express the insulin receptor transgene under control of the transthyretin promoter (16). In these mice, Insr expression is restricted to the brain, liver, and pancreas. In the IR+/− model, half the level of Insr expression, compared with WT mice, is seen in all tissues (17).
These mice provide unique models in which to study the specific effects of varying degrees of impaired insulin action. Although L1 and IR+/− females are known to be grossly fertile by random mating experiments (16), we undertook extensive reproductive and gestational phenotyping of these mice to determine whether a subfertility phenotype is present.
Materials and Methods
Animal production and genotyping
All animals were housed in the Russ Berrie Animal Care Facility and treated according to animal protocols approved by the Columbia University Institutional Animal Care and Use Committee. Mice were maintained on a 12-h light, 12-h dark cycle with free access to food and water, unless otherwise specified. Generation of L1 (Ttr-Insr−/−) mice has been described previously (16).
Metabolic analyses
Two- to 4-month-old female mice were bled in random fed or fasted state (4 h fast, with ad libitum access to water). Fasting blood was obtained via tail bleeding in unanesthetized mice. Random fed samples were obtained using retroorbital bleeds in mice anesthetized with ip injections of 1 μl/g of 50 mg/ml sodium pentobarbital. Pregnancy samples were always obtained in the fed state via tail bleeding. Fed and fasted glucose levels were assessed with the One-Touch Ultra glucometer (LifeScan, Milpitas, CA). Fed and fasted insulin levels were measured with sensitive and ultrasensitive insulin ELISA kits, respectively (Mercodia, Winston-Salem, NC). Leptin and adiponectin assays were conducted on fed blood samples by the Scherer Laboratory (University of Texas Southwestern).
Glucose tolerance test
After 15 h overnight fasting, mice were injected ip with 2 g/kg body weight of d-glucose. Plasma glucose measurements were made from tail vein bleeding at 0, 15, 30, 60, 90, and 120 min. The One-Touch Ultra glucometer (LifeScan) was used for glucose measurements.
Weight and body composition analysis
Mice were weighed with the Scout Pro Balance mouse scale (Ohaus, Pine Brook, NJ) at each interval. Percent body fat and body composition was measured with the PIXImus scanner (GE Lunar, Madison, WI). Mice were anesthetized with isoflurane before and during body composition analysis.
Protein expression studies
Extracts were prepared from brain, uterus, or ovarian tissues in lysis buffer containing 20 mm Tris-HCl, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, and protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentration of extracts was determined with BCA assay (Pierce, Rockford, IL). Equal amounts of protein were resolved with 10% SDS-PAGE and transferred onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Membranes were probed with the following antibodies: anti-Insr-β antibody (C-19; Santa Cruz Biotechnologies, Santa Cruz, CA) at a dilution of 1:1,000 and antiactin antibody (Calbiochem, La Jolla, CA) at a dilution of 1:10,000. Bound antibody was detected with the following secondary antibodies, respectively: antirabbit immunoglobulin horseradish peroxidase conjugate at 1:10,000 dilution and antimouse IgG immunoglobulin at (1:3,000) dilution (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). Antibody signal was detected using the ECL chemiluminescent detection system (Amersham Pharmacia Biotech).
Reproductive analyses
Assessment of estrous cycles
The duration of estrous cycles was assessed in 6- to 12-wk-old females with daily inspection of vaginal smears for 15 d. During this period, females were individually housed with ad libitum access to food and water and controlled photoperiod of 12 h light and 12 h darkness.
Ovarian histology and follicular analysis
Ovaries from 12-wk-old females were dissected, weighed on an analytical balance (Denver Instrument Co., Denver, CO), fixed in formalin, dehydrated, and embedded in paraffin. Five-micrometer serial sections were cut throughout the ovary and stained with hematoxylin and eosin. Every fifth section after random start was examined for presence of follicles or corpora lutea. Growing follicles were grouped as primordial, primary, preantral, antral, or graffian. Only follicles exhibiting the nucleus of the oocyte were counted, to avoid overestimating follicle numbers. Follicle counts were multiplied by a factor of 5 to determine the total number of each follicle subtype in the ovary (18). Thecal thickness was measured with light microscopy. Ten antral WT, IR+/−, polyovular IR+/−, and L1 follicles were analyzed. The average of three measurements were used for each follicle.
Response to superovulation with gonadotropins
Female mice, aged 2–4 months, were injected with 10 IU pregnant mare serum gonadotropin (Sigma-Aldrich, St. Louis, MO) ip at 1600 h. They were injected with 10 IU human chorionic gonadotropin (Sigma-Aldrich) ip at 48 h and killed by cervical dislocation 16 h thereafter. The oocytes were retrieved from bilateral ampullae of the oviducts and dissociated from the surrounding cumulus with 300 μg/ml hyaluronidase (Sigma-Aldrich) in M2 medium (Specialty Media, Phillipsburg, NJ). The oocytes were recovered in M2 medium and their number and morphology noted with a phase contrast T-2 microscope (Nikon, Melville, NY).
