Abstract
The TSH receptor (TSHR) ectodomain comprises a tubular leucine-rich repeat domain (LRD) and a hinge [or signaling specificity domain (SSD)]. TSH binds to both the LRD and SSD, leading to signal transduction by the transmembrane domain. The SSD structure and spatial orientation to the other components are unknown. We exploited a fortuitous observation to obtain mechanistic insight into the relationship between TSH binding and signal transduction. A mouse TSHR cDNA generated by PCR was found to express a receptor with poor TSH-induced cAMP generation despite normal TSH binding. Progressive reversion to wild-type of six mutations revealed E251K in the LRD to be critical for reduced signal transduction in both mouse and human TSHR. An I286F substitution in the SSD had a much weaker effect and was additive with E251K. To our knowledge, there are no previous examples of specific amino acid mutations in the TSHR LRD that dissociate TSH binding from TSHR signal transduction. To prevent flailing of the TSHR LRD, its position vis-à-vis the SSD must be stabilized by multiple amino acid interactions. The present data suggest that TSHR residue E251 is one of these residues involved in stabilizing the LRD relative to the SSD, thereby enabling ligand binding to transduce a signal by the latter. That the E251K mutation can reduce signal transduction despite high-affinity TSH binding comparable with the wild-type TSHR provides mechanistic insight into the coupling between ligand binding and receptor activation.
TSH receptor amino acid E251 at the junction of the extracellular leucine-rich repeat and hinge regions is involved in linking ligand binding to signal transduction.
The TSH receptor (TSHR), a member of the G protein-coupled receptor (GPCR) superfamily (reviewed in Refs. 1,2,3), plays a vital role in thyroid hormone homeostasis. The thyroid hormone servofeedback loop on pituitary TSH secretion, together with iodine autoregulation of the thyroid gland (4), maintains serum thyroid hormone levels within a narrow physiological range. In Graves’ disease, thyroid dysregulation occurs when thyroid-stimulating autoantibodies usurp the function of TSH and activate the TSHR (5,6). More rarely germline or somatic mutations in the TSHR gene can result in thyroid dysfunction, either hyperthyroidism or hypothyroidism (reviewed in Ref. 7). For all these reasons, understanding the structure-function relationship of the TSHR is of heuristic as well as clinical interest.
Like the gonadotropin members of the glycoprotein hormone receptor GPCR subfamily, the TSHR (764 amino acid residues less a 21 residue signal peptide) comprises three distinct components, the structure of two of which is well understood. The extracellular leucine-rich repeat domain (LRD; approximately residues 21-260) has been crystallized and its three-dimensional structure determined (8). The TSHR transmembrane domain (TMD) comprises 349 amino acid residues with seven membrane-spanning helices. Although the TSHR TMD has not been crystallized, its structure can be modeled with reasonable accuracy on the basis of existing crystal structures of other GPCR, in particular rhodopsin (9), the β2-adrenergic receptor (10,11,12) and the A2A adenosine receptor (13). The third TSHR component is an approximately 150 residue hinge region that links the LRD with the TMD and whose structure remains opaque. For reasons discussed below, rather than hinge, we prefer the term signaling specificity domain (SSD) coined by Moyle et al. (14). TSH binds in large part (but not exclusively) to the extracellular LRD, and a signal is transduced via the TMD. However, exactly how these two functions are related is poorly understood.
Obviously, mutations in the TMD, the distal, signal-generating component of the TSHR, can diminish signal transduction without altering TSH binding. However, it has long been known that chimeric substitutions of segments in the TSHR extracellular domain (the LRD and SSD) can similarly lead to a dissociation between high-affinity TSH binding and TSHR activation (for example, see Refs. 15 and 16). The present study investigating this phenomenon developed from a fortuitous observation with a mouse TSHR (mTSHR) cDNA obtained for a different purpose, establishment of an mTSHR thyroid-stimulating autoantibody assay (17). The mTSHR cDNA clone that we obtained functioned very poorly in terms of cAMP generation, and its DNA sequencing revealed a number of unexpected mutations. Investigation of these mutations has generated mechanistic insight into regions of the TSHR extracellular domain (ectodomain) that are important in linking ligand binding with signal transduction.
Materials and Methods
TSHR cDNA constructs and mutations
The mTSHR cDNA obtained by PCR from the normal allele in heterozygous hyt/wt mice (18) was kindly provided by Dr. Peter Kopp (Northwestern University, Chicago IL). This cDNA was reported to contain three polymorphisms (D143G, E251K, and I286F) in the ectodomain relative to the wild-type mTSHR sequence in GenBank U02602 (19). The cDNA was excised from the vector pSG5 using EcoRI, blunted, and transferred into the AfII and XhoI sites (both blunted) in the vector pcDNA5/FRT (Invitrogen, Carlsbad, CA). Introduction of the wild-type human TSHR (hTSHR) cDNA (20) (with the H601 polymorphism converted to Y601) into the vector pcDNA5/FRT was described previously (21). The additional TSHR cDNA mutations described below in the text were generated using the QuickChange site-directed mutagenesis kit (Stratagene, San Diego, CA). All mutations were confirmed by nucleotide sequencing.
