Abstract
We tested a model for the intergenerational transmission of depression integrating specific genetic (5-HTTLPR), cognitive (inferential style), and environmental (mother depressive symptoms and expressed-emotion criticism) risk factors. Supporting the hypothesis that maternal depression is associated with elevated levels of stress in children’s lives, mothers with a history of major depressive disorder (MDD) exhibited higher depressive symptoms across a 6-month multi-wave follow-up than mothers with no depression history. In addition, partially supporting our hypothesis, levels of maternal criticism during the follow-up were significantly related to mothers’ current depressive symptoms, but not history of MDD. Finally, we found support for an integrated gene × cognition × environment model of risk. Specifically, among children with negative inferential styles regarding their self-characteristics, there was a clear dose response of 5-HTTLPR genotype moderating the relation between maternal criticism and children’s depressive symptoms, with the highest depressive symptoms during the follow-up observed among children carrying two copies of the 5-HTTLPR lower expressing alleles (S or LG) who also exhibited negative inferential styles for self-characteristics and who experienced high levels of EE-Crit. In contrast, children with positive inferential styles exhibited low depressive symptoms regardless of 5-HTTLPR genotype or level of maternal criticism.
Keywords: cognitive vulnerability, attributional style, depression, 5-HTTLPR, expressed emotion
A family history of depression is one of the strongest risk factors for depression in youth and children of depressed mothers are 3–4 times more likely to experience major depressive disorder (MDD) by early adulthood than are individuals in the general population (for reviews, see Goodman, 2007; Goodman & Tully, 2008; Hammen, 2008; Weissman & Jensen, 2002). There is also evidence that children of depressed, compared to nondepressed, mothers have an earlier age of MDD onset, a more chronic course, and a greater risk of recurrence (for review, see Goodman, 2007; Hammen, 2008). Clearly, then, it is important to understand why children of depressed mothers are at such an increased risk for depression themselves.
Theorists (e.g., Goodman, 2007; Goodman & Gotlib, 1999) have suggested that this elevated risk is due to a combination of genetic, psychological, and environmental variables. In terms of environmental influences, there are a number of ways in which maternal depression is associated with increased stress in their children’s lives. For example, mothers’ depressive symptoms themselves serve as a stressor for children (Goodman & Tully, 2008; Hammen, 2009). Maternal depression is also associated with the presence of negative parenting behavior (for reviews, see Goodman, 2007; Goodman & Tully, 2008; Hammen, 2008). One potentially important form of behavior is expressed emotion (EE), which is a measure of family environment that is a robust predictor of relapse for a variety of disorders including depression (for a review, see Butzlaff & Hooley, 1998). Although initially used to study relapse rates among adults, researchers have begun to use measures of EE in studies of children and adolescents, including studies of interactions between depressed mothers and their children (e.g., Nelson, Hammen, Brennan, & Ullman, 2003; Schwartz, Dorer, Beardslee, Lavori, & Keller, 1990). These studies have suggested that one component of EE, criticism (EE-Crit), has the strongest validity among pediatric samples. Specifically, studies have found that mothers of depressed children exhibit more EE-Crit than do mothers of nondepressed children (e.g., Asarnow, Tompson, Hamilton, Goldstein, & Guthrie, 1994; Asarnow, Tompson, Woo, & Catwell, 2001; Hirshfeld, Biederman, Brody, Faraone, & Rosenbaum, 1997). Studies have also shown that depressed mothers exhibit more EE-Crit toward their children than do nondepressed mothers (Nelson et al., 2003; Schwartz et al., 1990). Even in the presence of these environmental stressors, however, not every child whose mother has a history of depression becomes depressed, suggesting the presence of other moderating variables.
Given the significant role played by negative life events in contributing risk for depression, theories of depression risk generally focus on factors hypothesized to increase reactivity to environmental stressors. According to cognitive theories of depression (e.g., Abramson, Metalsky, & Alloy, 1989; Clark, Beck, & Alford, 1999; Williams, Watts, MacLeod, & Mathews, 1997), individuals’ characteristic ways of attending to, interpreting, and remembering events in their lives may contribute vulnerability to the development of depression. For example, according to the hopelessness theory of depression (Abramson et al., 1989), individuals’ characteristic ways of interpreting the causes and implications of negative events may increase risk for the development of depression following the occurrence of negative life events. Cognitive vulnerability is defined in the hopelessness theory as the tendency to attribute negative events to stable, global causes and to infer negative consequences and negative self-characteristics following the events’ occurrence. In adults, these three negative inferential styles – causes, consequences, and self-characteristics – are highly correlated and reflect a single higher-order construct (Hankin, Lakdawalla, Carter, Abela, & Adams, 2007). Therefore, in adults, the three inferential styles are typically examined as a single composite vulnerability and research has provided strong support for the predictive validity of this composite for adults’ symptoms and diagnoses of depression (for reviews, see Gibb & Coles, 2005; Haeffel et al., 2008; Hankin & Abela, 2005). Among children, however, the three inferential styles are less highly correlated and do not appear to reflect a single, coherent vulnerability (for a review, see Abela & Hankin, 2008). Rather, researchers have suggested that a child’s level of cognitive vulnerability may be best defined as his or her most negative inferential style. According to this “weakest link” hypothesis (Abela & Sarin, 2002), children are as vulnerable to depression as their most negative inferential style makes them. A growing body of research has supported the hopelessness theory’s vulnerability-stress hypothesis and has suggested that children’s weakest link may be a stronger predictor of prospective changes in depressive symptoms following negative events than any of the three inferential styles considered individually (for a review, see Abela & Hankin, 2008). Supporting the hypothesis that mothers’ depressive symptoms may serve as a stressor within a cognitive vulnerability-stress framework, a recent study found that the relation between mother and child depressive symptoms over the course of a multi-wave follow-up was stronger among girls exhibiting negative inferential styles (weakest link) than among girls exhibiting positive inferential styles, though similar effects were not observed for boys (Abela, Skitch, Adams, & Hankin, 2006). A potential limitation of this study was that the authors only examined the role of children’s weakest link and did not examine the potential influence of the three inferential styles (causes, consequences, self-characteristics) individually. Therefore, it is unclear whether support for the vulnerability-stress hypothesis may also have been obtained for any of the other inferential styles.
An important question for cognitive models of depression is whether certain forms of cognitive vulnerability may be more likely to increase risk for depression following specific types of environmental stress. According to Beck’s (1987; Clark et al., 1999) event-congruency hypothesis, depressive reactions to environmental stress should be most likely when there is a match between the domain of cognitive vulnerability and the type of negative life event. Within the framework of the current study, therefore, we would predict that the children most likely to exhibit increases in depressive symptoms in the presence of maternal criticism (EE-Crit) would be those who exhibit negative inferential styles for self-characteristics since mothers’ criticism of their children should be more likely to “activate” cognitive vulnerability specifically for inferences regarding self-characteristics than inferences for causes or consequences.