Hormonal assays
Fed age-matched adult females were anesthetized for terminal bleed. Mice were then killed by cervical dislocation. Serum samples were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for assessment of testosterone and LH levels. This Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (Specialized Cooperative Centers Program in Reproduction and Infertility Research).
Fertility assessment
The 6- to 10-wk-old nulliparous females were housed with fertile WT males. The presence of vaginal plug was assessed daily. The presence of a plug was designated as d 0.5 of gestation. A subset of mice euthanized on d 17.5 of gestation. Embryos and placentae were identified and individually dissected. Individual placenta and embryo length was noted and weight assessed with an analytical scale. A small section of embryo tail was obtained for genotyping analysis.
Metabolic evaluations in pregnancy
Blood was obtained via tail bleeding for glucose and insulin assessments from unanesthetized random-fed mice. Samples were obtained before pregnancy and on gestational d 9.5, 14.5, and 17.5. Glucose and insulin levels were assessed as described above in Metabolic analyses. Weights were also measured at these time points using the Scout Pro Balance mouse scale (Ohaus).
Histological analysis of placentae
Placentae were sectioned, fixed in formalin, and embedded. Five-micrometer sections were cut, mounted onto slides, and stained with hematoxylin and eosin. Sections were analyzed under light microscopy with the Nikon Eclipse E400 microscope. Photo images were taken and analyzed with Spot Advanced Software (version 4.0; Diagnostic Instruments, Inc., Sterling Heights, MI).
Statistical analysis
Statistical analyses were performed with Microsoft Excel software using two-tailed Student’s t tests. P < 0.05 was considered to be statistically significant.
Results
Metabolic profile of L1 and IR+/− females
Glucose homeostasis
The metabolic phenotype of transgenic L1 mice has previously been characterized in males (16). However, female transgenics often have milder metabolic profiles than male counterparts (19). Therefore, we conducted experiments to study glucose metabolism in L1 and IR+/− females in comparison with WT littermates. The metabolic analyses were performed in 2- to 4-month-old females, the age group used for reproductive analyses.
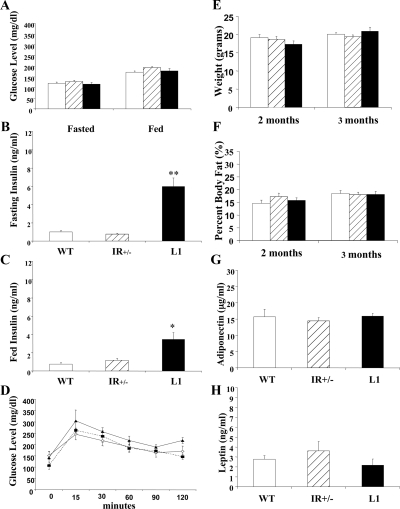
Unlike their male counterparts, 30% of whom develop diabetes by 6 months age (16), L1 females have fasted and fed glucose levels in the nondiabetic range (Fig. 1A). There were no statistically significant differences in glucose levels between L1, IR+/−, and WT females. L1 females demonstrate significantly higher fasting and fed insulin levels (Fig. 1, B and C). Intraperitoneal glucose tolerance testing was performed to assess glucose disposal (Fig. 1D). There was no significant difference among the three groups of mice.
Figure 1.
Metabolic analyses. The data represent the mean ± sem fasted and fed glucose (A) (n = 10–30) and insulin levels (B and C) (n = 6–10) in WT (open bar), IR+/− (striped bar), and L1 (closed bar) females. *, P < 0.01; **, P < 0.001. D, Intraperitoneal glucose tolerance test. The mean glucose level ± sem at each time point after glucose injection is presented. Open diamond, WT; closed square, IR+/−; closed triangle, L1. E and F, The mean ± sem weight and percent body fat in 2- and 3-month-old WT, IR+/−, and L1 females (n = 6–20). G and H, Adiponectin and leptin expression levels were assessed with nonfasting samples (n = 7–15).
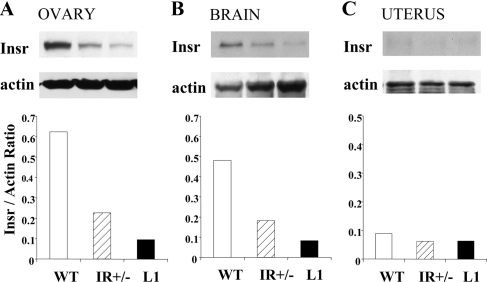
A partial tissue distribution of insulin receptor expression in L1 mice has been characterized (16); however, reproductive tissues were not assessed in these initial studies. We performed immunoblot experiments to document insulin receptor expression levels in L1, IR+/−, and WT females in the brain, ovary, and uterus.