TSHR expression
The mTSHR and hTSHR cDNAs were transfected into Flp-In-CHO cells (Invitrogen) using Fugene HD (Roche, Indianapolis IN). Cell lines stably expressing the TSHR were obtained by selection with hygromycin B (Invitrogen; 75–100 μg/ml), a concentration at the low end of the range recommended by the manufacturer. Transfected clones did not survive at higher concentrations. Cells were cultured in Ham’s F12 medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), gentamicin (50 μg/ml), and Fungizone (2.5 μg/ml).
Flow cytometry
CHO cells were harvested from six-well plates using 1 mm EDTA and 1 mm EGTA in PBS. After washing twice with PBS containing 10 mm HEPES (pH 7.4), 2% fetal bovine serum, and 0.05% NaN3, the cells were incubated for 30 min at room temperature in 100 μl of the same buffer containing 1 μg of either normal mouse IgG or murine monoclonal antibody 2C11 (Morphosys, Raleigh, NC). After rinsing, the cells were incubated for 45 min with 100 μl fluorescein isothiocyanate-conjugated goat antimouse IgG (1:100; Caltag, Burlingame, CA), washed, and analyzed using a FACScan flow cytofluorimeter (Becton Dickinson, Franklin Lakes, NJ). Cells stained with propidium iodide (1 μg/ml final concentration) were excluded from analysis.
Cultured cell cAMP assays
CHO cells stably or transiently expressing mouse or hTSHR were transferred into 96-well plates. For bioassay, the culture medium described above was replaced with F12 medium supplemented with 1 mm isobutyl methylxanthine, 10 mm HEPES, and, where indicated in the text, bovine (b) TSH or human (h) TSH (Sigma-Aldrich, St. Louis MO). Untransfected CHO cells (mock transfections with empty vector in the case of transient transfections) were included as controls. After 60 min at 37 C, the medium was aspirated and intracellular cAMP was extracted with 0.2 ml 95% ethanol. The extracts were evaporated to dryness, resuspended in 0.1 ml PBS (pH 7.5), and samples (12 μl) were assayed using the LANCE cAMP kit according to the protocol of the manufacturer (PerkinElmer, Shelton, CT). The effective dose of TSH required for half-maximal stimulation of intracellular cAMP levels (EC50) was calculated using GraphPad Prism software (La Jolla, CA).
TSH binding
CHO cells expressing the TSHR were cultured in 24-well plates. Medium was aspirated and replaced with 250 μl binding buffer (Hanks’ buffer with 250 mm sucrose substituting for NaCl to maintain isotonicity and 0.25% BSA) containing about 8,000–13,000 cpm 125I-TSH (BRAHMS, Berlin, Germany) and the indicated concentrations of unlabeled bTSH. After incubation for 4 h at room temperature, cells were rapidly rinsed three times with binding buffer (4 C), solubilized with 0.2 ml 1 n NaOH, and radioactivity then measured in a γ-counter. Nonspecific binding was determined using untransfected cells.
Results
Reduced signal transduction by a mTSHR with six amino acid substitutions
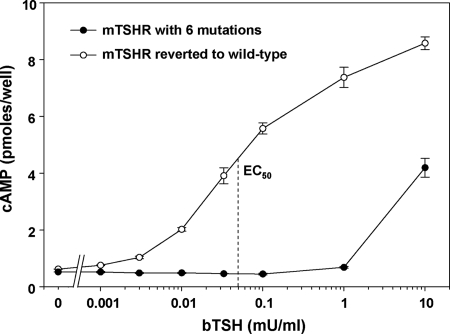
CHO cells stably expressing the mTSHR cDNA with three known mutations generated a cAMP response to TSH stimulation, confirming the previous report that it was functional (18). However, the sensitivity of this response was suboptimal for a thyroid-stimulating autoantibody assay (our original intent), with a strong, 8-fold increment attained only at a high TSH concentration (10 mU/ml) (Fig. 1). Consequently, we determined the nucleotide sequence of the entire mTSHR cDNA. In addition to the reported substitutions of D143G, E251K, and I286F, we observed E23K, R415K, and L732H, possibly PCR errors introduced during plasmid construction. Because of the possibility that one or more of these residues could contribute to the poor sensitivity to TSH stimulation, we reverted all six substitutions back to the wild-type mTSHR sequence. Sensitivity to TSH stimulation of the wild-type mTSHR was increased by approximately 2 orders of magnitude, with a clear cAMP response evident at 0.003 mU/ml TSH and with an EC50 at 0.05 mU TSH per milliliter (Fig. 1).