In addition to cognitive vulnerabilities, theorists have also suggested that specific genetic risk factors may contribute to the intergenerational transmission of depression (e.g., Firk & Markus, 2007). Genetic effects could be observed in terms of gene-environment interactions (G × E), gene-environment correlations (rGE), or a combination of both (see Jaffee & Price, 2007; Rutter, Moffitt, & Caspi, 2006). For example, certain genetic risk factors may increase reactivity to environmental stressors (G × E), including stress associated with having a depressed mother. Children with a genetic risk may also be exposed to more environmental stress (e.g., maternal depression or EE-Crit), which could be either independent of (passive rGE), or dependent on (active or evocative rGE), the child’s influence. It is also possible that certain genotypes are associated with the presence of specific cognitive risk factors such as inferential styles.
To date, the strongest evidence for a specific genetic risk factor for depression has been obtained for a putatively functional polymorphism in the serotonin transporter gene (5-HTTLPR). There are two common variants in 5-HTTLPR, a short allele (S) and a long allele (L), with the short allele exhibiting less transcriptional efficiency than the long allele (Lesch et al., 1996). More recently, studies have suggested a triallelic variation (S, LG, LA; e.g., Hu et al., 2005), with the LG allele exhibiting functional equivalence with the S allele. There is increasing evidence that carriers of these lower expressing alleles (S or LG) are more likely to develop depression following negative events than are individuals with two copies of the long (or LA) allele, an effect that has been observed in both adults and children (e.g., for reviews, see Rutter et al., 2006; Uher & McGuffin, 2008). There is also evidence from one study that children carrying the 5-HTTLPR S or LG allele exhibit more negative inferential styles for causes than children homozygous for the long allele (Sheikh et al., 2008). In contrast, research examining potential gene-environment correlations has provided no evidence that 5-HTTLPR genotype is correlated with levels of negative life events (e.g., Caspi et al., 2003; Kaufman et al., 2004; Kilpatrick et al., 2007), nor is there evidence for a main effect of 5-HTTLPR on depression (for a review, see Anguelova, Benkelfat, & Turecki, 2003). Although the precise mechanisms by which 5-HTTLPR genotype may confer risk in the context of environmental stressors are not well-established, 5-HTTLPR short allele carriers have been shown to exhibit stronger amygdala reactivity to emotional stimuli (for a review, see Munafò, Brown, & Hariri, 2008) and greater cortisol reactivity to a laboratory stressor (Gotlib, Joormann, Minor, & Hallmayer, 2008). Therefore, the presence of the 5-HTTLPR short allele appears to be related to stronger neurobiological reactivity to environmental stimuli.
Both inferential styles and 5-HTTLPR genotype, therefore, are hypothesized to increase reactivity to environmental stress. As such, they may represent overlapping, independent, or interactive risk factors in the intergenerational transmission of depression. Consistent with an interactive model of risk, there is evidence from a recent twin study that the link between adolescents’ inferential styles and depressive symptoms may be moderated by both genetic and environmental factors (Lau, Rijskijk, & Ely, 2006). In addition, we recently found evidence for a gene × cognition × environment interaction such that the relation between mother and child depressive symptom levels during the course of a multi-wave prospective study was strongest among children carrying the 5-HTTLPR S or LG allele who also exhibited a specific form of cognitive vulnerability to depression – attentional biases for sad faces (Gibb, Benas, Grassia, & McGeary, in press). Supporting Beck’s (1987; Clark et al., 1999) vulnerability-event congruency hypothesis, this effect was specific to children’s attentional biases for sad, but not happy or angry, faces.
Our goal in the current study was to extend previous findings examining the intergenerational transmission of depression by testing an integrative vulnerability-stress model of risk. Specifically, we predicted that children of mothers with a history of MDD during their children’s lives would exhibit higher depressive symptom levels across a multiwave prospective follow-up than would children of mothers with no depression history. We predicted that this increased risk for depression would be predicted, in part, by environmental stressors associated with mothers’ depression history – elevations in mothers’ depressive symptoms across the follow-up and higher levels of maternal criticism toward their child (EE-Crit). Finally, we predicted gene × cognition × environment interactions such that the link between environmental stress (mother depressive symptoms or EE-Crit) and children’s depressive symptoms would be stronger among children who exhibited both genetic risk (one or two copies of the lower expressing 5-HTTLPR alleles) and cognitive risk (negative inferential style). As noted above, Beck (1987; Clark et al., 1999) asserted that depressive reactions to environmental events should be strongest when there is a match between the domain of vulnerability and the type of negative events. Therefore, although gene × cognition × environment interactions were generally expected to be strongest for children’s weakest link, we predicted that the effects for EE-Crit would be driven primarily by children’s inferential styles for their self-characteristics. Finally, given the likelihood that at least some of the children in our study, particularly those whose mothers had a history of MDD, would have a lifetime history of MDD themselves, we examined whether any results obtained would be maintained after accounting for the potential influence of children’s MDD history.
Method
Participants
Participants in this study were 100 mothers and their children drawn from the community. To qualify for inclusion in the “depressed” group (n = 52), mothers were required to meet criteria for MDD during the child’s lifetime according to the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV; American Psychiatric Association, 1994). To qualify for inclusion in the “nondepressed” group (n = 48), mothers were required to have no lifetime diagnosis of any DSM-IV mood disorder. Exclusion criteria for both groups included symptoms of schizophrenia, organic mental disorder, alcohol or substance abuse within the last six months, or history of bipolar I disorder. Children’s participation was limited such that no more than one child per mother could participate and all children were between the ages of 8–12. The average age of mothers in our sample was 38.56 years (SD = 6.66, Range = 26–53) and 88% were Caucasian. The median family income was $50,000–55,000 and, in terms of education level, 45% of the mothers had graduated from college. For the children in our sample, the average age was 9.97 years (SD = 1.32), 59% were girls, and 82% were Caucasian. Maternal history of MDD was not significantly related to children’s age, sex, or race (Caucasian vs. non-Caucasian).
Measures
The Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS-L; Endicott & Spitzer, 1978) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) were used to assess for lifetime histories of DSM-IV Axis I disorders in mothers and their children, respectively. Both measures are widely used diagnostic interviews with well-established psychometric properties (Angold, 1989; Endicott & Spitzer, 1978; Kaufman et al., 1997). The SADS-L and K-SADS-PL were administered by separate interviewers. For the K-SADS-PL, mothers and children were interviewed separately. As noted above, 52 mothers met criteria for MDD during their child’s life; 8 mothers met criteria for current MDD. Eleven children met lifetime criteria for MDD (10 children of mothers in the depressed group), with 4 meeting criteria for current MDD. Although not the focus of this study, current and lifetime diagnoses of anxiety disorders were also assessed as part of the SADS-L and K-SADS interviews. Of the mothers, 25 (16 from the depressed group) met lifetime criteria for a DSM-IV anxiety disorder (9 mothers met criteria for a current anxiety disorder) and 16 children (13 children of mothers from the depressed group) met lifetime criteria for an anxiety disorder (9 children met criteria for a current anxiety disorder).1 A subset of 20 SADS-L and 20 K-SADS-PL interviews from this project were coded by a second interviewer and kappa coefficients for diagnoses of MDD in mothers and in children were excellent (κ = 1.00). Interrater reliability for lifetime diagnoses of anxiety disorders in mothers and children was κ = .78 and κ = .76, respectively.