In the brain and ovary, WT females have the highest, IR+/− females intermediate, and L1 females the lowest level of insulin receptor expression (Fig. 2, A and B). Analysis of uterus lysates, however, showed very low levels of Insr protein expression in all three mouse lines (Fig. 2C).
Figure 2.
Levels of insulin receptor expression in the ovary (A), brain (B), and uterus (C). Single brain and uterus samples were used to generate protein lysates; pooled samples from five animals were used for the ovarian lysate. Immunoblot panels represent expression levels of either insulin receptor (Insr) or the loading control protein (actin). The histograms present the ratio of Insr to actin expression.
These data show that the two mouse lines, L1 and IR+/−, represent normoglycemic mice with differing degrees of hyperinsulinemia and insulin receptor expression in important reproductive tissues, such as the brain and the ovary.
Adiposity and adipokine expression levels
Adipose tissue elaborates adipokines such as leptin and adiponectin that are linked to reproductive function (20,21,22). We evaluated fat mass and adipokine expression levels to determine whether differences in these parameters correlated with reproductive function in our mice. At 8 and 12 wk age, there were no statistically significant differences in body weight or fat mass, as assessed by dual-energy x-ray absorptiometry scanning (Fig. 1, E and F). Leptin and adiponectin levels in 2- to 4-month-old fed mice were also similar among L1, IR+/−, and WT females (Fig. 1, G and H). Therefore, differences in these parameters of adiposity are unlikely to account for altered reproductive function in the mice.
Reproductive outcomes in L1 and IR+/− females
Assessment of function of the HPG axis
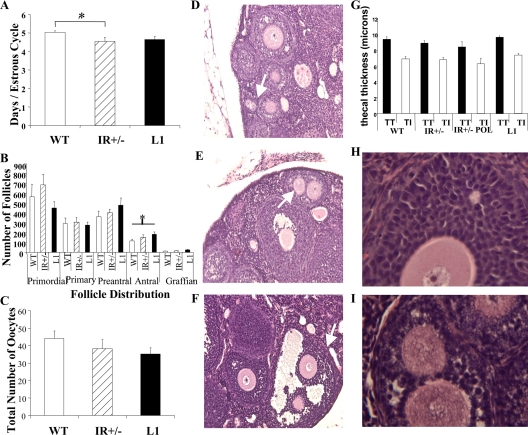
Visual inspection of ovaries and uteri did not reveal any gross differences in structure or size. Examination of daily vaginal smears for 15 d demonstrated that both L1 and IR+/− females cycle through the five stages of the mouse estrous cycle (23). However, the average duration of each cycle was significantly shorter in IR+/− mice when compared with WT (4.53 ± 0.23 vs. 5.03 ± 0.09 d/cycle, P = 0.04). No significant differences were noted between L1 and WT females (Fig. 3A).
Figure 3.
Assessment of ovarian function. A, Estrous cycling. The data represent the average number of days ± sem per estrous cycle (n = 6–11). B, Follicular analysis. The average number of healthy primordial, primary, preantral, antral, and graffian follicles (n = 6–8) is shown. C, Superovulation. The number of oocytes retrieved ± sem is shown (n = 7–9). *, P < 0.05. Polyovular follicles in IR+/− ovaries (indicated with white arrows). Photomicrographs of primary (D), preantral (E), and antral (F) follicles are depicted. For thecal dimensions, the mean ± sem of thecal thickness (TT, total thickness; TI, theca interna) in WT, IR+/−, polyovular IR+/− (IR+/− POL), and L1 follicles is presented (G). Granulosa cell morphology in WT (H) and a subset of IR+/− ovaries (I) is shown.
As a second measure of ovarian function, we performed histological analyses of ovarian sections from 12-wk-old virgin females. L1 and IR+/− ovaries contained similar numbers of primordial, primary, preantral, and graffian follicles compared with WT (Fig. 3B). There was also no difference in the average number of corpora lutea [9.6 ± 1.4 (L1), 11.3 ± 1.3 (IR+/−), 10 ± 2.6 (WT), n = 5–7]. However, L1 ovaries contained a significantly higher number of antral follicles than age-matched WT ovaries (185 ± 22.5 vs. 115.7 ± 18.7, P = 0.036). The number of antral follicles in IR+/− ovaries (153.6 ± 26.7) was intermediate but did not show a statistically significant difference from WT.
As a measure of ovarian follicular reserve, adult females were superovulated with exogenous gonadotropins. Although a decreasing trend in the number of extracted oocytes was noted in L1 and IR+/− mice when compared with WT (WT: 44 ± 4.3, IR+/−: 38 ± 5.4; L1: 35 ± 3.4), these differences did not reach statistical significance (Fig 3C).
