Abstract
Chitosan and collagen type I are naturally-derived materials used as cell carriers because of their ability to mimic the extracellular environment and direct cell function. In this study beta-glycerophosphate (beta-GP), an osteogenic medium supplement and a weak base, was used to simultaneously initiate gelation of pure chitosan, pure collagen, and chitosan-collagen composite materials at physiological pH and temperature. Adult human bone marrow-derived stem cells (hBMSC) encapsulated in such hydrogels at chitosan/collagen ratios of 100/0, 65/35, 25/75, and 0/100 wt% exhibited high viability at day 1 after encapsulation, but DNA content dropped by about half over 12 days in pure chitosan materials while it increased two-fold in materials containing collagen. Collagen-containing materials compacted more strongly and were significantly stiffer than pure chitosan gels. In monolayer culture, exposure of hBMSC to beta-GP resulted in decreased cell metabolic activity that varied with concentration and exposure time, but washing effectively removed excess beta-GP from hydrogels. The presence of chitosan in materials resulted in higher expression of osterix and bone sialoprotein genes in medium with and without osteogenic supplements. Chitosan also increased alkaline phosphatase activity and calcium deposition in osteogenic medium. Chitosan-collagen composite materials have potential as matrices for cell encapsulation and delivery, or as in situ gel-forming materials for tissue repair.
Keywords: chitosan, collagen, hydrogel, osteogenic, tissue engineering, biomaterials, composite, bone, β-glycerophosphate
1. Introduction
Composite biomaterials are increasingly being used in bone tissue engineering in order to mimic the complex properties of native extracellular matrices (ECM) [1]. Specific components in composite materials can offer different instructive cues to promote tissue-specific differentiation of cells via cell-ECM interactions, and also can provide desired mechanical properties [2]. Synthetic biomaterials have been modified chemically to provide specific cues and thereby guide cell function modification [3], and a range of naturally-derived materials also have been used for this purpose due to their biological compatibility and relevance [4–5]. Collagen type I is the most abundant extracellular matrix protein and is widely used as a biomaterial because of its support of cell adhesion and proliferation, as well as its robust mechanical properties that allow it to be handled and fashioned into scaffolds by various methods [6]. Recent findings also suggest that integrin-mediated adhesion to collagen type I enhances osteogenic differentiation of human bone marrow mesenchymal stem cells (hBMSC) [7–8]. Chitosan is a polysaccharide derived from the deacetylation of chitin, the main structural component of crustacean exoskeletons. It is therefore abundant and has been used as a biomaterial because of its intrinsic antibacterial activity, wound-healing properties, and low immunogenicity [9]. In orthopedic tissue engineering, chitosan has been combined with other materials such as calcium phosphate [10] and hydroxyapatite [11] to create bioactive composites that demonstrate osteoconductivity and enhanced mineralization [12].
In previous studies, collagen and chitosan have been combined using a freeze-drying process to create composite sponges that have been employed for stem cell culture [9], hemostatic dressings [13], periodontal regeneration [14], as well as other applications. A recent study using chitosan-collagen sponges showed that these composite materials exhibited increased osteoblastic differentiation of MSC and improved mechanical properties, relative to pure matrices [15]. However, scaffolds prepared by freeze-drying are limited by the fact that cells cannot be incorporated directly into the material at the time of fabrication, but rather must be exogenously added after drying and subsequent rehydration. This process can result in lower seeding efficiency and uneven cell distribution, compared to gelation methods that embed cells directly in hydrogel matrices. In addition, freeze-dried scaffolds are not malleable, and therefore are not suitable as conformal shape-filling materials that are required to full irregularly shaped tissue defects. Therefore, it is of great interest to fabricate a chitosan-collagen composite that supports cell survival but that also can be injected and gelled in situ to efficiently fill tissue defects.
Beta-glycerophosphate (β-GP) has been shown to be an osteogenic supplement when added to cultures of hBMSC. It also has been used as a catalyst to cause a sol-gel transition in chitosan solutions at physiological pH and temperature [16]. This hydrogel form of chitosan has been investigated for use in cartilage tissue engineering [17–20] because the polysaccharide nature of chitosan resembles the glycosaminoglycans in cartilage, and has demonstrated good cytocompatibility with chondrocytes [18] and hBMSCs [21]. Because β-GP is a weak base, we hypothesized that it could be used to neutralize preparations of acid-solubilized collagen type I and thereby cause reconstitution of collagen fibrils. The use of β-GP as an agent to cause both a sol-gel transition in chitosan as well as collagen fibrillogenesis presents the possibility of creating pure chitosan, pure collagen, and chitosan-collagen composites without the need for freeze-drying or chemical crosslinking. Furthermore, because these processes can be initiated and maintained at physiological temperature and pH, we included hBMSC at the time of gelation to form engineered tissue constructs consisting of cells directly surrounded by hydrogel matrix.
In the present study, we first characterized the concentration of β-GP (range: 2.5 to 17.5% wt%) needed to cause gelation of both pure and mixed composites of chitosan and collagen, and then made composites at a range of chitosan/collagen ratios (100/0, 65/35, 25/75, 0/100). The resulting materials were characterized by chemical composition, mechanical stiffness, and cytocompatibility. Finally, the effects of chitosan/collagen ratios on osteogenic differentiation were evaluated in vitro by alkaline phosphatase activity, calcium mineralization, and osteogenic gene expression.
2. Materials and methods
2.1 Preparation of chitosan-collagen hydrogels
Bovine type I collagen (MP Biomedicals, Solon OH) and chitosan (93% DDA; BioSyntech, Quebec, Canada) were dissolved in 0.02 N and 0.1 N acetic acid (Sigma, St. Louis, MO) to obtain 4.0 mg/ml collagen and 2.0 wt% chitosan stock solutions, respectively. These two solutions were mixed at various chitosan/collagen mass ratios of 100/0, 65/35, 25/75, or 0/100 and an appropriate amount of pre-cooled 58 wt% β-GP solution was added to the above mixture to obtain a solution including 2.5%, 5%, 7.5%, 10%, 12.5%, 15%, or 17.5% β-GP. This resulted in a panel of 28 different combinations of chitosan, collagen, and β-GP concentration. The solution was then used to resuspend hBMSC to achieve the desired cell density. All procedures were conducted on ice to maintain a liquid state before gelation. Gelation was initiated by incubation of the chitosan-collagen solution at 37 °C. Gel formation and gelation time were determined by the mobility of chitosan-collagen solution after inverting test tubes, and by monitoring the turbidity of the solution from clear in the liquid state to white/gray in the gel state. The pH of chitosan-collagen materials (n = 3) were recorded on ice before gelation and at 37 °C after gelation. Unless otherwise specified, 7.5% β-GP was used to fabricate all gels for the comparison of the effects of chitosan/collagen ratios on gel properties and biological response.
2.2 Energy dispersive X-ray spectroscopy (EDX)
EDX microanalysis was used to identify the presence of β-GP in formed gels. Specifically, two characteristic chemical elements in β-GP, sodium (Na) and phosphorus (P), were examined in a total of 8 different gels, including 4 chitosan/collagen ratios at 2 conditions. One condition was unwashed gels and the other was washed gels with de-ionized (DI) water. All gels were freeze-dried in a lyophilizer before examination. EDX spectroscopy was performed using an energy dispersive X-ray spectrometer (EDAX Inc., Mahwah NJ) installed in an environmental scanning electron microscope (ESEM; Quanta 200 FEG, FEI Company, Hillsboro OR) at 15 kV under a low vacuum mode without sputter coating. EDX data (n = 2) were collected and analyzed using genesis microanalysis software (EDAX Inc.).