Mothers’ and children’s symptoms of depression were assessed at each assessment point using the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) and Children’s Depression Inventory (Kovacs, 1981), respectively. Numerous studies have supported the reliability and validity of both measures (Beck et al., 1996; Kovacs, 1981, 1985; Smucker, Craighead, Craighead, & Green, 1986). In the current study, both the BDI-II and the CDI exhibited good internal consistency (αs = .92-.93 and .77-.86, respectively, across all time points).
The Five Minute Speech Sample (FMSS; Magaña et al., 1986) was used to assess mothers’ levels of Expressed Emotion-Criticism (EE-Crit). In administering the FMSS, the mother is asked to speak uninterrupted for five minutes about her child. The response is audiotaped and coded for EE-crit. Mothers are rated as high on EE-Crit if their initial statement about the child is negative, or if they report a negative relationship or one or more criticisms as defined by the FMSS coding system. Mothers are rated as borderline-high on EE-Crit if they express dissatisfaction with the child not severe enough to be rated as a criticism. Responses to the FMSS were assigned values of 2, 1, and 0 to reflect high, borderline-high, and low EE-Crit, respectively. A number of studies have supported the reliability as well as the concurrent and predictive validity of the FMSS EE-Crit subscale (e.g., Asarnow, Goldstein, Tompson, & Guthrie, 1993; Asarnow et al., 2001; Magaña et al., 1986; McCarty, Lau, Valerie, & Weisz, 2004). In this study, the FMSS was administered and coded by individuals blind to mother diagnostic status. Coders were trained to reliability standards by the creator of the FMSS (Ana Magaña-Amato). All samples were coded by two raters. When discrepancies arose, a third rater was consulted and a consensus rating was reached. A subsample of 20 FMSSs was also coded by Ana Magaña-Amato and inter-rater agreement for the three-level EE-Crit ratings was 95%.
Children’s inferential styles were assessed using the Children’s Attributional Style Questionnaire (CASQ; Seligman et al., 1984) and the Children’s Cognitive Style Questionnaire (CCSQ; Abela, 2001). The CASQ was used to assess children’s inferential styles for the causes of negative events and the CCSQ was used to assess children’s inferential styles for consequences and self-characteristics. The CASQ is a 48-item forced choice questionnaire and, for each item, a hypothetical event is presented and the child must pick one of two attributional explanations for the event. In each pair of attributional explanations, one of the attributional dimensions varies (internality, stability, or globality), while the other two are held constant. A number of studies have supported the reliability and validity of the CASQ (e.g., Abela, 2001; Abela & Payne, 2003; Nolen-Hoeksema, Girgus, & Seligman, 1986, 1992; Seligman et al., 1984). Consistent with the hopelessness theory, we focused on the 16 items assessing stable and global attributions for negative events and we created a composite score by summing responses to these 16 items. Scores on this composite can range from 0–16, with higher scores indicating a more negative inferential style for the causes of events. In the current study, the internal consistency for the CASQ was .58, which is similar to that obtained in previous research (e.g., Abela, 2001; Abela & Payne, 2003). The CCSQ is a two-part questionnaire. Part 1 assesses children’s tendency to infer negative consequences following negative events (CCSQ-Consequences) and Part 2 assesses children’s tendency to infer negative self-characteristics following the occurrence of negative events (CCSQ-Self). Both parts contain 12 items presenting hypothetical negative events involving the child. As with the CASQ, participants are instructed to imagine that the event happened to them and then to choose the response that would best describe the way they would think. Scores on the CCSQ-Consequences can range from 0–36 and scores on the CCSQ-Self can range from 0–24, with higher scores on both scales indicating more negative inferential styles. Studies have supported the reliability and validity of the CCSQ (e.g., Abela, 2001; Abela & Payne, 2003). In the current study, the internal consistencies for the CCSQ-Consequences and CCSQ-Self were .75 and .76, respectively. In addition to examining each of the inferential styles separately, we also examined children’s “weakest link”, or most negative of the three inferential styles (Weakest). To determine each child’s weakest link in this study, scores on the CASQ, CCSQ-Consequences, and CCSQ-Self were standardized (so that all three variables had a possible range of 0–36) and the highest score of the three inferential dimensions was coded as each child’s weakest link.2 In this sample, the self-characteristics dimension was the weakest link for 58% of children, consequences was weakest for 30%, and causes was weakest for 11%. For one child, self-characteristics and consequences were equally negative.
Finally, children provided buccal cells by rubbing swabs along their cheeks and gums and then rinsing their mouth with 10ml of distilled water. DNA was collected and isolated using published procedures (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988). The 5-HTTLPR S alleles were assayed using previously reported methods (Pooley, Houston, Hawton, & Harrison, 2003) and the rs25531 SNP genotypes (LA vs. LG) were obtained using a combination of published methods. Specifically, the primers used for PCR were those reported in Hu et al., (2005) and the MspI restriction site protocol follows Wendland et al. (2006). Samples were analyzed on an ABI PRISM® 3130×l Sequencer. Consistent with previous research (e.g., Zalsman et al., 2006), three groups of participants were formed based on their genotyping: (a) S′S′: children with two copies of the lower expressing 5-HTTLPR alleles (SS, SLG, or LGLG; n = 25), (b) S′L′: children with one copy of a lower expressing 5-HTTLPR allele (SLA or LGLA; n = 52), and (c) L′L′: children homozygous for the higher expressing LA allele (LALA; n = 23).
Procedure
Potential participants were recruited from the community through a variety of means (e.g., newspaper and bus ads, flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Those reporting either significant depressive symptoms during the child’s life or no significant lifetime symptoms of depression were invited to participate in the study. Upon arrival at the laboratory, mothers were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the mother was administered the FMSS and K-SADS-PL interview by a research assistant. During this time, the child completed questionnaires, including the CASQ, CCSQ, and CDI, in a separate room. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL to the child. While children were being administered the K-SADS-PL, mothers completed a series of questionnaires including the BDI-II and were then administered the SADS-L by a separate interviewer. Participation in this initial assessment took approximately 3 hours, which included frequent breaks for children to minimize fatigue effects. Follow-up assessments occurred 2, 4, and 6 months after the initial assessment, during which mothers were administered the FMSS and BDI-II and children were administered the CDI over the phone. Families were compensated $100 for their participation.
Results
Preliminary Analyses
Of the 100 mother-child pairs, 90, 89, and 90 participated at the 2, 4, and 6-month follow-ups, respectively (10% attrition). Given the presence of missing data, we examined whether the data were missing at random, thereby justifying the use of data imputation methods for estimating missing values (cf. Shafer & Graham, 2002). As a first step in examining the pattern of missing data, a series of t tests was conducted to determine if families who completed all of the assessments differed from those with missing data on any Time 1 variables. None of these analyses was significant. In addition, Little’s missing completely at random (MCAR) test, for which the null hypothesis is that the data are MCAR (Little & Rubin, 1987) was nonsignificant, χ2(457) = 437.03, p = .74, providing further support for the imputation of missing values. Given these results, maximum likelihood estimates of missing data were created and used in all subsequent analyses (see Shafer & Graham, 2002).