Figure 1.
Reduced sensitivity to bTSH stimulation of CHO cells stably expressing the mTSHR cDNA with six mutations. These mutations were corrected to generate a true wild-type mTSHR. Stably transfected CHO cells expressing either the mutated mTSHR or wild-type TSHR were incubated with the indicated concentrations of TSH for 1 h before assay of intracellular cAMP levels (see Materials and Methods). Each point is the mean ± range of values determined in duplicate wells of cells. This experiment is representative of four separate experiments (Fig. 3 depicts another experiment).
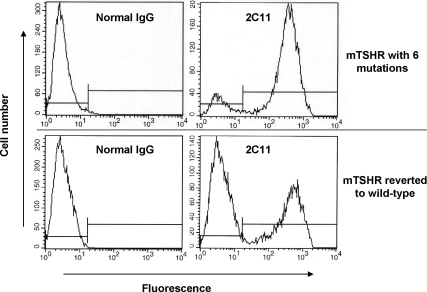
Flow cytometric analysis confirmed that the mTSHR with six substitutions trafficked normally to the cell surface with an expression level per cell comparable to that of the wild-type mTSHR (Fig. 2). Of interest, CHO cells stably expressing the wild-type mTSHR were less clonal than the polymorphic mTSHR, indicating the limitations of the Flp-In transfection system with some TSHR. In principle, with this vector all cells should express the transgene. Nevertheless, less than complete clonality (as with transient transfections) is not a problem for functional studies.
Figure 2.
Flow cytometric analysis indicates that the mTSHR with six mutations traffics normally to the cell surface with an expression level per cell comparable with that of the wild-type mTSHR. TSHR detection was with monoclonal antibody 2C11. The clonality of the mutated mTSHR cell line is greater than that of the wild-type mTSHR. Nevertheless, the degree of fluorescence for individual cells is similar for both cell lines. Incidentally, the Flp-In stable transfection system should theoretically generate clonal cell lines with identically located transgenomes. As is evident, in our experience this is not the case with some TSHR mutants.
Identification of mTSHR mutation(s) responsible for reduced sensitivity to TSH stimulation
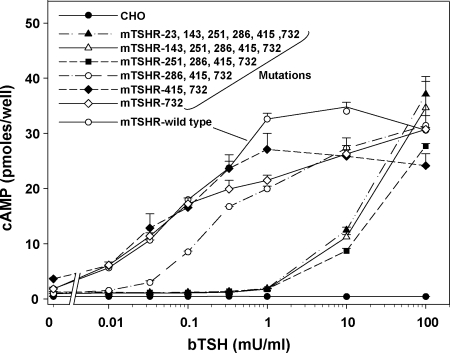
To determine which of the mTSHR mutations contributed to poor TSH stimulation, we progressively reverted these mutations to the wild type. Beginning at the N terminus, we first corrected E23K to E23. Then, using the latter as a template, we reverted D143G to D143, continuing in a cumulative manner until all six mutations were corrected. TSH stimulation of the mTSHR with E23 restored to wild type (five mutations remaining), and D143 restored (four mutations remaining) was similar to that of the initial poorly responsive mTSHR with all six mutations (Fig. 3). The major shift in TSH sensitivity toward that of the wild-type mTSHR (>1 order of magnitude) occurred with the reversion of residue K251 to E251 (three mutations remaining). Subsequently, correction of I286 to F286 had a smaller additive effect, fully restoring TSH sensitivity to that of the wild-type mTSHR.
Figure 3.
Identification of mTSHR mutations(s) responsible for reduced sensitivity to bTSH stimulation. Six mTSHR mutations were progressively and cumulatively reverted to the wild-type, beginning with E23K converted to E23, and then D143G converted to D143, E251K converted to E251, I286F converted to I286, R415K converted to R415, and L732H converted to L732. For simplicity, description of the mutations in these receptors indicate only their position, not the amino acid. Stably transfected CHO cells were incubated for 1 h with the indicated bTSH concentrations and intracellular cAMP levels were measured. CHO, Untransfected cells. Each point indicates the mean ± range of cAMP levels in duplicate wells of cells. These data were replicated in a second experiment. Please note that the apparent differences in constitutive (ligand independent) activity among receptors simply reflects different levels of receptor expression, as described in the legend to Fig. 4.