2.3 Scanning electron microscopy (SEM)
For SEM observation, gels containing 4 different chitosan/collagen ratios were fabricated. For sample preparation, all gels were snap-frozen in liquid nitrogen and then freeze-dried overnight. Dried gels were cut with a sharp blade to expose internal microstructure and sputter coated with platinum-gold for SEM imaging at 15 kV using a FEI Nova Nanolab scanning electron microscope (FEI Company, Hillsboro OR).
2.4 Mechanical testing
Hydrated samples (n = 4) were used to perform unconfined compression tests using a mechanical testing system (800LM instrument, TestResources Inc., Shakopee MN). Samples were placed under a Delrin platen and a small tare load of 0.01 N was applied to ensure that each sample received the same degree of compression. Samples were then compressed at a ramp speed of 20% strain per second until reaching 10% strain. Force and deformation data were collected by the test instrument and were converted to stress and strain values. Elastic modulus was determined from the slope of the linear portion of the stress-strain curve for all samples.
2.5 Cell culture, viability assessment and DNA quantification in chitosan-collagen gels
hBMSCs at passage 1 (P1; Lonza Inc., Walkersville MD) were thawed, cultured, and expanded in complete medium consisting of Dulbecco's Modified Eagle Medium - low glucose (DMEM-LG; Invitrogen, Carlsbad CA), 10% MSC-qualified fetal bovine serum (FBS; Invitrogen), and 1% penicillin/streptomycin (PS; Invitrogen). Cells were used at P5 and were encapsulated at a density of 5.0×105 cells/ml and 400 µl of formed solution, corresponding to 2.0×105 cells per gel, was transferred into each well of a 24-well plate. After gel formation, all gels were washed three times with cell culture medium every 10 min and then cultured in complete medium. Culture medium was changed every two days. Cell viability and DNA quantification (n =4) were conducted at days 1 and 12. For cell viability assessment, constructs were washed in sterile PBS three times for 10 min and then incubated for 1 h at room temperature with 4 µM calcein-AM and 4 µM ethidium homodimer (Molecular Probes, Eugene OR) in PBS. After incubation, constructs were washed again for fluorescence microcopy. For DNA quantification, samples were digested in 1.0 ml of 4.0 M guanidine hydrochloride buffer (pH = 7.5) and a Quant-iT™ PicoGreen® kit from Invitrogen was used to quantify DNA content following the manufacturer’s protocol. Briefly, 50 µl samples or DNA standards were incubated with 150 µl of 1X PicoGreen® reagents and then used for fluorescence measurements at 560/590 nm (excitation/emission).
2.8 Evaluation of cytotoxicity induced by β-GP solution
To evaluate cytotoxicity of β-GP solution (n = 3), hBMSC were seeded as monolayers at a density of 12.5×103 cells per well in 96-well plates and cultured for 24 h. The medium was then replaced with 100 µl of 25 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-buffered media (Invitrogen) supplemented with the appropriate concentration of β-GP: 0%, 2.5%, 5%, 7.5%, 10%, 12.5%, and 15%. Cytotoxicity assays were conducted after 0.5, 4 and 8 h incubations with β-GP using alamarBlue® cell viability reagents (Invitrogen). In brief, 10.0 µl of 10× alamarBlue® solution was added into each well and incubated at 37 °C for 5 h. Ninety microliters of the above solution was used for fluorescence measurements at 560/590 nm (excitation/emission). In this assay, fluorescence intensity varies with metabolic activity of the cells.
2.7 Gel compaction assay
For gel compaction assays, P5 cells were encapsulated at a density of 1.0×106 cells/ml in gels (n = 4). Following gel formation, all constructs were released from their culture wells using a sterile spatula to allow unrestrained cell-mediated gel compaction. All constructs were cultured in complete media for 3 weeks. Gel images were taken at days 0, 1, 4, 6, 8, 10, 14, and 21 and analyzed using Image Pro software to obtain gel diameters. Gel compaction was reported as a reduction in the diameter of the gels with time.
2.8 Osteogenic differentiation of hBMSCs
To examine the effects of chitosan/collagen ratios on osteogenic differentiation, P5 hBMSCs were encapsulated at a density of 1.0×106 cells/ml in gel solution containing different chitosan/collagen ratios: 65/35, 25/75, or 0/100. Constructs were cultured in an osteogenic medium consisting of DMEM-LG, 10% FBS, 1% PS, 100 nM dexamethasone (Sigma), 5.0 mM β-GP, and 50 µg/ml ascorbic acid 2-phosphate (Sigma). As a control, gels were cultured in complete medium without osteogenic supplements. All media were changed every two days. Osteogenic differentiation was evaluated by ALP activity, calcium content, and gene expression levels, as described below.
2.9 RNA isolation and RT-PCR analysis
At days 1, 12, and 21, samples (n = 4) were collected in RNase-free cryovials, snap-frozen in liquid nitrogen, and stored at −80 °C. All frozen samples were pulverized in a BioPulverizer (Bartlesville OK) pre-cooled in liquid nitrogen. Pulverized sample powders were used for RNA extraction using a cetlytrimethylammonium bromide (CTAB)-based method reported previously [22]. Briefly, sample powders were mixed with 600 µl pre-warmed CTAB extraction buffer in RNase-free eppendorf tubes. An equal volume of chloroform-isoamyl alcohol (24:1) (Fisher Scientific, Pittsburgh PA) was added, well mixed, and centrifuged for 5 min at 15,000×g at room temperature. After centrifugation, the clear upper phase was extracted again with an equal volume of chloroform-isoamyl alcohol. The upper phase was then precipitated with an equal amount of isopropanol (IPA), and the obtained RNA pellets were washed in 75% ethanol and dissolved in 30–50 µl RNase-free water. The dissolved RNA was further purified using a Qiagen RNeasy Mini kit (Qiagen Inc., Valencia CA). For quantitative RT-PCR, messenger RNA (mRNA) was converted to cDNA using a High-Capacity cDNA Archive kit (Applied Biosystems Inc., Foster City CA) following the manufacturer’s protocol. TaqMan gene expression assay kits (Applied Biosystems) were used for transcript levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), type I collagen (CI), bone sialoprotein (BSP), and osterix (OSX) in an Applied Biosystems 7500 Fast System. The ID numbers of the kits were Hs99999905_m1 for GAPDH, Hs00164004_m1 for CI, Hs00173720_m1 for BSP, and Hs00541729_m1for OSX. The 2−ΔΔCt method was used to evaluate relative mRNA expression levels for each target gene [23]. Briefly, ΔCt values were obtained by the difference between the Ct values of target genes and the GAPDH gene. They were then normalized by subtracting the ΔCt value of the calibrator sample, their respective Ct values at day 1, to obtain ΔΔCt values.
2.10 Biochemical assays
At day 21, samples (n = 4) were processed in the same manner as for RNA isolation to obtain pulverized powders for biochemical assays. To assess ALP activity, pulverized sample powders were lysed in 500 µl of 0.2% Triton X-100 (Sigma) using two freeze–thaw cycles. The lysate was used to determine alkaline phosphatase activity following a previously described method [24]. In brief, 20.0 µl lysate was combined with 80.0 µl of 1.5 M 2-amino-2-methyl-1-propanol (AMP; Sigma) buffer (pH = 10.3) containing 5.0 mM p-nitrophenol phosphate substrate (Sigma). For the standard curve, serial dilutions of 0–20 nM p-nitrophenol (Sigma) were made in Triton solution. The reaction was stopped by adding 100 µl of 0.5 M NaOH. The plate was then read spectrophotometrically at 405 nm. For calcium quantification, an OCPC (orthocresolphthalein complex one) method was used as previously described [24]. In brief, sample powders were digested in 1.0 N acetic acid overnight. Twenty microliters of the digested solution was mixed with 250 µl of working solution consisting of 0.05 mg/ml OCPC solution and ethanolamine/boric acid/8-hydroxyquinoline buffer (Sigma), incubated for 10 min at room temperature, and finally read at 575 nm. Both ALP activity and calcium contents were normalized to DNA contents measured by the PicoGreen® kit.