Correlations and descriptive statistics for study variables are presented in Table 1. Of note, children’s 5-HTTLPR genotype was not significantly correlated with any of the other study variables (except T2 BDI-II scores). Preliminary analyses were also conducted to determine whether any of the study variables were significantly related to children’s age or sex. The only significant sex difference was for 5-HTTLPR genotype, χ2(1, N = 100) = 7.27, p = .03. Comparing the three genotype groups, this difference was due to girls being more likely than boys to carry the S´S´ genotype (78% were girls) than the L´L´ genotype (40% were girls), χ2(1, N = 48) = 7.22, p = .007, reffect size = .39. The proportion of girls with the S´L´ genotype did not differ significantly from those with S´S´, χ2(1, N = 77) = 2.61, p = .11, reffect size = .18, or L´L´, χ2(1, N = 75) = 2.45, p = .12, reffect size = .18. Finally, children’s age was negatively correlated with two of the measures of inferential style. Specifically, younger children reported more negative inferences regarding self-characteristics, r = −.31, p = .002, and more negative weakest links, r = −.29, p = .004.
Table 1.
Correlations and Descriptive Statistics for Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Mom MDD | - | ||||||||||||||||||
| 2. | Child MDD | .27** | - | |||||||||||||||||
| 3. | 5-HTTLPR | .03 | .04 | - | ||||||||||||||||
| 4. | CASQ | .20 | .10 | .00 | - | |||||||||||||||
| 5. | CCSQ-Cons | −.03 | .06 | .07 | .38** | - | ||||||||||||||
| 6. | CCSQ-Self | .00 | .09 | −.10 | .32** | .43** | - | |||||||||||||
| 7. | Weakest | −.02 | .11 | −.11 | .46** | .63** | .88** | - | ||||||||||||
| 8. | T1 EE-Crit | −.04 | .20* | −.06 | .11 | −.06 | −.08 | .01 | - | |||||||||||
| 9. | T2 EE-Crit | −.01 | −.09 | .07 | .16 | .02 | .12 | .18 | .45** | - | ||||||||||
| 10. | T3 EE-Crit | .10 | .22* | −.02 | .09 | −.12 | −.02 | −.05 | .42** | .31** | - | |||||||||
| 11. | T4 EE-Crit | −.01 | .09 | .12 | .08 | .11 | −.01 | .10 | .38** | .41** | .53** | - | ||||||||
| 12. | T1 BDI-II | .54** | .42** | .12 | .20* | .02 | .05 | .12 | .10 | .01 | .19 | .14 | - | |||||||
| 13. | T2 BDI-II | .42** | .42** | .22* | .15 | .03 | −.05 | .02 | .02 | .02 | .16 | .10 | .75** | - | ||||||
| 14. | T3 BDI-II | .42** | .59** | .11 | .22* | −.01 | .03 | .08 | .15 | .03 | .23* | .03 | .70** | .80** | - | |||||
| 15. | T4 BDI-II | .38** | .35** | .07 | .17 | .00 | −.02 | .08 | .15 | .07 | .19 | .07 | .67** | .78** | .82** | - | ||||
| 16. | T1 CDI | .19 | .42** | .02 | .57** | .40** | .48** | .55** | .19 | .14 | .18 | .16 | .22* | .22* | .27** | .25* | - | |||
| 17. | T2 CDI | .29** | .21* | .13 | .45** | .37** | .34** | .41** | .11 | .31** | .13 | .13 | .21* | .17 | .24* | .25* | .74** | - | ||
| 18. | T3 CDI | .26** | .22* | .14 | .41** | .36** | .25* | .32** | .12 | .22* | .20 | .02 | .17 | .27** | .31** | .29** | .59** | .70** | - | |
| 19. | T4 CDI | .16 | .09 | .05 | .33** | .36** | .38** | .44** | .23* | .24* | .19 | .08 | .09 | .15 | .22* | .15 | .54** | .59** | .75** | |
| % | 52 | 11 | 25 | - | - | - | - | 16 | 11 | 15 | 13 | - | - | - | - | - | - | - | - | |
| Mean | - | - | - | 3.78 | 12.67 | 9.60 | 16.21 | - | - | - | - | 7.70 | 7.63 | 6.79 | 6.73 | 6.71 | 4.39 | 3.25 | 3.06 | |
| SD | - | - | - | 2.33 | 4.68 | 4.21 | 5.26 | - | - | - | - | 8.51 | 8.25 | 7.88 | 7.95 | 6.19 | 4.34 | 3.51 | 3.42 | |
Note. MDD = lifetime major depressive disorder (coded 1 = yes, 0 = no). 5-HTTLPR = serotonin transporter genotype (coded 2 = S´S´, 1 = S´L´, 0 = L´L´). CASQ = Children’s Attributional Style Questionnaire. CCSQ = Children’s Cognitive Style Questionnaire. Weakest = Most negative inferential style. EE-Crit = Expressed Emotion-Criticism (coded 2 = high, 1 = borderline, 0 = low). BDI-II = Beck Depression Inventory-II. CDI = Children’s Depression Inventory. Percentages for MDD = % with lifetime MDD. Percentage for 5-HTTLPR = % with S´S´ genotype. Percentages for EE-Crit = % coded as high EE-Crit at each time point.
p < .05.
p < .01.
Trajectories in Depressive Symptoms and EE-Crit
Next, hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002; Raudenbush, Bryk, Cheong, & Congdon, 2004) was used to examine trajectories in children’s and mothers’ depressive symptoms and EE-Crit across the follow-up. Specifically, we tested the hypotheses that maternal history of MDD would be associated with elevated trajectories in children’s and mothers’ depressive symptoms across the follow-up and with higher levels of EE-Crit across the follow-up. Focusing first on children’s depressive symptoms, the Level 1 model for these analyses was:
where CDIij represents the CDI score on week i for participant j, π0j is the CDI intercept (CDI score at the initial assessment point), π1j is the slope of the linear relation between Time (in weeks) and CDI scores across the follow up for participant j, and eij represents the error term.
The Level 2 model was:
where β01 is the cross-level interaction term representing the effect of Mother MDD on the CDI intercept and β11 is the cross-level interaction term representing the effect of Mother MDD on the slope of the relation between Time and CDI scores. Finally, β00 and β10 represent the intercepts of their respective equations and r0j and r1j represent the error terms. In these analyses, Mother MDD was significantly related to the CDI intercept, t(98) = 2.52, p = .01, reffect size = .25. There was also a significant effect of Time, t(98) = −7.09, p < .001, reffect size = −.58, but Mother MDD did not moderate this effect, t(98) = −1.53, p = .13, reffect size = −.15. Therefore, children of mothers with a history of MDD during the children’s lives, compared to children of control mothers, exhibited higher depressive symptom levels across the follow-up although, as is typical in multi-wave studies with the CDI (for a review, see Twenge & Nolen-Hoeksema, 2002), scores for all children decreased over time. A similar HLM model was used to examine trajectories in mothers’ depressive symptoms across the follow-up. In this analysis, mother MDD was significantly related to the BDI-II intercept, t(98) = 6.57, p < .001, reffect size = .55. Although there was no significant linear change in depressive symptoms across time for the sample as a whole, t(98) = −1.80, p = .07, reffect size = .18, mother MDD status significantly moderated this effect, t(98) = −2.53, p = .01, reffect size = .25. Therefore, mothers with a history of MDD, compared to control mothers, exhibited higher depressive symptom levels at the initial assessment, though the magnitude of the group difference decreased over the course of the follow-up. This said, however, the mother MDD group difference in BDI-II scores remained significant at the 6-month follow-up, t(98) = 4.01, p < .001, reffect size = .38. Next, we examined trajectories in EE-Crit. Contrary to hypothesis, mother MDD was not significantly related to the EE-Crit intercept, t(98) = −0.21, p = .84, reffect size = −.02. There was also no significant linear change in levels of EE-Crit over time, t(98) = 1.30, p = .20, reffect size = .13, nor did mother MDD history significantly moderate variability around the Time slope, t(98) = 0.47, p = .64, reffect size = .05. Finally, we examined the relation between mothers’ depressive symptoms and levels of EE-Crit across the follow-up. In this analysis, levels of EE-Crit at each time point served as the dependent variable, mothers’ BDI-II score was the Level 1 predictor variable, and mother MDD was the Level 2 predictor. The slope of the relation between BDI-II and EE-Crit during the follow-up was significant, t(98) = 2.17, p = .03, reffect size = .21, and was not significantly moderated by mothers’ MDD history, t(98) = −1.48, p = .14, reffect size = −.15. Therefore, in the current sample, levels of EE-Crit during the follow-up were related to mothers’ levels of depressive symptoms at each assessment point, but not their history of MDD during their children’s lives.