Polyovular follicles in IR+/− mice
The histological analyses revealed an abnormality in follicular morphology in IR+/− females: six of seven of these ovaries (86%) contained polyovular follicles (follicles containing more than one oocyte). The polyovular phenotype was noted in all stages of follicular development, from primordial follicles to graffian follicles (Fig. 3, D–F). However, only one of seven of both L1 and WT ovaries (14%) demonstrated this aberrant phenotype.
Hormonal and morphological parameters
In human syndromes of insulin resistance and subfertility, such as polycystic ovary syndrome (PCOS), there is evidence of hyperandrogenism, thecal abnormalities, and altered expression of gonadotropins (24). Therefore, we examined key aspects of these parameters in our mice. Comparison of the thecal layer in WT, IR+/−, LI, and polyovular IR+/− follicles showed no significant differences in morphology or thickness of the theca interna, the steroid-producing layer, or in total thecal thickness (Fig. 3G).
Examination of the granulosa cells in a subset of IR+/− ovaries showed lipid vacuoles suggestive of steroidogenic activity (Fig. 3, H and I). This was not seen in L1 ovaries. Therefore, we compared steroid hormone levels in the IR+/− and age-matched WT females. There were no significant differences noted in testosterone levels (nanograms per deciliter) [IR+/−, 16.0 ± 4.0 (n = 5); WT, 28.6 ± 7.5 (n = 6); P = 0.2] or LH levels (nanograms per milliliter) [IR+/−, 0.32 ± 0.13 (n = 5); WT, 0.44 ± 0.12 (n = 5); P = 0.6] between the two groups of mice.
Assessment of reproductive capacity
Increased insulin resistance and hyperinsulinemia are known characteristics of the late stages of gestation (25,26). Although we showed earlier that L1 and IR+/− females remain normoglycemic during the reproductive period, we examined whether they maintain euglycemia despite the metabolic stresses of gestation.
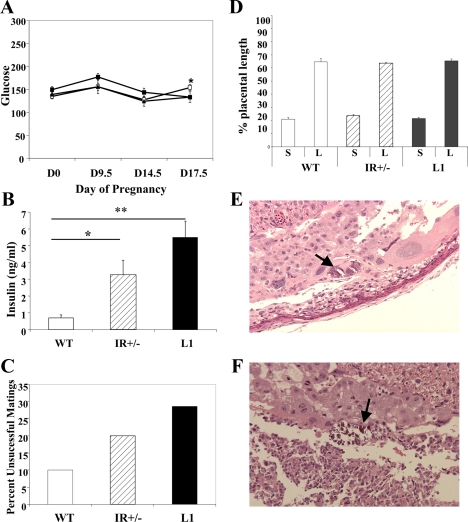
We therefore examined random glucose levels from unanesthetized mice throughout pregnancy (d 0, 9.5, 14.5, and 17.5 of gestation) (Fig 4A). All three mouse lines remained euglycemic throughout the pregnancy. There was no significant difference in mean glucose values between L1, IR+/−, and WT females before pregnancy (d 0) or at d 9.5 or 14.5. At d 17.5, there was a significant increase in glucose level in IR+/− females in comparison with WT, but both means were well within the euglycemic range.
Figure 4.
Gestational analyses and metabolic characterization. A, Fed glucose levels from gestational d 0, 9.5, 14.5, and 17.5 remained in the nondiabetic range in WT (closed square), IR+/− (open square), and L1 (closed triangle) mice. B, The data represent the mean ± sem fed insulin level in late gestation, d 17.5 (n = 5–8). *, P < 0.05; **, P < 0.005. C, Pregnancy outcomes and unsuccessful matings. The data represent the percentage of plugged females that did not proceed to successful pregnancies (n = 7–10). D, Placental histology. Histological sections of placentae from gestational d 17.5 were evaluated with light microscopy. Percentage of placental diameter consisting of labyrinthian (L) and spongiform (S) layers is presented. Foci of calcifications in IR+/− (E) and L1 (F) placentae are indicated with black arrows. Photomicrographs were taken under ×20 magnification.
Insulin levels on d 17.5, representative of late gestation, were also assessed (Fig. 4B). Both L1 and IR+/− females were significantly more hyperinsulinemic than their WT counterparts (L1, 5.5 ± 0.97; IR+/−, 3.2 ± 0.88; WT, 0.7 ± 0.18; P = 0.03 for WT vs. IR+/− , P = 0.0003 for WT vs. L1). L1 females displayed a greater degree of hyperinsulinemia than IR+/− females, but the increase did not reach statistical significance.
Nulliparous females were mated with fertile WT males. Copulatory plugs were noted in all three groups. However, whereas 10% of WT females failed to maintain successful pregnancies, 20% of IR+/− females and 28.6% of L1 females failed to deliver progeny despite the presence of a copulatory plug (Fig. 4C). In these matings, the females did not show weight gain in the 2-wk observation period after copulation. In successful pregnancies, the mean duration of gestation (19.5 d) did not vary among L1, IR+/−, and WT females.