2.11 Statistical analysis
All quantitative data were expressed as mean ± standard deviation, and analyzed by analysis of variance (ANOVA), followed by Tukey’s post hoc tests. A level of significance of p < 0.05 was used to indicate statistical differences between treatment groups.
3. Results
3.1 Gel fabrication
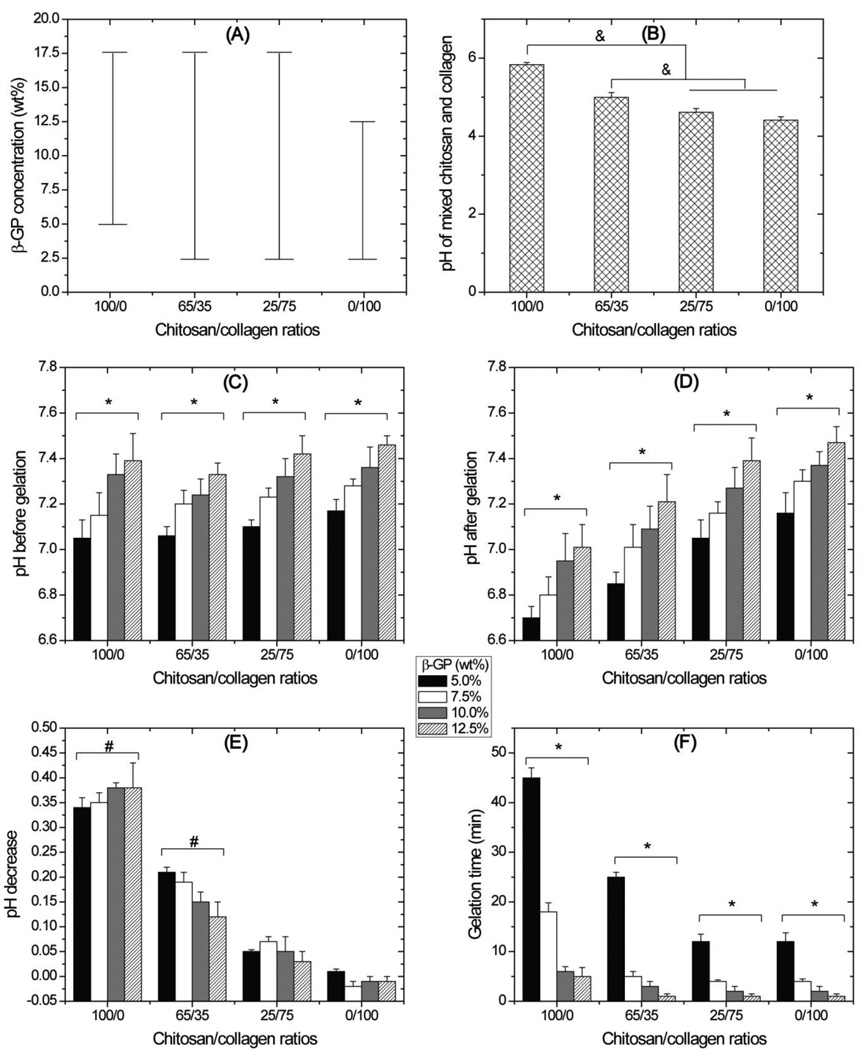
Figure 1 shows gelation parameters for chitosan and collagen hydrogels formed using β-GP as an initiator. The range of β-GP concentrations tested was from 2.5% to 17.5% and Fig. 1A shows the concentrations that produced solid gels for each of the four chitosan/collagen ratios examined. Pure chitosan (100/0) formed gels at β-GP concentrations ranging from 5.0% to 17.5%, and gels maintained their original volume immediately after gelation. No gels were formed from pure chitosan at 2.5% β-GP. Pure collagen (0/100) formed stable gels at β-GP concentrations ranging from 2.5% to 12.5%. At β-GP concentrations of 15.0% and 17.5%, pure collagen formed firm gels instantly on ice and rapidly compacted to less than 20% of their original volume within 2 min. Composite chitosan-collagen formulations (65/35, 25/75) formed gels at all tested β-GP concentrations between 2.5% to 17.5%.
Figure 1.
Gel formation parameters. A: Bars show range of β-GP concentration at which firm hydrogels were formed. B: pH of the chitosan and collagen solutions before β-GP addition. C: pH of the chitosan and collagen solutions after β-GP addition but before gelation, D: pH of the chitosan and collagen solutions after gelation. E: chart of pH decreases after gelation. F: gelation time of chitosan and collagen hydrogels. In general, high β-GP concentrations decreased pH and increased gelation time. High chitosan/collagen ratios decreased both pH and gelation time. (n = 3 separate experiments for all parameters; & = statistically significant differences; * = statistically significant differences existing among the groups containing different β-GP concentrations; # = statistically significant decrease in pH after gelation)
For experiments examining pH changes and gelation time (Fig. 1B to 1F), pure and composite hydrogels were made using β-GP concentrations between 5.0% and 12.5%. Before the addition of β-GP (Fig. 1B), pH values ranged from 4.4 to 5.8 with higher pH values at higher chitosan concentrations (p < 0.05). After the β-GP addition and before gelation (Fig. 1C), pH values of all tested groups ranged from 7.0 to 7.5 with higher pH values at higher β-GP concentrations (p < 0.05) though different chitosan/collagen ratios did not lead to significant difference in pH values. After gelation (Fig. 1D), a distinct pH prolife was observed with lower pH at higher chitosan/collagen ratios. Fig. 1E shows the change in pH during the gelation process, i.e. before versus after gel formation. A significant drop in pH occurred in pure chitosan and 65/35 groups (p < 0.05) while this drop was not significant for the two groups with higher collagen contents. Low β-GP concentrations and high chitosan/collagen ratios slowed down gelation process (Fig. 1F). The groups with either 5% β-GP or pure chitosan had significantly longer gelation time than other groups (p < 0.05).
3.2 EDX and SEM analyses
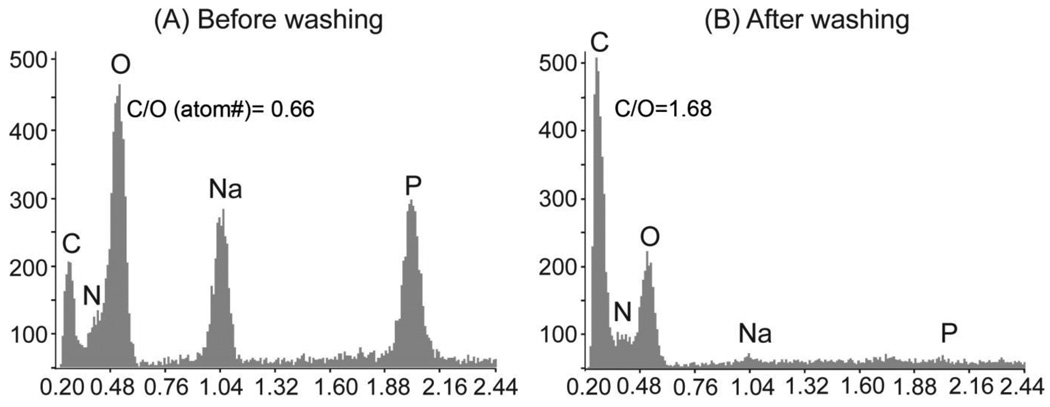
X-ray microanalysis results are shown in Figure 2 and demonstrated the presence of C, N, O, Na and P in all groups after gel formation (Fig. 2A). After washing with DI water (Fig. 2B), the Na and P peaks diminished substantially. These elements distinguish the presence of β-GP, since they are not chemical components of chitosan or collagen. The C:O ratios were also increased from 0.66 to 1.68 after the wash, as would be expected upon removal of residual β-GP. All formed gels shared a similar profile and the chitosan/collagen ratio did not affect the reduction of then Na and P peaks.