Vulnerability-Stress Analyses
Next, we tested the vulnerability-stress (gene × cognition × environment) hypotheses. The generic Level 1 model for these HLM analyses was:
where CDIij represents the CDI score on week i for participant j, π0j is the CDI intercept, π1j is the slope of the linear Time effect, π2j is the slope of the relation between Stress (BDI-II or EE-Crit) and CDI scores at a given time point for participant j, and eij represents the error term.
The Level 2 was:
where the primary relations of interest are β22, β23, and β24, which represent the main effects of 5-HTTLPR genotype, Inferential Style, and their interaction on the relation between Stress and CDI scores. Mother MDD was included as a covariate in these analyses since participants were chosen based on mothers’ MDD history. Separate HLM models were run for each of the two forms of Stress (EE-Crit and BDI-II) and for the four inferential styles (causes, consequences, self-characteristics, and weakest link).
First, we examined the role of maternal criticism (EE-Crit). Before testing the vulnerability-stress models, however, we examined the relation between EE-Crit and CDI scores over the follow-up with mother MDD history as the only Level 2 predictor. In this HLM model, we found that, statistically controlling for the linear effects of time, levels of EE-Crit were significantly related to CDI scores across the follow-up, t(98) = 3.23, p = .002, reffect size = .31, and this effect was not significantly moderated by mother MDD history, t(98) = −0.38, p = .71, reffect size = −.01. We then tested the gene × cognition × environment hypothesis. The 5-HTTLPR × Inferential Style × EE-Crit interaction was significant for CCSQ-Self, t(95) = 2.80, p = .007, reffect size = .28, and Weakest Link, t(95) = 2.06, p = .04, reffect size = .21, but not for CASQ, t(95) = 1.47, p = .15, reffect size = .15, or CCSQ-Consequences, t(95) = 0.54, p = .59, reffect size = .06. We should note that, in these analyses, the Inferential Style × EE-Crit interactions were also not significant for CASQ or CCSQ-Cons. Importantly, the 5-HTTLPR × CCSQ-Self × EE-Crit interaction remained significant even after excluding children who met criteria for current MDD at the initial assessment (n = 4), t(91) = 2.57, p = .01, reffect size = .26, and after statistically controlling for the influence of children’s lifetime history of MDD, t(94) = 2.73, p = .008, reffect size = .27, suggesting that the results were not due simply to the influence of children’s current or past diagnoses of MDD. In contrast, the 5-HTTLPR × Weakest × EE-Crit interaction was reduced to nonsignificant when we excluded children who met criteria for current MDD at the initial assessment, t(91) = 1.93, p = .06, reffect size = .20, and when we statistically controlled for the influence of children’s lifetime history of MDD, t(94) = 1.83, p = .07, reffect size = .19.
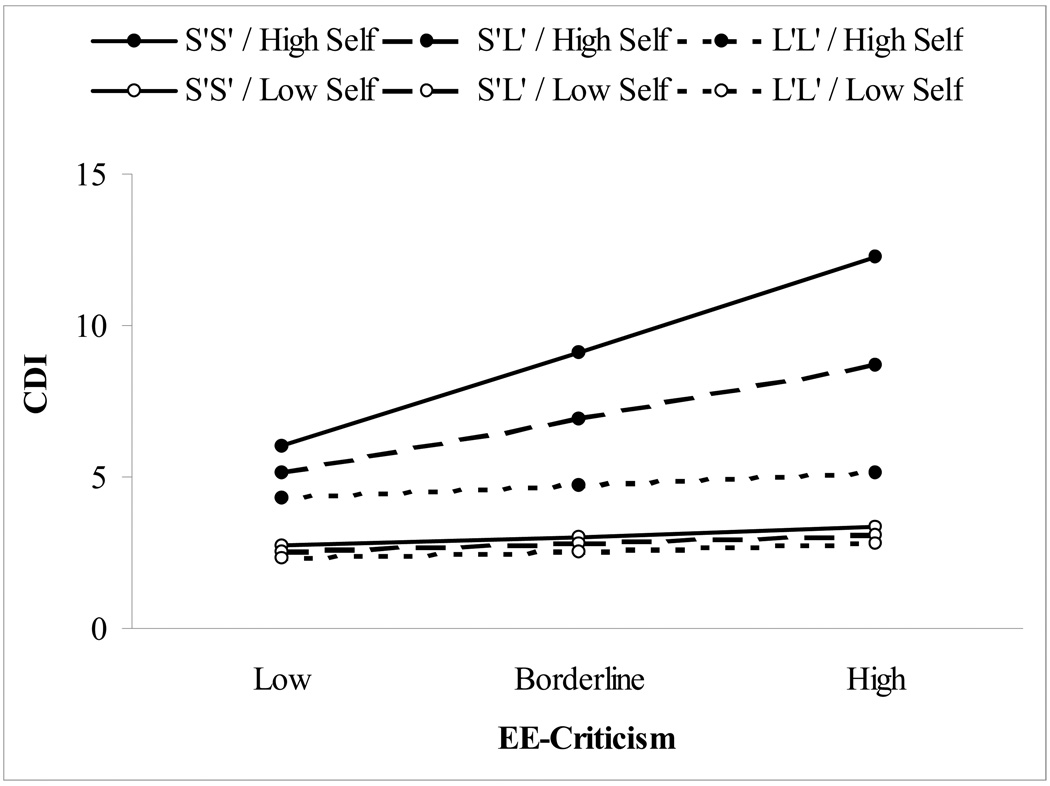
To determine the form of the significant 5-HTTLPR × CCSQ-Self × EE-Crit interaction, we examined the CCSQ-Self × EE-Crit two-way interactions separately among the three 5-HTTLPR genotype groups (S′S′, S′L′, and L′L′). The CCSQ-Self × EE-Crit interaction was significant among carriers of the 5-HTTLPR S′S′ genotype, t(22) = 2.41, p = .03, reffect size = .46, and among carriers of the S′L′ genotype, t(49) = 2.22, p = .03, reffect size = .30, but not among carriers of the L′L′ genotype, t(20) = −1.36, p = .19, reffect size = −.29. The form of this interaction is depicted in Figure 1, created by solving the HLM equation for values 1 standard deviation above and below the mean for CCSQ-Self (cf. Aiken & West, 1991). As can be seen in the figure, among children with negative inferential styles for self-characteristics (relatively high CCSQ-Self scores), there was a clear dose response of 5-HTTLPR genotype moderating the relation between EE-Crit and CDI scores, with the highest CDI scores during the follow-up observed among children carrying the 5-HTTLPR S′S′ genotype who also exhibited negative inferential styles for self-characteristics and who experienced high levels of EE-Crit. In contrast, children with positive inferential styles exhibited low CDI scores regardless of 5-HTTLPR genotype or level of EE-Crit.