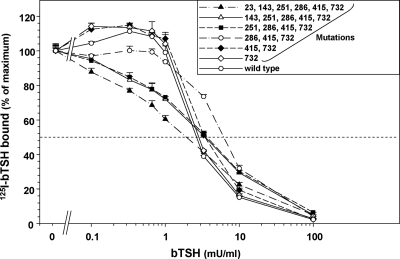
We investigated the relationship between 125I-TSH binding and TSH stimulated cAMP generation in the same stable cell lines depicted in Fig. 3. Because of differences in maximum specific TSH binding for the different TSHR variants (20.6–48.8% in the experiment shown; see legend to Fig. 4), the data for each receptor are normalized to 100%. In contrast to the very large range in EC50 for TSH stimulated cAMP generation (>100-fold), the EC50 for TSH binding inhibition encompassed a much narrower range (∼3-fold; 2–7 mU TSH per milliliter) (Fig. 4). Indeed, the step-up in sensitivity to TSH stimulation with the correction of the E251K mutation was not associated with any increase in sensitivity to inhibition of TSH binding. These data clearly indicated a dissociation between TSH binding and function in the mTSHR cDNA with six mutations, attributable in large part to the mutation at amino acid residue 251 (E251K).
Figure 4.
TSH binding to mTSHR with six mutations progressively converted to the wild type. Stably transfected CHO cells expressing the indicated TSHR (see legend to Fig. 3) were incubated for 3 h at room temperature in buffer containing 125I-bTSH supplemented with the indicated concentrations of unlabeled bTSH (see Materials and Methods). To compensate for variations in the levels of TSHR expression, data are normalized to 100% (125I-bTSH binding in the absence of stable bTSH). In the experiment shown, total 125I-bTSH counts per minute added per well were 9351 cpm and nonspecific binding (untransfected cells in the presence of 100 mU bTSH/ml) was 1.1%. Absolute binding for TSHR with the following number of mutations at the indicated sites was: six mutations (original cDNA), 22.0%; five mutations, 23.2%; four mutations, 20.6%; three mutations, 48.8%; two mutations, 48.7%; one mutation, 48.1%; no mutations (wild type), 47.1%. Similar data were obtained in a second experiment.
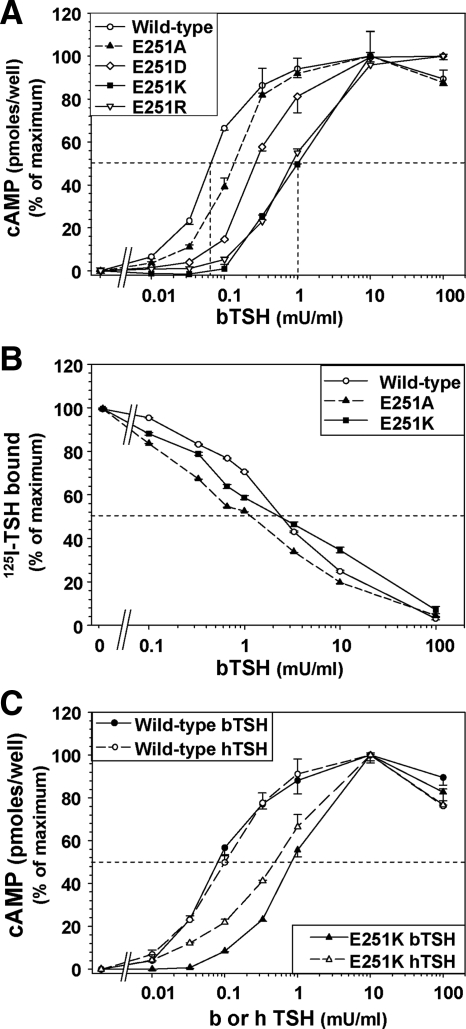
To determine whether the E251K substitution on it own (isolated from the other five substitutions) would increase the EC50 to TSH stimulation, we mutated the wild-type mTSHR and hTSHR to E251K and expressed these cDNAs in CHO cells. A similar effect was observed in both TSHR species. With the hTSHR, the wild-type TSHR was 14-fold more sensitive to TSH stimulation than TSHR-E251K (mean 14.0 ± 3.3 sem in four experiments). In the representative experiment shown (Fig. 5A), the EC50 for TSH stimulated cAMP generation in hTSHR-E251K was 10-fold higher than in the wild-type TSHR (3 vs. 0.3 mU/ml, respectively). Unlike TSHR-E251K, an E251A substitution had a minimal effect on sensitivity to TSH stimulation (Fig. 5A). A TSHR with E251 replaced by R, with a positively charged side chain slightly longer than K, behaved similarly to E251K. TSHR-E251D (negatively charged side chain slightly shorter than E) decreased sensitivity to TSH stimulation but to a lesser degree than E251K and E251R (Fig. 5A). Despite its reduced sensitivity to ligand activation, TSH binding to TSHR-E251K was similar to that of the wild-type TSHR and TSHR-E251A, confirming that the E251K mutation conferred a dissociation between TSH binding and function (Fig. 5B). All experiments described above were performed with bTSH. Even though there is no direct, experimental evidence that TSHR E251 is a ligand contact residue, we examined the TSHR E251K response to hTSH. The specific activity (units per milligram protein) of hTSH is less than that of bTSH. However, as expected in terms of biopotency units, bTSH and hTSH were equipotent in stimulating intracellular cAMP generation (Fig. 5C). As with bTSH, the EC50 for hTSH stimulation of TSHR E251K cAMP generation was also increased (reduced potency) relative to the wild-type.