A subset of pregnant females was killed on d 17.5. The number of embryos, embryo weight, and length as well as placental weight, length, and morphology was recorded (Table 1 and Fig. 4).
Table 1.
Pregnancy outcomes is successful matings
| WT pregnancies | IR+/− pregnancies | L1 pregnancies | |
|---|---|---|---|
| Litter weight (g) | 7.086 ± 0.51 (7) | 8.572 ± 0.67 (6) | 7.427 ± 0.64 (5) |
| Embryos/gestation | 7.4 ± 0.7 (9) | 9.3 ± 0.5 (11)a | 8.2 ± 0.6 (5) |
| Embryo weight (g) | 0.973 ± 0.01 (51) | 0.970 ± 0.02 (53) | 0.887 ± 0.02 (32)b |
| Embryo length (mm) | 21.35 ± 0.17 (44) | 21.156 ± 0.20 (61) | 20.97 ± 0.18 (39) |
| Placenta weight (mg) | 86.3 ± 1.9 (51) | 87.1 ± 1.7 (60) | 80.0 ± 2.5 (37) |
| Placenta length (mm) | 7.96 ± 0.09 (37) | 8.06 ± 0.07 (53) | 7.98 ± 0.11 (23) |
The 7- to 10-wk-old nulliparous females were mated with fertile WT males. Data represent the mean ± sem for matings that resulted in delivery of progeny. The number of samples (n) is noted in parentheses.
P < 0.05 in comparison with WT pregnancies.
P < 0.005 in comparison with WT and IR+/− pregnancies.
IR+/− females produced a significantly greater number of progeny than their WT counterparts, with individual embryo weights that were similar in IR+/− and WT gestations. The average embryo weight in L1 gestations, however, was significantly less than their WT counterparts (Table 1). Embryo length did not vary among the three different gestations.
Because of the difference in maternal genotype, there was a difference in genotype distribution among the offspring. WT mothers produced Insr+/+ offspring, IR+/− mothers produced both Insr+/+ and Insr+/− offspring, and L1 mothers produced Insr+/− offspring with or without the transthyretin transgene. To determine whether the decrease in average embryo weight in L1 gestations was due to differences in embryonic genotype rather than maternal genotype, we performed PCR analysis to denote offspring genotype in each pregnancy.
There were no significant differences in Insr+/+ vs. Insr+/− embryonic weight in IR+/− gestations. Similarly, no differences were detected between Insr+/− and Ttr-Insr+/− progeny from L1 mothers. Additionally, Insr+/− embryos weighed less in L1 gestations than in IR+/− gestations (Table 2). This suggests that the lower average embryonic weight in L1 gestations was due to maternal genotype rather than embryonic genotype.
Table 2.
Comparison of embryonic weights by genotype
| Dams | Embryonic weights (g)
|
||
|---|---|---|---|
| Insr+/+ | Insr+/− | Ttr-Insr+/− | |
| WT | 0.97 ± 0.01 | ||
| IR+/− | 0.943 ± 0.03 | 0.967 ± 0.03 | |
| L1 | 0.841 ± 0.05a | 0.776 ± 0.08 | |
Embyronic genotype was determined by PCR analysis of tail DNA. The data, mean embryonic weight ± sem, was analyzed by both embryonic and dam genotype.
P < 0.05 when compared with Insr+/− embryos from IR+/− dams.
Evaluation of placental weight and length did not reveal any differences among L1, IR+/−, and WT pregnancies (Table 1). However, we also examined placental morphology, as gestational abnormalities can result from altered placental architecture rather than difference in size. We analyzed histological sections of the placentae to determine relative thickness of the labyrinthian and spongiform layers. We found no differences in this aspect of placental architecture (Fig. 4D). However, we did note the presence of crystallized material, likely calcifications, in d 17.5 placentae from all of the IR+/− and L1 conceptuses (five of five and four of four, respectively) but in only one of four of WT placentae (25%) (Fig. 4, E and F).
Discussion
The obesity epidemic is associated with comorbidities such as hypertension, diabetes, and sleep apnea. In women, there appears to be an additional phenotype of subfertility. It is well documented that hyperglycemia and obesity have detrimental effects on the HPG axis and pregnancy outcomes. It is difficult, however, to tease out the effects of insulin resistance from that of accompanying adiposity or hyperglycemia.
To dissect the independent role of insulin signaling, we undertook a systematic evaluation of the reproductive phenotype in mice that are lean and normoglycemic with varying degrees of hyperinsulinemia.
HPG axis function
In our analyses of the HPG axis, the insulin-resistant females, L1 and IR+/−, maintain regular estrous cycles. However, shorter estrous cycles in the IR+/− females suggests subtle alterations in the HPG axis.