Figure 1.
Form of 5-HTTLPR × CCSQ-Self × EE-Crit interaction for the three 5-HTTLPR genotype groups (S′S′, S′L′, L′L′) as a function of negative inferential style for self-characteristics (High versus Low) and levels of expressed emotion-criticism (Low, Borderline, High). CDI = Children’s Depression Inventory.
Next, we examined the role of maternal depressive symptoms (BDI-II scores) within the vulnerability-stress models. Paralleling our previous analyses, we first examined the relation between BDI-II and CDI scores over the follow-up with mother MDD history as the only Level 2 predictor. In this HLM model, we found that, statistically controlling for the linear effect of time, BDI-II and CDI scores were not significantly related during the course of the follow-up, t(97) = 0.44, p = .66, reffect size = .05, nor was this relation significantly moderated by maternal MDD history, t(97) = −0.72, p = .47, reffect size = −.07. We then tested whether inferential styles, alone or interacting with 5-HTTLPR genotype, would moderate the relation between mothers and children’s depressive symptoms across the follow-up. None of the main effects or interactions was significant in these vulnerability-stress analyses (lowest p = .24).3
Finally, exploratory analyses were conducted to determine whether any of the vulnerability-stress relations was moderated by children’s age or sex or mothers’ histories of MDD. None of these analyses was significant.
Discussion
The goal of this study was to examine specific cognitive, genetic, and environmental factors that may help to explain why children of depressed mothers are at increased risk for depression themselves. We obtained partial support for our proposed integrated model for the intergenerational transmission of depression. Specifically, as predicted, mothers with a history of MDD during their children’s lives, compared to control mothers, exhibited higher levels of depressive symptoms across the follow-up. However, mothers’ levels of criticism toward their children (EE-Crit) were significantly related to mothers’ current depressive symptoms, but not their MDD history. Therefore, maternal criticism appeared to be a correlate of current depression rather than a stable characteristic of mothers with a history of MDD, though mothers with a history of MDD were more likely to also exhibit current depressive symptom elevations. Consistent with our hypothesis, we also found support for a gene × cognition × environment model of risk. Specifically, we found that children with negative inferential styles for self-characteristics were more likely to exhibit depressive reactions to experiences of maternal criticism than were children with positive inferential styles, but only if they carried one or two copies of the lower expressing 5-HTTLPR alleles (S or LG). Among children homozygous for the LA allele, there was no evidence of a cognitive vulnerability-stress interaction. Indeed, we found evidence of a dose-response effect in which the magnitude of the cognitive vulnerability-stress relation varied as a function of the number of lower expressing 5-HTTLPR alleles the children exhibited (0 vs. 1 vs. 2). We also found support for Beck’s (1987; Clark et al., 1999) cognitive vulnerability-event congruency hypothesis in that the vulnerability-stress interaction was significant only when there was a match between the type of cognitive vulnerability (inferential style for self-characteristics) and the type of environmental stressor (maternal criticism). Finally, supporting the robustness of these findings, they were maintained even after accounting for the potential influence of children’s current or lifetime diagnoses of MDD.
We should also note that children’s 5-HTTLPR genotype was not significantly related to any of the measures of inferential style in this study. This is in contrast to findings from a recent study suggesting that children carrying the 5-HTTLPR S or LG alleles (n = 31) exhibited more negative inferential styles for the causes of negative events than children homozygous for the LA allele (n = 7) (Sheikh et al., 2008). The reason for this discrepancy is unclear and may have been due to the nature of our sample (children were chosen for inclusion based on their mothers’ MDD history). However, it also seems unlikely that a single gene would code for anything as complex as inferential styles just as it is unlikely a single gene would code for something as complex as depression. Rather, various candidate polymorphism including 5-HTTLPR appear to be associated with increased reactivity to salient environmental stressors (see Bradley et al., 2008; Kaufman et al., 2004; Kaufman et al., 2006; Kim et al., 2007; Rutter et al., 2006; Uher & McGuffin, 2008). Therefore, research focused on the presence/development of cognitive vulnerability to depression may benefit from an increased focus on genetic factors that heighten risk for the development of a cognitive vulnerability in the context of certain negative life events (cf. Gibb et al., in press; Lau et al., 2006).
The current results may help to further refine and extend cognitive models of depression in youth in several ways. First, they suggest that the integration of additional known risk factors for depression (i.e., 5-HTTLPR genotype) into cognitive models may increase the predictive validity of cognitive vulnerabilities. Specifically, as noted above, the cognitive vulnerability-stress interaction was significant among children with one or two copies of the 5-HTTLPR S or LG allele, but not among those homozygous for the LA allele. Second, the current findings support the utility of examining the different inferential styles separately in children as well as different types of environmental stressors. The current lack of significant vulnerability-stress findings in the analyses focused on mothers’ depressive symptoms as the stressor and the analyses focused on inferences for causes or consequences interacting with maternal criticism may have been due, in part, to our relatively small sample size. This said, however, the current results support Beck’s (1987; Clark et al., 1999) hypothesis that the strongest cognitive vulnerability-stress effects would be observed when there is a specific match between domain of cognitive vulnerability and type of stressor. Thus, despite the relatively small sample size, support for the vulnerability-stress hypothesis was observed for children’s negative inferential styles for self-characteristics when combined with the occurrence of maternal criticism of the child. Importantly, this cognitive vulnerability-event congruency model of risk appears to be particularly likely among children carrying a genetic risk factor (5-HTTLPR S or LG allele) associated with heightened reactivity to environmental stress (cf. Gotlib et al., 2008; Munafò et al., 2008). Conclusions regarding the cognitive vulnerability-event congruency hypothesis are strengthened given our previous results indicating that children’s attentional biases for sad, but not happy or angry, faces moderated the strength of the relation between mothers’ depressive symptoms and children’s depressive symptoms over time among children carrying one or two copies of the 5-HTTLPR S or LG allele, but not among those homozygous for the LA allele (Gibb et al., in press).