Figure 5.
A, Effect of the TSHR E251K mutation on the cAMP response to bTSH stimulation. Human TSHR with single substitutions of E251K, E251A, E251R, and E251D, as well as the wild-type hTSHR, were stably expressed in CHO cells. Cells were incubated for 1 h in the indicated concentrations of TSH and intracellular cAMP measured (see Materials and Methods). Each point represents the mean ± ranges of cAMP values in duplicate wells of cells. The TSH EC50 is indicated by the vertical dashed lines. Similar data were obtained in five additional experiments for E251K and E251A and two additional experiments for E251R and E251D. B, TSH binding to hTSHR E251K and E251A is similar to that with the wild-type hTSHR. The same stably transfected CHO cell lines shown in A were incubated for 3 h at room temperature in buffer containing 125I-TSH supplemented with the indicated concentrations of unlabeled TSH (see Materials and Methods). Data are normalized to 100% of maximal 125I-bTSH binding in the absence of stable TSH. In the experiment shown, total 125I-bTSH counts per minute added per well were 6943 cpm. Absolute binding values were: wild-type TSHR, 28.9%; TSHR-E251K, 17.3%; and TSHR-E251A, 31.9%. Similar data were obtained in two additional experiments. C, Comparison of bTSH and hTSH potency on the wild-type TSHR and TSHR E251K. CHO cells stably expressing the wild-type TSHR and TSHR E251K were incubated for 1 h in the indicated concentrations of either bovine or human TSH and intracellular cAMP measured (see Materials and Methods). Each point represents the mean ± ranges of cAMP values in duplicate wells of cells. Similar data were obtained in one additional experiment.
Discussion
The present study originated with the unexpected observation that TSHR cDNA obtained by PCR from the normal allele in heterozygous hyt/wt mice (18) had poor TSH-induced signal transduction (cAMP generation) despite a TSH binding affinity similar to the wild-type TSHR. That is, this mTSHR exhibited a dissociation between TSH binding and function. Of the six mutations identified (possibly cloning artifacts), E251K (and to a lesser extent I286F) contributes to this phenomenon. The novelty of this observation is that, to our knowledge, there are no previous examples of specific amino acid mutations in the TSHR LRD region that uncouple ligand binding and function. Although mutation or deletion of a number of amino acids in the TSHR ectodomain, notably Y385 and adjacent residues in the hinge region, have previously been observed to decrease ligand-induced signal transduction (22,23), these effects are attributable to decreased TSH binding affinity; that is, there is no dissociation between ligand binding and function. Of course, mutations in the TMD, including the extracellular loops (24,25) as well as TSHR residues E409 and D410 at the ectodomain/membrane junction (24,26,27), can also reduce signal transduction without affecting ligand binding. TSHR E251 is of particular interest and was pursued in the present study because it is located in the LRD close to the junction with the hinge region.
Previously molecular modeling of TSH binding to the TSHR LRD crystal structure (8) suggested that E251is a contact residue in the TSH binding site on the concave surface of the last leucine-rich repeat, forming a salt bridge with TSH β-chain residue K44 (28,29). Our experimental data obtained with TSHR E251K do not support this hypothetical model. Indeed, there is prior evidence, confirmed in our study, that an E251A mutation has no effect on TSH binding or function (30). Also in regard to this model of TSH binding, despite overwhelming evidence for ligand binding to the LRD in the TSH holoreceptor, unlike for the closely related gonadotropin receptors, TSH binding to the isolated TSHR LRD cannot be observed experimentally (31). Presumably the affinity of TSH binding to the isolated TSHR LRD is too low for experimental detection. Indeed, there is much evidence that the TSHR hinge region linking the LRD to the TMD is also involved in TSH binding and signal transduction (for example, see Refs. 15,16,21,22,23,32 and 33). For these reasons, the hinge region is more accurately termed a SSD (14). How the SSD fulfills this role is poorly understood and very difficult to study because its structure and spatial relationship to the LRD and TMD are unknown.