Figure 2.
Representative EDX microanalysis after gelation without washing (A) and with washing using DI water (B). Washing with DI water resulted in the diminishment of Na and P in all gels.
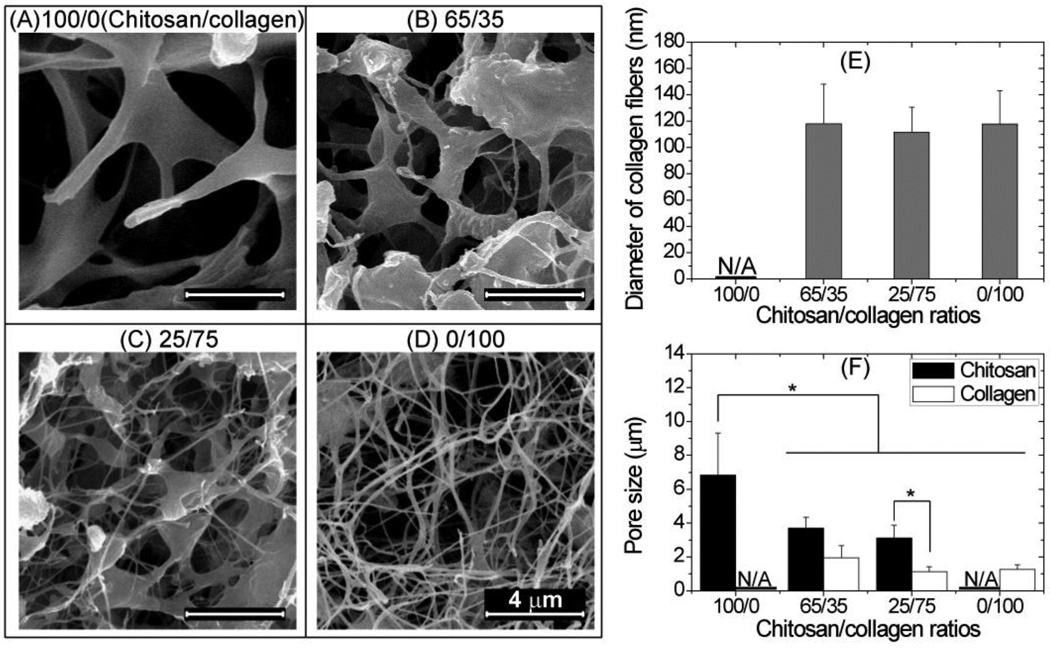
SEM images of the inside of composite materials are shown in Figure 3, and the structure of the matrix clearly changed with composition (Fig. 3A–3D). Pure chitosan materials exhibited an open, porous structure (Fig. 3A), while pure collagen gels exhibited an interconnected fibrous network (Fig. 3D) typical of reconstituted collagen gels. Mixed chitosan-collagen materials (Fig. 3B and 3C) exhibited a composite structure with collagen fibrils interspersed within the chitosan matrix. In 65/35 chitosan/collagen composite gels (Fig. 3C), most collagen fibers were embedded in chitosan matrix, while in 65/35 chitosan/collagen gels a larger fraction of collagen fibers were observed separate from the chitosan. In all collagen-containing gels, the collagen fibrils were of similar diameter (Fig. 3E), around 100 nm. Quantitation of pore sizes (Fig. 3F) showed that pure chitosan materials had the largest pores (p<0.05) while pure collagen had the smallest (p<0.05) and in general the pores formed by chitosan were larger than the ones formed by collagen fibers (p < 0.05).
Figure 3.
SEM analysis. Pure chitosan showed a porous structure (A), pure collagen gels exhibited a fibrous network (D), and mixed chitosan-collagen materials exhibited a composite structure with collagen fibrils interspersed with chitosan matrix (B and C). The collagen-containing gels had a similar collagen fiber diameter (E). Pure chitosan gels had the largest pores among all groups (F). (* = statistically significant differences; scale bar = 4 µm)
3.3 Mechanical testing
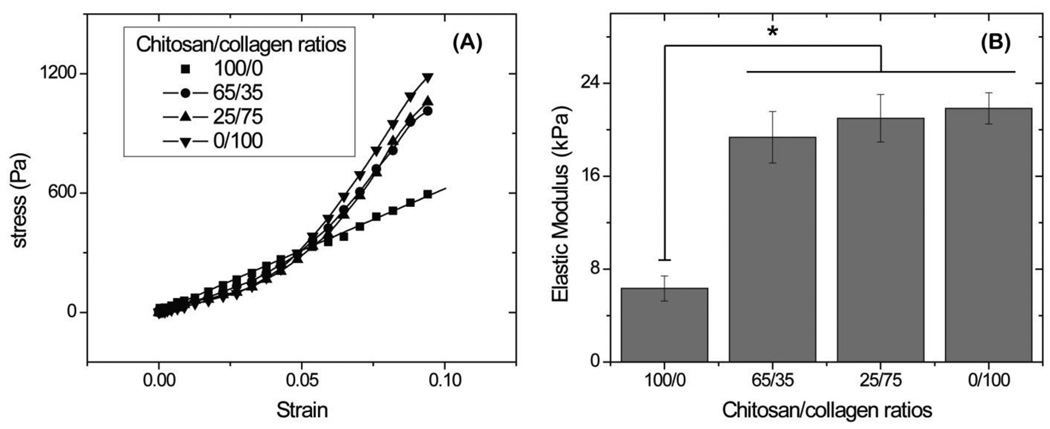
Results of unconfined compression testing on chitosan and collagen materials are shown in Figure 4. Stress-strain profiles (Fig 4A) showed that pure chitosan gels exhibited a uniformly linear strain-stress relationship from 0% to 8% strain. Composites containing collagen as well as pure collagen matrices exhibited a toe-in region from 0% to 5% strain, followed by a linear region between 5% and 8% strains. Determination of the linear modulus showed that all collagen-containing materials were approximately 3 times stiffer than pure chitosan (p<0.05), which had the lowest modulus of 6.3 ± 1.1 kPa. There was no significant difference in modulus between the collagen-containing composites or pure collagen.
Figure 4.