We should note that a significant 5-HTTLPR × inferential style × EE-Crit interaction was also observed for children’s weakest link. However, this relation was reduced to nonsignificant once we accounted for the influence of children’s current or lifetime diagnoses of MDD. These findings stand in contrast to those from previous studies, which have found that children’s weakest link exhibited stronger predictive validity than any of the other inferential styles (for a review, see Abela & Hankin, 2008). We believe that this discrepancy can be explained in terms of the cognitive vulnerability-event congruency hypothesis. Specifically, previous studies testing the weakest link hypothesis have typically focused on a broad range of negative life events experienced by children (see Abela & Hankin, 2008). When focused on negative events generally, then, children’s weakest link may provide the best measure of cognitive vulnerability. However, when focused on a specific type of stressor likely to directly “activate” a certain form of cognitive vulnerability (e.g., experiences of criticism), stronger results should be obtained for the domain of cognitive vulnerability specific to the type of stress (e.g., negative inferential styles regarding self-characteristics). The reason that analyses with children’s weakest link received some support in the current study may also have been due to the high proportion of children for whom inferences regarding self-characteristics was their weakest link (58%).
As noted above, our hypothesis regarding pathways for the intergenerational transmission of depression was only partially supported. Specifically, we predicted that maternal history of MDD would be associated with higher levels of ongoing environmental stress in the children’s lives, specifically higher levels of current depressive symptoms and EE-Crit. Although mothers with a history of MDD during their children’s lives, compared to mothers with no history of depression, did exhibit higher depressive symptom levels across the follow-up, levels of EE-Crit were related to mothers’ current depressive symptoms but not their history of MDD. These findings contrast with those of previous studies, which have supported the relation between maternal criticism and mothers’ depressive symptom levels as well as their history of MDD (e.g., Goodman, Adamson, Riniti, & Cole, 1994; Nelson et al., 2003; Rogosch, Cicchetti, & Toth, 2004; Schwartz et al., 1990; Webster-Stratton & Hammond, 1988). Although the exact reason for the pattern of findings in the current study is unclear, it is possible that some third variable may have moderated the link between mother MDD history and current levels of expressed emotion criticism. For example, there is increasing evidence that successfully treating mothers’ depression reduces risk for depression in children (for a review, see Gunlicks & Weissman, 2008) and some treatment efforts may focus specifically on improving mother-child interactions. It is possible, therefore, that mothers who received specific treatments for their depression became less likely to criticize their child in the future, even with recurrences of depressive symptoms. Research has also suggested the presence of different depression presentations in mothers, with some mothers exhibiting a more critical presentation and other presenting as more withdrawn (see Lyons-Ruth, Lyubchik, Wolfe, & Bronfman, 2002). In this case, only the former would be expected to express high levels of criticism toward their child. Future studies are needed to determine whether treatment utilization or specific differences in depression presentation may moderate risk for expressed emotion-criticism among mothers with a history of MDD.
Strengths of this study include the focus on integrating known risk factors for child depression that are typically examined independently (genetic, cognitive, and environmental risk factors), our use of interviewer-coded levels of maternal criticism, and our prospective multi-wave design. There were, however, limitations as well, which should be acknowledged. First, the assessment of children’s and mothers’ depressive symptoms during the follow-up was based solely upon their self-reports and future studies should include interviewer-administered, as well as questionnaire-based, measures of depressive symptoms. Second, future research is needed to determine whether the supported gene × cognition × environment model of risk will generalize to predicting the onset of depressive diagnoses in children. Third, our sample size was relatively small, limiting our statistical power, particularly for the analyses examining the potential moderating role of children’s age or sex or mother MDD history. Our sample size was large enough, however, to observe the hypothesized 5-HTTLPR × inferential style for self-characteristics × maternal criticism interaction, a finding that was maintained even after controlling for the influence of mothers’ and children’s MDD history. This said, future research would benefit from larger samples that would allow a more statistically powerful exploration of potential moderators of these relations (cf. Abela et al., 2006).
Fourth, although there is some evidence for the specificity of inferential styles (e.g., Brozina & Abela, 2006; Hankin & Abramson, 2002; Hankin, Abramson, Miller, & Haeffel, 2004; Robinson, Garber, & Hillsman, 1995) and, to a lesser extent EE-Crit (e.g., Asarnow et al., 1994; Asarnow et al., 2001; but see also Hirshfeld et al., 1997), to depression versus other forms of psychopathology, maternal depression is a nonspecific risk factor for various forms of psychopathology in children (for reviews, see Goodman, 2007; Hammen, 2008). In addition, there is evidence that 5-HTTLPR genotype may be associated with heightened reactivity to emotional stimuli generally, rather than depression specifically (for a review, see Hariri & Holmes, 2006). Therefore, it will be important to determine in future research whether the supported gene × cognition × environment model of risk is specific to depression versus other forms of psychopathology in children (e.g., anxiety).
Fifth, there is always the possibility in any genetic association study of an unmeasured genetic or non-genetic third variable accounting for the associations reported (e.g., population stratification or linkage disequilibrium between measured variant and actual functional variant). Future studies, therefore, would benefit from the inclusion of a genomic control. A final limitation is that we focused exclusively on one genetic risk factor – 5-HTTLPR – and it is unlikely that the observed genotype effects are unique to 5-HTTLPR. Indeed, previous studies have identified other candidate polymorphisms associated with reactivity to environmental stress including the brain derived neurotrophic factor (BDNF) gene (Kaufman et al., 2006; Kim et al., 2007) and the corticotrophin-releasing hormone type 1 receptor (CRHR1) gene (Bradley et al., 2008). Future research is needed to determine whether these or other genetic risk factors may also interact with cognitive vulnerabilities (in combination with environmental stress) to increase risk for depression.
In summary, the current results provide initial support for a model of the intergenerational transmission of depression risk that integrates cognitive, genetic, and environmental risk factors. A number of previous studies have supported the roles of these risk factors individually (for reviews, see Abela & Hankin, 2008; Goodman & Tully, 2008; Uher & McGuffin, 2008), and theorists have proposed integrative models of risk (e.g., Goodman, 2007; Goodman & Gotlib, 1999). The current findings, however, are among the first of which we are aware to test and support a model of depression risk in children combining specific cognitive, genetic, and environmental risk factors (see also Gibb et al., in press). Specifically, they suggest that mothers experiencing current elevations in depressive symptoms may be more likely to exhibit criticism toward their children and that these current depressive symptom elevations are more likely among mothers with a history of MDD during their children’s lives. They also suggest that a specific subgroup of children – those with negative inferential styles regarding self-characteristics who also carry one or two copies of the 5-HTTLPR S or LG allele – may be particularly likely to exhibit depressive symptom elevations in the presence of maternal criticism. If replicated, these findings could help to better delineate which children are at greatest risk for depression so that targeted interventions can be offered to those in greatest need.
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD048664 and by funding from the Center for Developmental Psychobiology, Binghamton University, awarded to B.E. Gibb as well as 1S10RR023457-01A1 and Shared equipment grant (ShEEP) and a Research Career Development award from the Medical Research Service of the Department of Veteran Affairs, awarded to J. McGeary. We would like to thank Sarah Crossett for her help in conducting assessments for this project as well as Meredith E. Coles for her comments on an earlier draft of this article. We would also like to thank Andrew Smolen for his assistance with genotyping
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
All significant effects were maintained even after statistically controlling for the influence of mother and child lifetime anxiety diagnoses and when excluding mother-child pairs with a current anxiety disorder.