TSHR residue E251 is also of interest from a number of other perspectives. First, there is preliminary evidence that the dual mutation of TSHR E251R and R255T (termed T21) is less responsive to TSH stimulation than the wild-type TSHR (34) (Supplemental Fig. 3A, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Which of these two residues contributes to this phenomenon was not defined, in retrospect, most likely the former. Furthermore, in the absence of TSH binding data, it could not be concluded that these combined mutations introduce a dissociation between TSH binding and signal transduction. Second, there is considerable variation among the glycoprotein hormone receptors at the position comparable with human TSHR E251 (35). For example, all subspecies of FSH receptor have a conserved lysine at this position. However, there is less conservation for the other glycoprotein hormone receptors, particularly for the LH receptor. For example, the bTSHR has a lysine rather than a glutamic acid, whereas the hLH receptor has an arginine residue. In our opinion, variability among receptors at the position corresponding to TSHR residue E251 does not negate the potential importance of this residue in coupling ligand binding with signal transduction (discussed below), as shown experimentally in the present study. This is because there is also much variability among receptors in the N-terminal half of the SSD region adjacent to the C-terminal region of the LRD containing TSHR residue E251. Amino acid changes in this region can alter the conformation or charge of the latter, thereby permitting a complementary fit for a different residue at position 251, i.e. E in the TSHR and K in the FSH receptor.
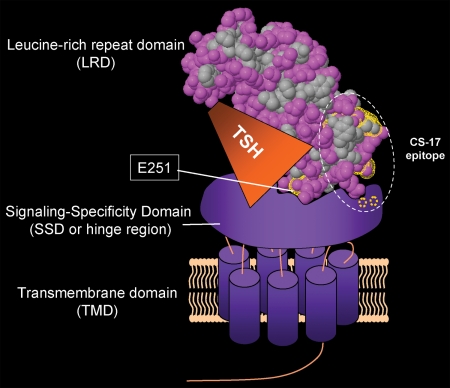
That the E251K substitution can reduce signal transduction despite high-affinity TSH binding comparable with the wild-type TSHR provides mechanistic insight into the coupling between ligand binding and receptor activation. Based on experimental evidence and molecular modeling, there is general agreement that the SSD sits on top of the extracellular loops in the TMD (14,21,33,36,37,38). However, there is diversity of opinion as to the steric relationship between the LRD and SSD. The model of Moyle et al. (14) projects the LRD to lie horizontally to the membrane, wrapped around the SSD, obscuring an evolutionary ancient cryptic ligand binding site on the concave surface of the LRD (39). Most reports, however, envision the TSHR LRD orientated vertically to the SSD, permitting ligand binding to the exposed concave surface of the LRD (33,36,40). To prevent flailing of the TSHR LRD, its position, vis-à-vis the SSD, must be stabilized by multiple amino acid interactions. The crystal structure of the TSHR LRD (residues 22–260) reveals E251 to be a projection from the concave surface of the LRD at the C terminus of the latter (8). With the LRD vertically orientated to the SSD, TSHR E251 is ideally situated to be a residue involved in stabilizing the LRD attachment to the SSD, with the LRD conceptually depicted as a squat banana stuck nearly vertically into a bowl of ice cream (SSD) (Fig. 6). We suggest that the E251A substitution, replacing the longer E251 side chain with a very small methyl group, is tolerated because other residues at the LRD-SSD interface are sufficient to maintain stability of the LRD relative to the SSD.
Figure 6.
Proposed model of how TSHR LRD residue E251 plays a role in TSH-mediated signal transduction. TSHR residues are depicted on the TSHR LRD (8) (Protein Data Base 3G04). The LRD is shown in a hydrophobic (gray)/polar (pink) plot (http://firstglance.jmol.org). Ligand binding transversely to the concave surface of the forward leaning LRD also contacts the top surface of the SSD. The TSHR LRD attachment to the SSD is likely to be stabilized by multiple amino acid interactions, of which E251 may be only one. TSHR E251 near the C terminus of the LRD is ideally positioned to immobilize the latter as well as to couple ligand binding with subsequent TSHR activation. Being on the concave surface of the LRD, E251 contributes to its forward leaning position and is adjacent to residues in the TSH binding site (34,36,44). It is noteworthy that TSHR E251 projects from the surface of the LRD. Because the K side chain is longer than that of E, we suggest that the TSHR E251K mutation reduces TSH-mediated signal transduction by modifying the flexibility of the LRD relative to the SSD. The TSHR monoclonal antibody CS-17 with inverse agonist activity (41) contains epitopic components on the LRD (convex surface opposite to E251) as well as in the SSD (residues T273 and R274 shown in dotted circles) (42,43). In the proposed model, the suppressive effect of the TSHR ectodomain on constitutive activity (21,45) is enhanced by CS-17 binding to both LRD and SSD, slightly altering their steric relationship.
In our proposed model, based on extensive evidence that TSH contacts both the TSHR LRD and SSD (see above), we depict TSH lying horizontally and contacting both the LRD and SSD (Fig. 6). Residue E251 is closely adjacent to the TSH binding site, yet projects into and stabilizes the LRD attachment to the SSD. Consistent with the evidence that TSHR residue E251 does not contribute to the TSH binding site (see above), it is difficult to visualize TSHR residue E251 binding TSH and simultaneously interfacing with the SSD. Also shown on this model are known epitopic components of monoclonal antibody CS-17 with TSHR inverse agonist activity (41,42,43). CS-17 binds to the convex surface of the LRD (opposite to E251) as well as to the SSD (residues T273 and R274). We suggest that CS-17 binding to both components suppresses TSHR constitutive activity by subtly altering their steric relationship.