A second phenotype in IR+/− females is the increased prevalence of polyovular follicles. This follicular variant is rarely found in WT females (27). Increased prevalence is seen with in utero exposure to excessive estrogen or testosterone (28,29) and is also associated with altered ratios of inhibin to activin expression (27). Furthermore, the presence of polyovular follicles is associated with subfertility (30).
The increased incidence of polyovular follicles is not seen in L1 ovaries. Our mating strategy used similar parental genotypes to generate experimental females. Therefore, there is little justification to invoke differences in in utero hormonal environments in the development of these phenotypes. However, it is possible that insulin signaling within the embryo itself, determined by its genotype, independently alters the inhibin to activin ratio or neonatal testosterone or estrogen levels, leading to a polyovular phenotype.
Despite the fact that L1 females are more hyperinsulinemic and have fewer insulin receptors in the brain and ovary than IR+/− females, these mice do not exhibit shorter estrous cycles or increased polyovular follicles. This would suggest that insulin resistance in reproductive organs is not directly related to these phenotypic abnormalities.
However, it is important to note that L1 and IR+/− females do not differ only by serum insulin levels but also by pattern of insulin receptor expression. Transgenic L1 females have complete absence of insulin receptors in many tissues, including inflammatory cells such as macrophages, whereas the IR+/− mice have half the level of insulin receptor expression found in WT mice in all tissues (17,31).
Recent work has shown that despite exhibiting hyperinsulinemia and effective insulin resistance at the level of the macrophage, L1 males do not demonstrate the increase in inflammatory milieu (increased circulating cytokines and increased macrophage density in adipose tissue) that is seen in other insulin-resistant models (32). Because many side effects of obesity are attributed to a proinflammatory phenotype, we can speculate that the presence of polyovular follicles and altered menstrual cycles also requires the presence of a proinflammatory milieu.
Histological analyses of L1 ovaries showed a significant increase in the number of antral follicles. There was also an increasing trend in the number of preantral follicles. Furthermore, a nonsignificant increase in preantral and antral follicles was noted in IR+/− mice. This gradation of phenotype correlates with the degree of hyperinsulinemia, suggesting a role for insulin action in proper follicular development.
Syndromes of insulin resistance and subfertility are often accompanied by signs of hyperandrogenism, such as ovarian thecal hypertrophy (24). In our analyses, however, there were no differences noted in thecal dimensions of ovarian follicles. However, because of the presence of lipid laden granulosa cells, altered menstrual cycles, and polyovular abnormalities in the IR+/− females, we compared sex steroid hormone and gonadotropin levels between IR+/− and WT females. The lack of difference in these parameters suggests that insulin resistance and hyperinsulinemia can affect these ovarian functions independent of hyperandrogenism.
Despite the lack of statistical significance, the gonadotropin and androgen levels in IR+/− females are lower than in WT females. This trend is also seen in neuron-specific Insr knockout and Irs2 null mice (12,13), linking altered insulin action and subfertility with decreased gonadotropin and sex steroid expression. This is contrary to the trend seen in human syndromes of insulin resistance and subfertility, such as PCOS. Mouse models of altered insulin signaling are present from the embryonic stage, whereas human syndromes of insulin resistance generally worsen with age and weight gain. It is possible that differential timing and exposure to altered insulin action affects the HPG axis by different mechanisms.
Pregnancy outcomes
Early gestation
Both L1 and IR+/− females are able to copulate and produce viable offspring. However, a greater percentage of matings for both L1 and IR+/− females fails to proceed to successful gestations. The percentage of failed matings increases with degree of hyperinsulinemia and insulin resistance at the central and gonadal level. The lack of significant weight gain after copulation suggests an early defect in the progression of pregnancy, i.e. improper oocyte maturation, failed fertilization, or poor implantation.
Women with PCOS have an increased risk of early pregnancy complications (33). Treatment with insulin-sensitizing agents such as metformin has been shown to decrease the incidence of first-trimester miscarriages in these women, suggesting a role for insulin signaling in early fetal development or maintenance of pregnancy (34,35). It has been shown that oocytes contain insulin receptors (36). It has also been shown that insulin receptor numbers increase during oocyte maturation. This is consistent with the notion that insulin resistance may have detrimental effects on oocyte development and ensuing fertilization and early development.
Late gestation
Evaluation of embryo weights on gestational d 17.5 shows that offspring from L1 dams have decreased weight when compared with embryos from WT or IR+/− dams. This effect is independent of embryonic genotype. Moreover, embryos of the same genotype (Insr+/−) weigh less when resulting from L1 pregnancies than from IR+/− pregnancies. These data implicate the gestational milieu of the more hyperinsulinemic and insulin-resistant L1 dams in development of decreased embryonic weights.