Representative strain-stress curves (A) and linear modulus (B) obtained by unconfined compressive mechanical tests. Pure chitosan gels had a lower modulus than collagen-containing materials. (n = 4; * = statistically significant differences from pure chitosan gels)
3.4 Cell viability, morphology, and proliferation
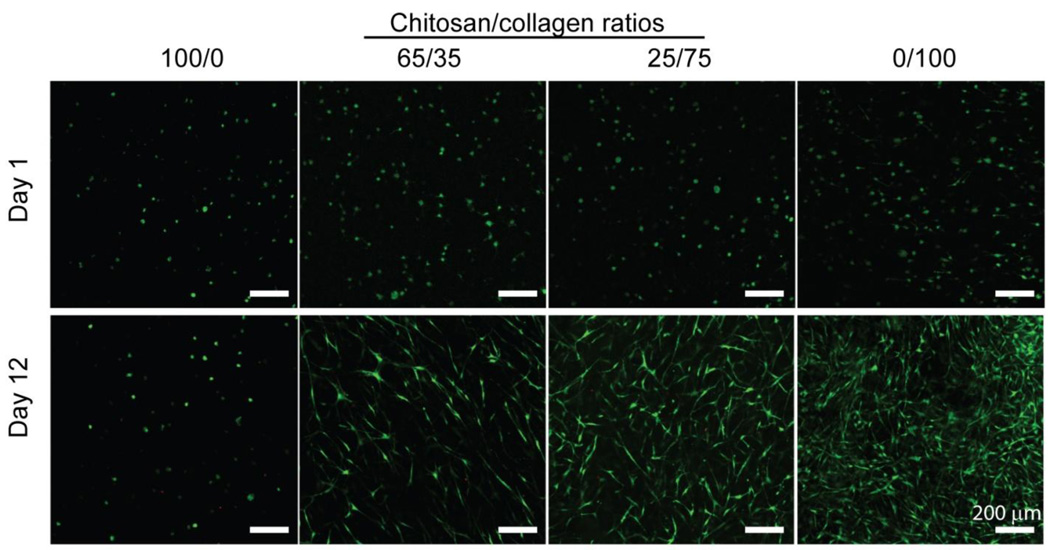
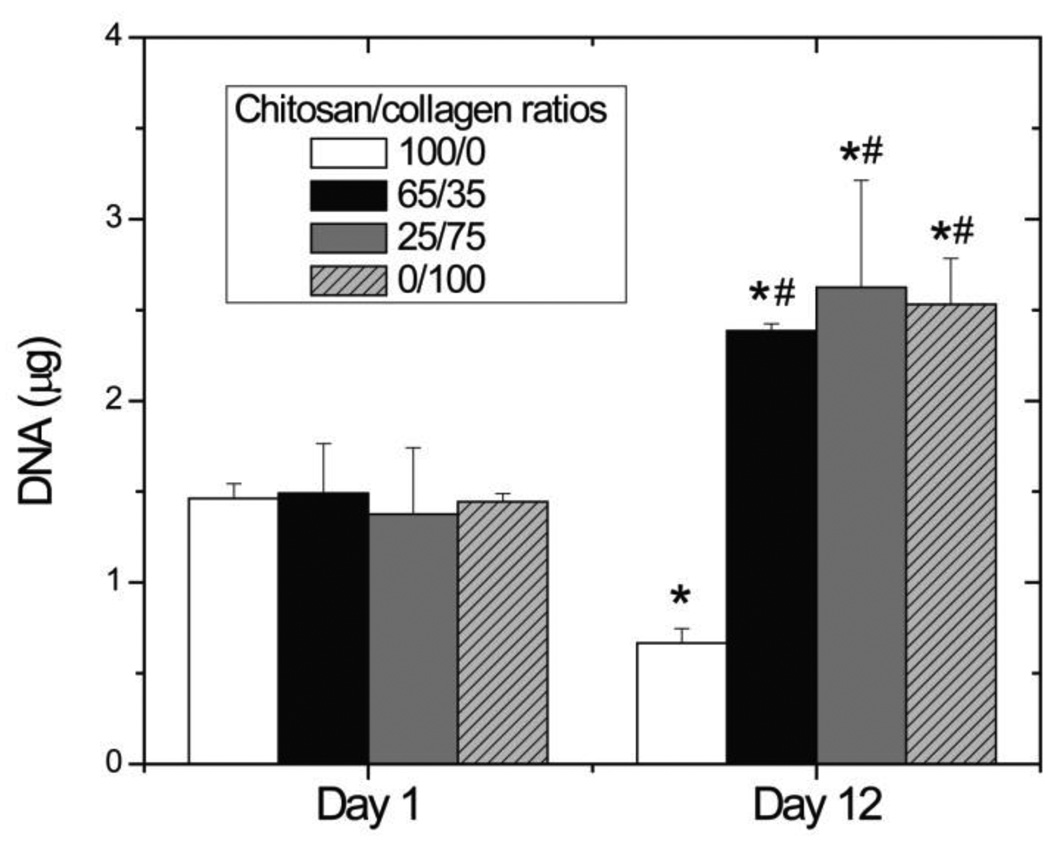
Vital staining of hBMSC embedded in chitosan and collagen materials, shown in Figure 5, revealed that >90% of cells survived the gel fabrication process and there was no observable difference in cell viability between materials at day 1 (Fig. 5, top panels). At day 12 in culture (Fig 5. bottom panels) it was evident that collagen-containing materials promoted spreading of embedded cells, resulting in a spindle-shaped morphology. In contrast, cells in pure chitosan gels maintained a rounded morphology with little evidence of interaction with the surrounding matrix. These images also suggested that cell proliferation was increased in collagen-containing gels, an observation that was confirmed by quantitatively assessing DNA content in gels, shown in Figure 6. At day 1, there was no difference in DNA content between materials. By day 12, DNA content in pure chitosan gels had decreased by approximately 50% (p < 0.05) while in collagen-containing materials it increased by approximately 70% (p < 0.05). There was no statistically significant difference in DNA contents between collagen-containing gels.
Figure 5.
Viability of hBMSC encapsulated in 3D hydrogels. hBMSC demonstrated increased cell spreading and proliferation in collagen-containing gels than in pure chitosan gels. (scale bar = 200 µm)
Figure 6.
DNA content at days 1 and 12. Collagen-containing gels had a higher DNA content than pure chitosan gels at day 12. (n = 4; * = statistically significant differences from day 1; # = statistically significant differences from pure chitosan gels)
3.5 Cytotoxicity of β-GP solution
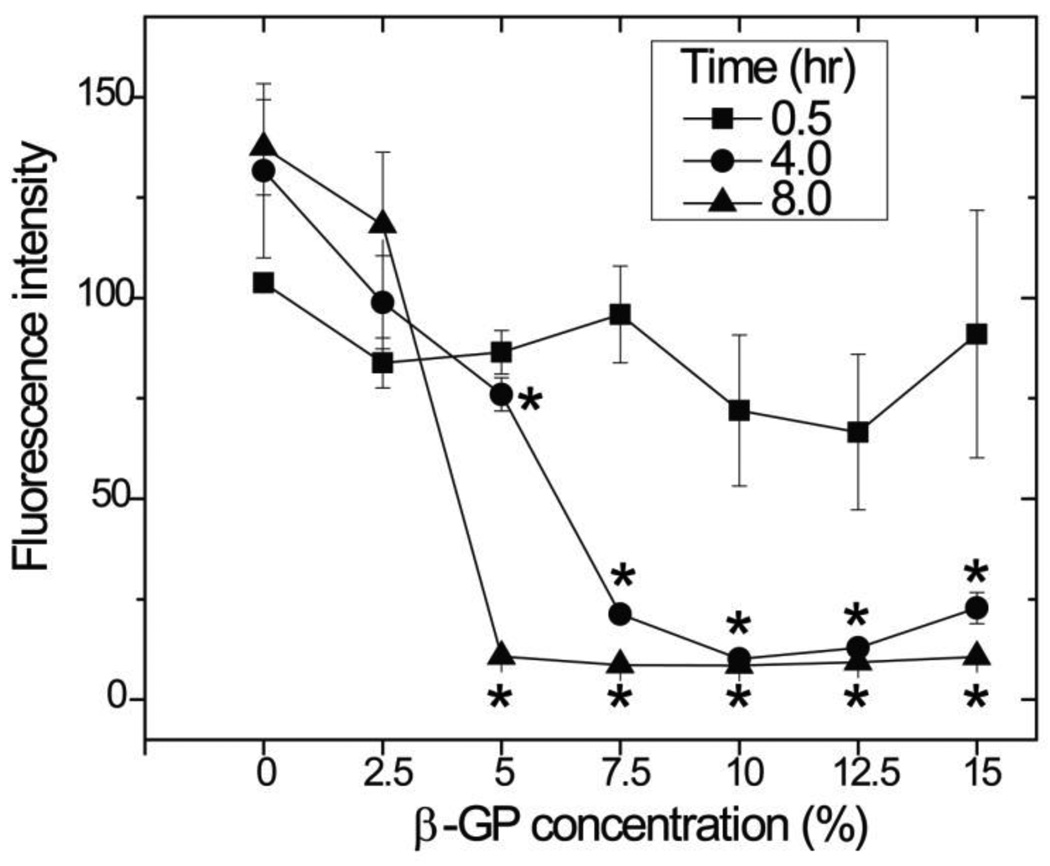
The metabolic activity of hBMSC grown in monolayer exposed to a range of β-GP concentrations over time is shown in Figure 7. A 0.5 h incubation with β-GP did not significantly affect the metabolic activity of cells, relative to controls (0% β-GP), regardless of β-GP concentration. When 4 and 8 h incubations were used, there was a clear and statistically significant (p < 0.05) drop in metabolic activity when β-GP concentration was above 2.5 wt%. The drop was particularly abrupt at the 8 h incubation, which essentially completely inhibited cellular activity. The lowest concentration of β-GP, 2.5 wt%, did significantly affect cell metabolic activity compared to the controls, regardless of incubation time.