In terms of ligand activation of signal transduction, E251 at the tenth β-strand of the LRD might be situated in a spatial neighborhood that is potentially involved in conformational changes during TSHR activation. The drastic change in the side-chain property of E251K disturbs this activation. In this critical position, the longer side chain of K compared with the wild-type E (TSHR-E251K), but not the small, single methyl side chain of A (TSHR-E251A), would alter the orientation between the LRD and SSD and could explain why only the former is associated with partial uncoupling of TSH binding and receptor activation. Replacement of E251with R produces a receptor functionally similar to TSHR-E251K, raising the possibility that E251 forms a salt bridge with a positively charged TSHR residue in the SSD region, of which there are very many candidates. Indeed, TSHR-E251D, retaining a negatively charged side chain, has a lesser effect than E251K or R on the sensitivity to TSH stimulation. However, the sensitivity to TSH stimulation of TSHR-E251A is very similar to that of the wild-type TSHR, and despite having similar charges, residues K/R and E/D have conformationally different side chains. Therefore, although the present study clearly demonstrates the importance of TSHR residue E251 in coupling TSH binding with receptor function, whether this process involves a salt bridge cannot be determined.
In summary, reversion to wild type of mutations in a TSHR cDNA reveals E251 to be an important TSHR LRD amino acid residue involved in signal transduction without making a discernible contribution to TSH binding. These data suggest that TSHR residue E251 is one residue that contributes to stabilizing the LRD with the SSD, thereby enabling ligand binding to transduce a signal by the TMD mediated via the SSD.
Acknowledgments
We thank Dr. J. Froehlich (B.R.A.H.M.S., Hennigsdorf, Germany) for generously providing radiolabeled TSH. We are also grateful for contributions by Dr. Boris Catz (Los Angeles, CA).
Footnotes
This work was supported by National Institutes of Health Grants DK 19289 (to B.R.) and DK 54684 (to S.M.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 24, 2010
Abbreviations: b, Bovine; GPCR, G protein-coupled receptor; h, human; hTSHR, human TSHR; LRD, leucine-rich repeat domain; mTSHR, mouse TSHR; SSD, signaling specificity domain; TMD, transmembrane domain; TSHR, TSH receptor.
References
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin receptor: Interaction with thyrotropin and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S 2004 A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci 29:119–126 [DOI] [PubMed] [Google Scholar]
- Ingbar SH 1972 Autoregulation of the thyroid. Response to iodide excess and depletion. Mayo Clin Proc 47:814–823 [PubMed] [Google Scholar]
- Adams DD, Purves HD 1956 Abnormal responses in the assay of thyrotropins. Proc Univ Otago Med Sch 34:11–12 [Google Scholar]
- Kriss JP, Pleshakov V, Chien JR 1964 Isolation and identification of the long-acting thyroid stimulator and its relation to hyperthyroidism and circumscribed pretibial myxedema. J Clin Endocrinol Metab 24:1005–1028 [DOI] [PubMed] [Google Scholar]
- Corvilain B, Van Sande J, Dumont JE, Vassart G 2001 Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol (Oxf) 55:143–158 [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M 2000 Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK 2007 Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK 2007 GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318:1266– 1273 [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC 2007 High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258– 1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC 2008 The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle WR, Xing Y, Lin W, Cao D, Myers RV, Kerrigan JE, Bernard MP 2004 Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem 279:44442–44459 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Wadsworth HL, Chazenbalk GD, Russo D, Seto P, Rapoport B 1991 Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for TSH receptor function. Proc Natl Acad Sci USA 88:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Rapoport B 1992 Role of the carboxyl-terminal half of the extracellular domain of the human thyrotropin receptor in signal transduction. Endocrinology 131:548–552 [DOI] [PubMed] [Google Scholar]
- Misharin A, Hewison M, Chen CR, Lagishetty V, Aliesky HA, Mizutori Y, Rapoport B, McLachlan SM 2009 Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology 150:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WX, Du GG, Kopp P, Rentoumis A, Albanese C, Kohn LD, Madison LD, Jameson JL 1995 The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology 136:3146–3153 [DOI] [PubMed] [Google Scholar]