To understand the etiology of this difference, we examined placental weight, length, and morphology. Whereas length and weight were the same among L1, IR+/−, and WT gestations, there was an increase in the incidence of crystallized materials, similar to calcifications, noted in the placentae of IR+/− and L1 pregnancies.
Calcifications are a normal feature of placental maturation in both mice (37) and humans (38,39). Placental calcification increases as this organ develops in both human and murine gestations. However, studies indicate that increased or earlier development of placental calcifications can be associated with pregnancy complications such as intrauterine growth retardation and proteinuric hypertension (40,41). An increased incidence of proteinuric hypertension is correlated with states of increased insulin resistance such as obesity, metabolic syndrome, PCOS, and diabetes (42,43). It would be interesting, therefore, to explore whether there is a direct and causative correlation between altered insulin signaling and the development of these pregnancy complications.
In summary, we examined three lines of euglycemic, nonobese mice representing varying degrees of hyperinsulinemia and insulin action at the central and gonadal level. Our results indicate that insulin action, independent of hyperglycemia and adipose tissue mass, appears to affect functioning of the HPG axis and the reproductive cycle, including menstrual cycling, follicular development, and early progression of gestation. It may also play a role in later gestation, leading to decreased embryonic weight and pathological development of the placenta. This study provides the basis for further mechanistic evaluations of the role of insulin signaling in female reproductive function.
Acknowledgments
We thank the members of the Accili and Wolgemuth laboratories for their teaching, technical assistance, and helpful discussions. We also thank Katia Manova-Todorova (Sloan-Kettering Institute) for her aid in ovarian follicular analyses, V. A. Papaioannou (Columbia University) for demonstration of embryo dissection techniques, and Harsh Thaker (Columbia University) for critical review of mouse placenta slides. We acknowledge the Scherer laboratory (University of Texas Southwestern) for performing the leptin and adiponectin assays; the University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core (supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934) for performing the LH and testosterone assays.
Footnotes
This work was supported in part by National Institutes of Health Grants 2RO1-DK58282-09 (to D.A.) and 1RO1-HD039915 (to D.J.W.).
Disclosure Summary: A.N., X.W., D.A., and D.J.W. have nothing to declare.
First Published Online February 22, 2010
Abbreviations: HPG, Hypothalamic-pituatry-gonadal; PCOS, polycystic ovary syndrome; WT, wild type.
References
- Dunaif A 1997 Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- Legro RS 2001 Polycystic ovary syndrome: the new millennium. Mol Cell Endocrinol 184:87–93 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kandarakis HA 2003 Conservative management of gynecological diseases: insulin sensitizing agents in polycystic ovary syndrome. Ann NY Acad Sci 997:322–329 [DOI] [PubMed] [Google Scholar]
- Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM 2006 Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 91:4459–4466 [DOI] [PubMed] [Google Scholar]
- Kjaer K, Hagen C, Sandø SH, Eshøj O 1992 Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J Clin Endocrinol Metab 75:524–529 [DOI] [PubMed] [Google Scholar]
- Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, Karbaat J 1993 Fat and female fertility: prospective study of effect of body fat distribution on conception rates. BMJ 306:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew R, Hamilton-Fairley D 1997 Obesity and female reproductive function. Br Med Bull 53:341–358 [DOI] [PubMed] [Google Scholar]
- Eriksson UJ 1995 The pathogenesis of congenital malformations in diabetic pregnancy. Diabetes Metab Rev 11:63–82 [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Ruvkun G 1998 An insulin-like pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics 148:703–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A 2003 The endocrine regulation of aging by insulin-like signals. Science 299:1346–1351 [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvé AR, Efstratiadis A 1996 Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10:903–918 [DOI] [PubMed] [Google Scholar]
- Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF 2000 IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377–382 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Weiss EP, Holloszy JO 2007 Improvements in body composition, glucose tolerance, and insulin action induced by increasing energy expenditure or decreasing energy intake. J Nutr 137:1087–1090 [DOI] [PubMed] [Google Scholar]