Figure 7.
Cytotoxicity of β-GP solution. Exposure of hBMSC to β-GP solution for 30 min did not result in cytotoxic effects, while exposure for 4 or 8 h decreased cell metabolic activity. (n =3; * = statistically significant difference compared to control without β-GP)
3.6 Gel compaction
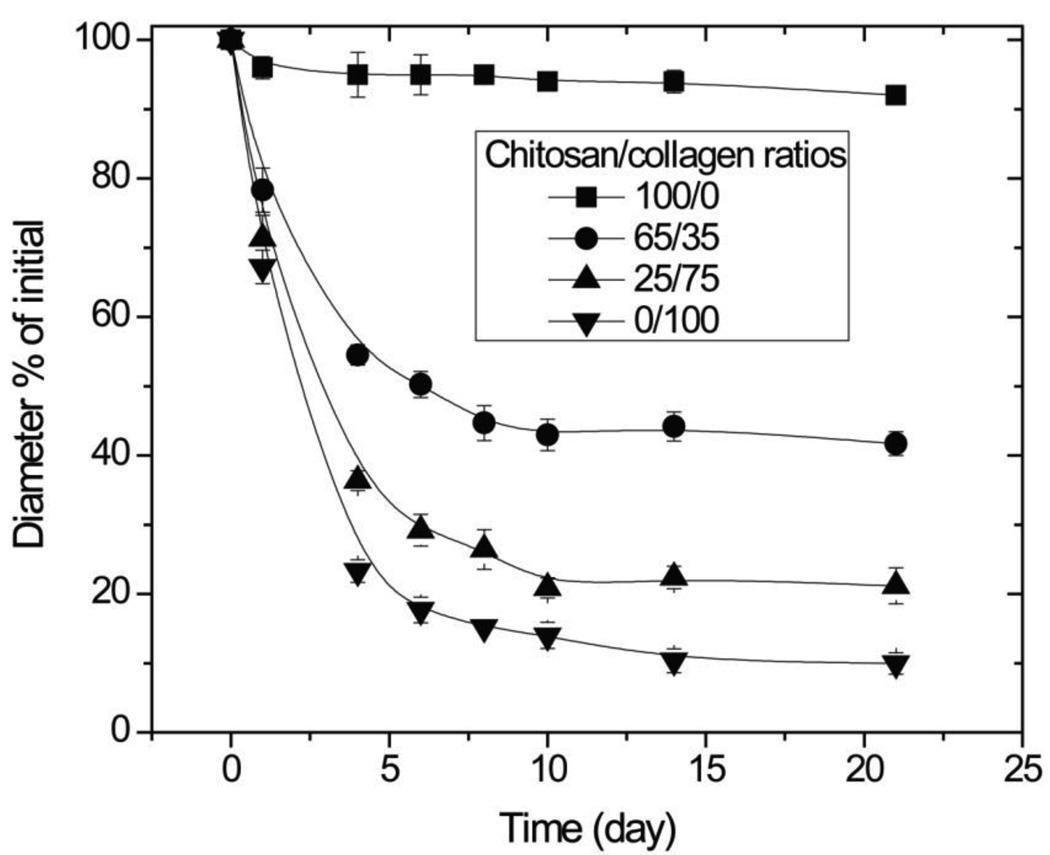
The change in construct diameter over time in culture is shown in Figure 8. Pure chitosan gels compacted only slightly over the 3-week culture period, to 92% of their original diameter. Collagen-containing gels compacted much more vigorously with time in an asymptotic manner. From days 0 to 4, gel compaction proceeded rapidly and materials with chitosan/collagen ratios of 65/35, 25/75, and 0/100 compacted to 55%, 36%, and 23% of their original diameters, respectively. From days 4 to 10, the compaction continued at a much lower rate, and after day 10 compaction essentially ceased and construct size reached a plateau. Among collagen-containing gels, the rate and degree of compaction increased with increasing collagen content. After 21 days of culture, pure collagen materials compacted to about 10% of their original diameter, while 25/75 and 65/35 chitosan/collagen constructs compacted to about 20% and 40% of their original diameters, respectively.
Figure 8.