- Stein SA, Oates EL, Hall CR, Grumbles RM, Fernandez LM, Taylor NA, Puett D, Jin S 1994 Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol Endocrinol 8:129–138 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Kaufman KD, Seto P, Rapoport B 1989 Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun 165:1184–1190 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Chen CR, McLachlan SM, Rapoport B 2008 The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol 22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ban T, Akamizu T, Kohn LD 1991 Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J Biol Chem 266: 19413–19418 [PubMed] [Google Scholar]
- Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G 2002 Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J 21:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus M, Jaeschke H, Kleinau G, Neumann S, Krause G, Paschke R 2005 A hydrophobic cluster in the center of the third extracellular loop is important for thyrotropin receptor signaling. Endocrinology 146:5197–5203 [DOI] [PubMed] [Google Scholar]
- Kleinau G, Claus M, Jaeschke H, Mueller S, Neumann S, Paschke R, Krause G 2007 Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem 282:518–525 [DOI] [PubMed] [Google Scholar]
- de Roux N, Misrahi M, Brauner R, Houang M, Carel JC, Granier M, Le Bouc Y, Ghinea N, Boumedienne A, Toublanc JE, Milgrom E 1996 Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocrinol Metab 81:4229–4235 [DOI] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Neumann S, Krause G, Paschke R 2006 Significance of ectodomain cysteine boxes 2 and 3 for the activation mechanism of the thyroid-stimulating hormone receptor. J Biol Chem 281:31638–31646 [DOI] [PubMed] [Google Scholar]
- Núñez Miguel R, Sanders J, Chirgadze DY, Blundell TL, Furmaniak J, Rees Smith B 2008 FSH and TSH binding to their respective receptors: similarities, differences and implication for glycoprotein hormone specificity. J Mol Endocrinol 41:145–164 [DOI] [PubMed] [Google Scholar]
- Núñez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B 2009 Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol 42:381–395 [DOI] [PubMed] [Google Scholar]
- Sanders J, Bolton J, Sanders P, Jeffreys J, Nakatake N, Richards T, Evans M, Kiddie A, Summerhayes S, Roberts E, Miguel RN, Furmaniak J, Smith BR 2006 Effects of TSH receptor mutations on binding and biological activity of monoclonal antibodies and TSH. Thyroid 16:1195–1206 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Jaume JC, McLachlan SM, Rapoport B 1997 Engineering the human thyrotropin receptor ectodomain from a non-secreted form to a secreted, highly immunoreactive glycoprotein that neutralizes autoantibodies in Graves’ patients’ sera. J Biol Chem 272:18959–18965 [DOI] [PubMed] [Google Scholar]
- Kleinau G, Jäschke H, Neumann S, Lättig J, Paschke R, Krause G 2004 Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem 279:51590–51600 [DOI] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Paschke R, Krause G 2008 Extended hormone binding site of the human thyroid stimulating hormone receptor: distinctive acidic residues in the hinge region are involved in bovine thyroid stimulating hormone binding and receptor activation. J Biol Chem 283:18048–18055 [DOI] [PubMed] [Google Scholar]
- Smits G, Campillo M, Govaerts C, Janssens V, Richter C, Vassart G, Pardo L, Costagliola S 2003 Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J 22:2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Durme J, Horn F, Costagliola S, Vriend G, Vassart G 2006 GRIS: glycoprotein-hormone receptor information system. Mol Endocrinol 20:2247–2255 [DOI] [PubMed] [Google Scholar]
- Núñez Miguel R, Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Blundell TL, Rees Smith B, Furmaniak J 2004 Analysis of the thyrotropin receptor-thyrotropin interaction by comparative modeling. Thyroid 14:991–1011 [DOI] [PubMed] [Google Scholar]
- Moyle WR, Lin W, Myers RV, Cao D, Kerrigan JE, Bernard MP 2005 Models of glycoprotein hormone receptor interaction. Endocrine 26:189–205 [DOI] [PubMed] [Google Scholar]
- Kleinau G, Krause G 2009 Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev 30:133–151 [DOI] [PubMed] [Google Scholar]
- Lin W, Bernard MP, Cao D, Myers RV, Kerrigan JE, Moyle WR 2007 Follitropin receptors contain cryptic ligand binding sites. Mol Cell Endocrinol 260–262:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TF, Ando T, Lin RY, Tomer Y, Latif R 2005 Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2008 Identification of key amino acid residues in a thyrotropin receptor monoclonal antibody epitope provides insight into its inverse agonist and antagonist properties. Endocrinology 149:3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2009 A monoclonal antibody with thyrotropin (TSH) receptor inverse agonist ant TSH antagonist activities binds to the receptor hinge region as well as to the leucine-rich domain. Endocrinology 150:3401–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Russo D, Wadsworth HL, Chazenbalk GD, Rapoport B 1991 Eleven amino acids (Lys-201 to Lys-211) and 9 amino acids (Gly-222 to Leu-230) in the human thyrotropin receptor are involved in ligand binding. J Biol Chem 266:14926–14930 [PubMed] [Google Scholar]
- Zhang M, Tong KP, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW 2000 The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517 [DOI] [PubMed] [Google Scholar]