- Holliday R 1989 Food, reproduction and longevity: is the extended life span of calorie-restricted animals an evolutionary adaptation? Bioessays 10:125–127 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D 2004 Transgenic rescue of insulin receptor-deficient mice. J Clin Invest 114:214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, José PA, Taylor SI, Westphal H 1996 Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 12:106–109 [DOI] [PubMed] [Google Scholar]
- Takai Y, Canning J, Perez GI, Pru JK, Schlezinger JJ, Sherr DH, Kolesnick RN, Yuan J, Flavell RA, Korsmeyer SJ, Tilly JL 2003 Bax, caspase-2, and caspase-3 are required for ovarian follicle loss caused by 4-vinylcyclohexene diepoxide exposure of female mice in vivo. Endocrinology 144:69–74 [DOI] [PubMed] [Google Scholar]
- Leiter EH, Coleman DL, Hummel KP 1981 The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. Effect of H-2 haplotype and sex. Diabetes 30:1029–1034 [DOI] [PubMed] [Google Scholar]
- Nandi A, Kitamura Y, Kahn CR, Accili D 2004 Mouse models of insulin resistance. Physiol Rev 84:623–647 [DOI] [PubMed] [Google Scholar]
- Mitchell M, Armstrong DT, Robker RL, Norman RJ 2005 Adipokines: implications for female fertility and obesity. Reproduction 130:583–597 [DOI] [PubMed] [Google Scholar]
- Tortoriello DV, McMinn JE, Chua SC 2007 Increased expression of hypothalamic leptin receptor and adiponectin accompany resistance to dietary-induced obesity and infertility in female C57BL/6J mice. Int J Obes (London) 31:395–402 [DOI] [PubMed] [Google Scholar]
- Rugh R 1968 The mouse: its reproduction and development. Minneapolis: Burgess; 38 [Google Scholar]
- Chang RJ 2007 The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 3:688–695 [DOI] [PubMed] [Google Scholar]
- Ryan EA, O'Sullivan MJ, Skyler JS 1985 Insulin action during pregnancy. Studies with euglycemic clamp technique. Diabetes 34:380–389 [DOI] [PubMed] [Google Scholar]
- Homko CJ, Sivan E, Reece EA, Boden G 1999 Fuel metabolism during pregnancy. Semin Reprod Endocrinol 17:119–125 [DOI] [PubMed] [Google Scholar]
- Guillette Jr LJ, Moore BC 2006 Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med 24:134–141 [DOI] [PubMed] [Google Scholar]
- Kirigaya A, Hayashi S, Iguchi T, Sato T 2006 Developmental effects of ethinyl estradiol on reproductive organs of female mice. In Vivo 20:867–873 [PubMed] [Google Scholar]
- Iguchi T, Todoroki R, Takasugi N, Petrow V 1988 The effects of an aromatase inhibitor and a 5α-reductase inhibitor upon the occurrence of polyovular follicles, persistent anovulation, and permanent vaginal stratification in mice treated neonatally with testosterone. Biol Reprod 39:689–697 [DOI] [PubMed] [Google Scholar]
- Iguchi T, Kamiya K, Uesugi Y, Sayama K, Takasugi N 1991 In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo 5:359–363 [PubMed] [Google Scholar]
- Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR 2004 Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 113:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, Accili D 2007 Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes 56:1969–1976 [DOI] [PubMed] [Google Scholar]
- Homburg R 2006 Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab 20:281–292 [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Wang P 2007 Metformin before and during pregnancy and lactation in polycystic ovary syndrome. Expert Opin Drug Saf 6:191–198 [DOI] [PubMed] [Google Scholar]
- Heyner S, Farber M, Rosenblum IY 1990 The insulin family of peptides in early mammalian development. Curr Top Dev Biol 24:137–159 [DOI] [PubMed] [Google Scholar]
- Acevedo N, Ding J, Smith GD 2007 Insulin signaling in mouse oocytes. Biol Reprod 77:872–879 [DOI] [PubMed] [Google Scholar]
- Akirav C, Lu Y, Mu J, Qu DW, Zhou YQ, Slevin J, Holmyard D, Foster FS, Adamson SL 2005 Ultrasonic detection and developmental changes in calcification of the placenta during normal pregnancy in mice. Placenta 26:129–137 [DOI] [PubMed] [Google Scholar]
- Poggi SH, Bostrom KI, Demer LL, Skinner HC, Koos BJ 2001 Placental calcification: a metastatic process? Placenta 22:591–596 [DOI] [PubMed] [Google Scholar]
- Spirt BA, Cohen WN, Weinstein HM 1982 The incidence of placental calcification in normal pregnancies. Radiology 142:707–711 [DOI] [PubMed] [Google Scholar]
- McKenna D, Tharmaratnam S, Mahsud S, Dornan J 2005 Ultrasonic evidence of placental calcification at 36 weeks’ gestation: maternal and fetal outcomes. Acta Obstet Gynecol Scand 84:7–10 [DOI] [PubMed] [Google Scholar]
- Hopper KD, Komppa GH, Bice P, Williams MD, Cotterill RW, Ghaed N 1984 A reevaluation of placental grading and its clinical significance. J Ultrasound Med 3:261–266 [DOI] [PubMed] [Google Scholar]
- Ray JG, Vermeulen MJ, Schull MJ, McDonald S, Redelmeier DA 2005 Metabolic syndrome and the risk of placental dysfunction. J Obstet Gynaecol Can 27:1095–1101 [DOI] [PubMed] [Google Scholar]
- Howarth C, Gazis A, James D 2007 Associations of type 1 diabetes mellitus, maternal vascular disease and complications of pregnancy. Diabet Med 24:1229–1234 [DOI] [PubMed] [Google Scholar]