Gel compaction. Higher amounts of collagen in composite materials resulted in increased gel compaction. (n =4)
3.7 Osteogenic differentiation
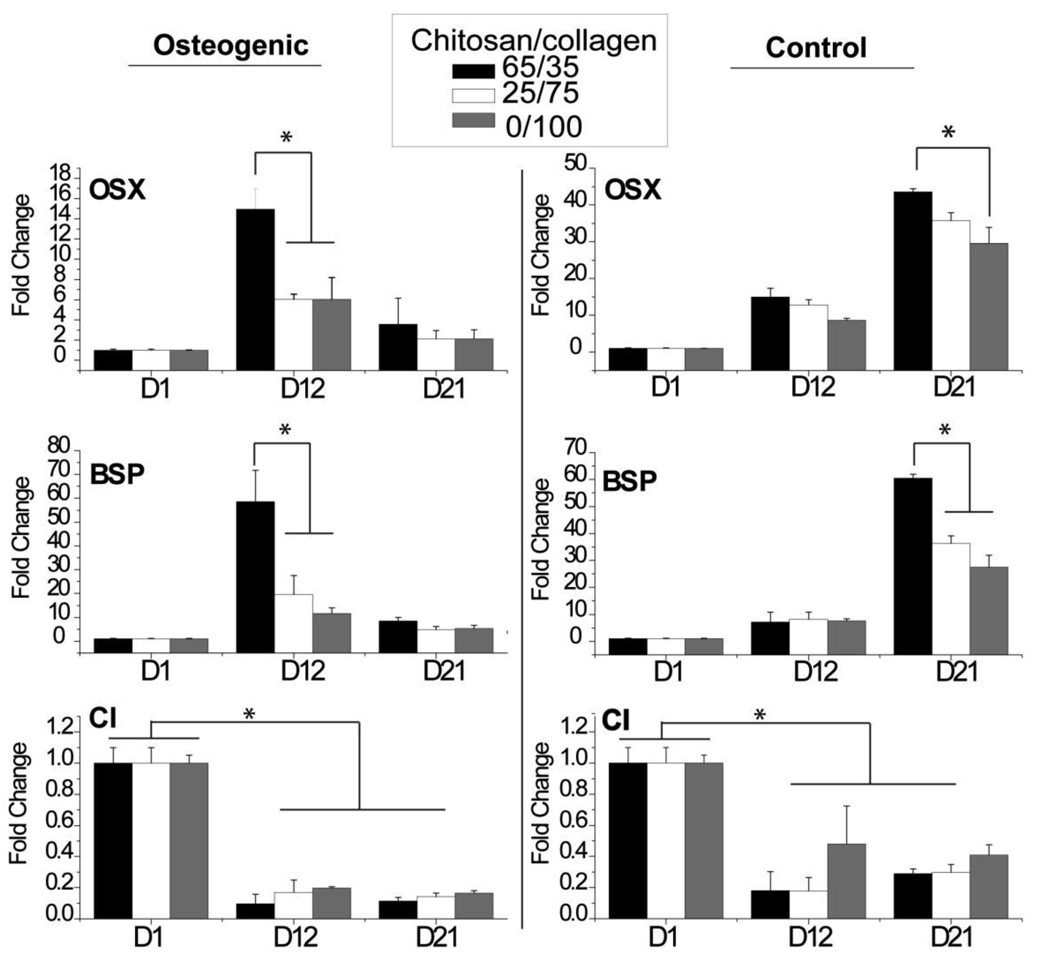
Because of their improved mechanical properties and ability to support cell spreading and proliferation, only collagen-containing materials were assessed for effects on osteogenic gene expression, as shown in Figure 9. Levels of osterix (OSX), bone sialoprotein (BSP) and collagen type I (CI) expression were compared in the presence and absence of osteogenic medium. Both OSX and BSP expression increased over time in both osteogenic and control medium. In osteogenic groups, OSX and BSP genes increased from day 0 to 12 and dropped from days 12 to 21. In control groups, OSX and BSP genes continued to increase during the culture period. At day 12 in osteogenic groups and at day 21 in control groups, materials containing 65/35 chitosan/collagen exhibited significantly higher OSX and BSP gene expression levels relative to materials with lower chitosan content (p < 0.05). CI gene expression in both osteogenic and control groups decreased from day 0 to 12 (p < 0.05), and then leveled off. Chitosan/collagen ratio had no effect on CI gene expression in either treatment group.
Figure 9.
Gene expression. Gels with 65/35 chitosan/collagen ratio exhibited higher osteogenic gene expression levels than other materials. (n = 4; * = statistically significant differences)
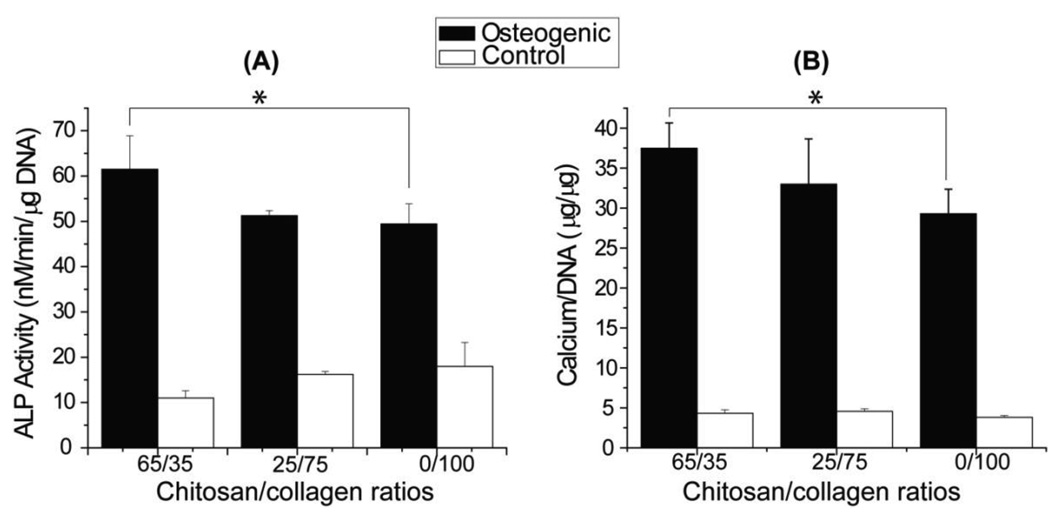
Figure 10 shows alkaline phosphatase activity (ALP) and calcium content for chitosan-collagen constructs cultured in osteogenic and control media. In all treatments, materials cultured in osteogenic medium exhibited higher ALP activity and calcium content than controls. In osteogenic medium, materials with 65/35 chitosan/collagen ratio resulted in higher ALP activity and calcium content than pure collagen materials (p < 0.05), while in control medium there was no significant difference between materials.
Figure 10.
ALP activities and calcium contents. Gels with 65/35 chitosan/collagen ratio exhibited higher osteogenic marker levels than pure collagen. (n = 4; * = statistically significant differences)
4. Discussion
In this study, pure chitosan, pure collagen, and chitosan-collagen composite materials were fabricated by thermal gelation and initiation using β-GP. Gel formation was accomplished at physiological pH and temperature, suggesting that such materials can be used for cell encapsulation and potentially as injectable in situ gel-forming scaffolds for tissue engineering. In contrast to chitosan-collagen sponges created using freeze-drying techniques, the composite materials created in this study contained self-assembled fibrillar collagen interspersed with the chitosan matrix. The porous structure of the materials provided space for embedded cells, which could form direct interactions with the matrix. Composites containing collagen were shown to promote cell survival and gel remodeling, while the chitosan component had an osteogenic effect.
β-GP was a critical component in gel formation. Previous studies have shown that chitosan hydrogels initiated by β-GP form through a physical gelation process, in which the functional groups of chitosan are not altered. However, the mechanism of gel formation is not clear. Several studies have suggested that gels form due to increased hydrophobic interactions between β-GP and chitosan caused by an increase in ionic strength at elevated temperature [16, 25–26]. However a recent study found that ionic strength decreased as temperature increased, and suggest that elevated temperatures induced proton transfer between the cationic amine groups in chitosan and the anionic phosphate groups in β-GP, result in gel formation [27]. In agreement with a previous study [28], X-ray microanalysis in our study showed that Na and P are removed from formed gels by washing, suggesting that β-GP was not ionically or covalently bound to amine groups in chitosan. In addition, the C:O ratio increased after washing with DI water, a finding consistent with removal of β-GP (formula: C3H7Na2O6P) since it has a higher proportion of oxygen atoms relative to carbon. Physical gelation of chitosan offers the potential to further chemically modify the polysaccharide, in particular through its abundant amine groups.
Addition of β-GP solution also was involved in initiating the self-assembly of collagen molecules to form protein fibers. We used acid-solublized collagen type I, which can be reconstitute by raising the temperature and pH [29]. Therefore the basic β-GP solution served to neutralize the collagen solution and in combination with elevated temperature this caused collagen fibrillogenesis. It is interesting that concentrations of β-GP greater than 12.5% induced almost instantaneous gelation of pure collagen matrices, followed by very rapid gel compaction. The mechanism of this phenomenon is unclear but it suggests that these materials are highly hydrophobic and unsuitable for tissue applications.
The cytocompatibility of is an important consideration for injectable biomaterials for cell encapsulation, since cell viability must be maintained during gel fabrication and upon implantation toxicity to surrounding tissue cells must also be low. Chitosan and collagen are naturally-derived materials that have been widely studied due to their ability to support cell growth and to integrate with surrounding ECM. Previous studies have demonstrated high cell viability in pure chitosan gels induced by β-GP when seeded with chondrocytes [17–19] and fibro-chondrocytes [30]. In the present study, cytocompatibility was enhanced by the addition of collagen to the composite materials, as demonstrated by increased cell proliferation.
It has been suggested that β-GP, and in particular concentrations above 10 wt%, can be detrimental to cell proliferation [21]. In order to examine this effect, we exposed hBMSC in monolayer culture to various concentrations of β-GP for differing lengths of time. Our results revealed that β-GP can inhibit cell metabolic activity, but that the effect was dependent on concentration and exposure time. β-GP concentrations lower than 2.5% did not result in significant changes in cell activity, but also were too low to initiate chitosan gelation. Exposure for 0.5 h also was not cytotoxic, suggesting that timely removal of excess β-GP after gel formation is sufficient to maintain cytocompatibility. However, our finding of cytotoxicity caused by β-GP has implications for use of injectable chitosan biomaterials, since it implies that removal or inactivation of excess β-GP is required for biocompatibility.
Adhesion to extracellular matrix proteins has effects on important cell functions, including cellular morphogenesis, proliferation, and differentiation. Type I collagen has multiple specific cell binding sites [31], as well as well characterized effects on cell function [6]. In contrast, chitosan does not provide such binding sites for cell attachment, since it is a polysaccharide derived from crustacean exoskeletons. In our study, hBMSC embedded in pure chitosan materials maintained their initial rounded morphology over 3 weeks in culture, and a decrease in DNA content over time suggested that cells were dying in these materials. The observed morphology was consistent with a previous study in which rat muscle-derived stem cells in β-GP-initiated chitosan gels remained rounded both in vitro and in vivo over a 4 week period [32]. The addition of type I collagen to chitosan gels dramatically increased cell spreading and hBMSC became spindle-shaped and proliferated more than in pure chitosan materials. In chitosan-collagen composites, higher collagen content did not lead to increased DNA content of gels suggesting that the cell number in these materials was similar. However, cell concentration did increase with increasing collagen concentration, since increased collagen resulted in greater gel compaction and therefore a decreased overall construct volume. Addition of collagen also resulted in a stiffer material, relative to pure chitosan.
Osteogenic differentiation of hBMSC was correlated to chitosan content in chitosan-collagen materials. It has been shown previously that rat progenitor cells deposit calcium when embedded in pure chitosan gels initiated by β-GP addition. [32], however such materials have not been investigated widely for use in bone tissue engineering. In the present study, pure chitosan gels initiated by β-GP were not conducive to maintaining cell viability, though it was observed that calcium was deposited over time (data not shown). However it is likely that the observed calcium was a result of calcium release as a result of cell necrosis, since we also observed decreasing DNA content over time in such gels. In chitosan-collagen composites, hBMSC proliferated and increasing chitosan content resulted in higher expression of osteogenic genes, increased ALP activity and higher calcium content. Complementary results have been observed in chitosan-collagen sponges created in the absence of β-GP [15]. Higher chitosan content resulted in increased osteogenesis, however, this effect was observed only in 3D gels. In 2D monolayer culture, chitosan did not promote osteogenic differentiation in vitro with or without osteogenic supplements and β-GP [33]. The role of chitosan in hBMSC differentiation has yet to be fully understood, however it is evident that this matrix material can be used alone and combination with ECM proteins to modulate progenitor cell differentiation.
5. Conclusions
Hydrogels containing varying ratios of chitosan and collagen have been fabricated by initiating gelation using β-GP and temperature. Importantly, this process was performed at physiological pH and temperature, such that living cells could be incorporated directly into the hydrogel matrix. The presence of collagen in chitosan-collagen materials was associated with increased cell spreading and proliferation, as well as increased gel compaction and a resulting stiffer matrix. Chitosan content was associated with improved osteogenic differentiation as assessed by gene expression and the presence of osteogenic markers. These chitosan-collagen composite hydrogel materials have potential applications in regenerative medicine, particularly in applications where injectable cell carriers are advantageous. Such materials can be used for cell encapsulation and delivery, or as in situ gel-forming materials for tissue repair.
Acknowledgements
This project was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases through grant R01-AR053231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed. 2008;19:1279–1293. doi: 10.1163/156856208786052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 7.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu AK, Putnam AJ. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:347–357. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 9.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res. 2001;55:304–312. doi: 10.1002/1097-4636(20010605)55:3<304::aid-jbm1018>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami T, Antoh M, Hasegawa H, Yamagishi T, Ito M, Eda S. Experimental study on osteoconductive properties of a chitosan-bonded hydroxyapatite self-hardening paste. Biomaterials. 1992;13:759–763. doi: 10.1016/0142-9612(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Wang X. A comparison of chitosan and collagen sponges as hemostatic dressings. J Bioact Compat Polym. 2006;21:39. [Google Scholar]
- 14.Peng L. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J Bioact Compat Polym. 2006;21:207–220. [Google Scholar]
- 15.Arpornmaeklong P, Pripatnanont P, Suwatwirote N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int J Oral Maxillofac Surg. 2008;37:357–366. doi: 10.1016/j.ijom.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Cho J, Heuzey MC, Begin A, Carreau PJ. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6:3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 17.Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671–2686. doi: 10.2106/JBJS.D.02536. [DOI] [PubMed] [Google Scholar]
- 18.Hoemann CD, Chenite A, Sun J, Hurtig M, Serreqi A, Lu Z, et al. Cytocompatible gel formation of chitosan-glycerol phosphate solutions supplemented with hydroxyl ethyl cellulose is due to the presence of glyoxal. J Biomed Mater Res A. 2007;83:521–529. doi: 10.1002/jbm.a.31365. [DOI] [PubMed] [Google Scholar]
- 19.Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78–89. doi: 10.1016/j.joca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316–327. doi: 10.1016/j.joca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadi R, de Bruijn JD. Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J Biomed Mater Res A. 86;2008:824–832. doi: 10.1002/jbm.a.31676. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Stegemann JP. Extraction of high quality RNA from polysaccharide matrices using cetlytrimethylammonium bromide. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.11.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Singh M, Bonewald LF, Detamore MS. Signalling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 25.Chenite A, Buschmann M, Wang D, Chaput C, Kandani N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr Polym. 2001;46:39–47. [Google Scholar]
- 26.Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 27.Lavertu M, Filion D, Buschmann MD. Heat-induced transfer of protons from chitosan to glycerol phosphate produces chitosan precipitation and gelation. Biomacromolecules. 2008;9:640–650. doi: 10.1021/bm700745d. [DOI] [PubMed] [Google Scholar]
- 28.Iliescu M, Hoemann CD, Shive MS, Chenite A, Buschmann MD. Ultrastructure of hybrid chitosan-glycerol phosphate blood clots by environmental scanning electron microscopy. Microsc Res Tech. 2008;71:236–247. doi: 10.1002/jemt.20545. [DOI] [PubMed] [Google Scholar]
- 29.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, et al. Multiple binding sites in collagen type I for the integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2000;275:38981–38989. doi: 10.1074/jbc.M007668200. [DOI] [PubMed] [Google Scholar]
- 32.Kim KS, Lee JH, Ahn HH, Lee JY, Khang G, Lee B, et al. The osteogenic differentiation of rat muscle-derived stem cells in vivo within in situ-forming chitosan scaffolds. Biomaterials. 2008;29:4420–4428. doi: 10.1016/j.biomaterials.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Guzman-Morales J, El-Gabalawy H, Pham MH, Tran-Khanh N, McKee MD, Wu W, et al. Effect of chitosan particles and dexamethasone on human bone marrow stromal cell osteogenesis and angiogenic factor secretion. Bone. 2009;45:617–626. doi: 10.1016/j.bone.2009.06.014. [DOI] [PubMed] [Google Scholar]