We should note that this approach to computing weakest link scores is different than that employed by Abela and Sarin (2002). Specifically, Abela and Sarin first converted children’s scores on the CASQ, CCSQ-Cons, and CCSQ-Self into z-scores and determined children’s weakest link as the highest of the three z-scores. Using this approach, however, children’s weakest link is influenced by the scores of the other children who happen to be in the sample. By instead converting scores on the CASQ, CCSQ-Cons, and CCSQ-Self into a common metric, we were able to take a more ideographic approach to calculating each child’s weakest link. This said, we also conducted all analyses using Abela and Sarin’s method. Results using this approach were somewhat weaker than those obtained using the ideographic approach.
All analyses were also conducted using classifications based on biallelic variation in 5-HTTLPR (SS, SL, LL). None of these analyses was significant (lowest p = .22). These findings support the utility of focusing on the more recently recognized triallelic variation in 5-HTTLPR and suggest that combining LG and LA carriers into a broader L group may result in reduced statistical power, which is consistent with evidence that the LG allele exhibits functional equivalence to the S allele (see Hu et al., 2005).
Contributor Information
Brandon E. Gibb, Binghamton University (SUNY)
Dorothy J. Uhrlass, Binghamton University (SUNY)
Marie Grassia, Binghamton University (SUNY).
Jessica S. Benas, Binghamton University (SUNY)
John McGeary, Providence Veterans Affairs Medical Center and Center for Alcohol and Addiction Studies, Brown University.
References
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York: Guilford; 2008. pp. 35–78. [Google Scholar]
- Abela JRZ, Payne AVL. A test of the integration of the hopelessness and self-esteem theories of depression in schoolchildren. Cognitive Therapy and Research. 2003;27:519–535. [Google Scholar]
- Abela JRZ, Sarin S. Cognitive vulnerability to hopelessness depression: A chain is only as strong as its weakest link. Cognitive Therapy and Research. 2002;26:811–829. [Google Scholar]
- Abela JRZ, Skitch SA, Adams P, Hankin BL. The timing of parent and child depression: A hopelessness theory perspective. Journal of Clinical Child and Adolescent Psychology. 2006;35:253–263. doi: 10.1207/s15374424jccp3502_9. [DOI] [PubMed] [Google Scholar]
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Angold A. Structured assessments of psychopathology in children and adolescents. In: Thompsen C, editor. The instruments of psychiatric research. Chichester: Wiley; 1989. pp. 271–394. [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Molecular Psychiatry. 2003;8:574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Goldstein MJ, Tompson M, Guthrie D. One-year outcomes of depressive disorders in child psychiatric in-patients: Evaluation of the prognostic power of a brief measure of expressed emotion. Journal of Child Psychology and Psychiatry. 1993;34:129–137. doi: 10.1111/j.1469-7610.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Tompson M, Hamilton EB, Goldstein MJ, Guthrie D. Family expressed-emotion, childhood-onset depression, and childhood-onset schizophrenia spectrum disorders: Is expressed emotion a nonspecific correlate of child psychopathology or a specific risk factor for depression? Journal of Abnormal Child Psychology. 1994;22:129–137. doi: 10.1007/BF02167896. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Tompson M, Woo S, Cantwell DP. Is expressed emotion a specific risk factor for depression or a nonspecific correlate of psychopathology? Journal of Abnormal Child Psychology. 2001;29:573–583. doi: 10.1023/a:1012237411007. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Beck AT, Steer RA, Brown GK. 2nd ed. San Antonio, TX: Psychological Corporation; 1996. Beck Depression Inventory manual. [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair H, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotrophin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozina K, Abela JRZ. Symptoms of depression and anxiety in children: Specificity of the hopelessness theory. Journal of Clinical Child and Adolescent Psychology. 2006;35:515–527. doi: 10.1207/s15374424jccp3504_3. [DOI] [PubMed] [Google Scholar]
- Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: A meta-analysis. Archives of General Psychiatry. 1998;55:547–552. doi: 10.1001/archpsyc.55.6.547. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Antony Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific foundations of cognitive theory and therapy of depression. New York: Wiley; 1999. [Google Scholar]
- Endicott J, Spitzer RA. A diagnostic interview: The Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Firk C, Markus CR. Serotonin by stress interaction: A susceptibility factor for the development of depression? Journal of Psychopharmacology. 2007;21:538–544. doi: 10.1177/0269881106075588. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374410902851705. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Coles ME. Cognitive vulnerability-stress models of psychopathology: A developmental perspective. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage; 2005. pp. 104–135. [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Adamson LB, Riniti J, Cole S. Mothers’ expressed attitudes: Associations with maternal depression and children’s self-esteem and psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1265–1274. doi: 10.1097/00004583-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A development model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Tully E. Children of depressed mothers: Implications for the etiology, treatment, and prevention of depressionin children and adolscents. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York: Guilford; 2008. pp. 415–440. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlicks ML, Weissman MM. Change in child psychopathology with improvement in parental depression: A systematic review. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:379–389. doi: 10.1097/CHI.0b013e3181640805. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Gibb BE, Metalsky GI, Alloy LB, Abramson LY, Hankin BL, Joiner TE, Jr, Swendsen JD. Measuring cognitive vulnerability to depression: Development and validation of the Cognitive Style Questionnaire. Clinical Psychology Review. 2008;28:824–836. doi: 10.1016/j.cpr.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen C, editors. Handbook of depression. 2nd ed. New York: Guilford; 2008. pp. 275–297. [Google Scholar]
- Hankin BL, Abela JRZ. Depression from childhood through adolescence and adulthood: A developmental vulnerability and stress perspective. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage; 2005. pp. 245–288. [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child and Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haeffel GJ. Cognitive vulnerability-stress theories of depression: Examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cognitive Therapy and Research. 2004;28:309–345. [Google Scholar]
- Hankin BL, Lakdawalla Z, Carter IL, Abela JRZ, Adams P. Are neurotocism, cognitive vulnerabilities and self-esteem overlapping or distinct risks for depression? Evidence from exploratory and confirmatory factor analyses. Journal of Social and Clinical Psychology. 2007;26:29–63. [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Biederman J, Brody L, Faraone SV, Rosenbaum JF. Associations between expressed emotion and child behavioral inhibition and psychopathology: A pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:205–213. doi: 10.1097/00004583-199702000-00011. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: A review of the evidnece and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang B-Z, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen K, Ruggiero JJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Archives of General Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim J-M, Stewart R, Kim S-W, Yang S-J, Shin I-S, Kim Y-H, Yoon J-S. Interaction between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lau JYF, Rijsdijk F, Eley TC. I think, therefore I am: A twin study of attributional style in adolescents. Journal of Child Psychology and Psychiatry. 2006;47:696–703. doi: 10.1111/j.1469-7610.2005.01532.x. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- Lyons-Ruth K, Lyubchik A, Wolfe R, Bronfman E. Parental depression and child attachment: Hostile and helpless profiles of parent and child behavior among families at risk. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. Washington, DC: American Psychological Association; 2002. pp. 89–120. [Google Scholar]
- Magaña AB, Goldstein MJ, Karno M, Miklowitz DJ, Jenkins J, Falloon IRH. A brief method for assessing expressed emotion in relatives of psychiatric patients. Psychiatry Research. 1986;17:203–212. doi: 10.1016/0165-1781(86)90049-1. [DOI] [PubMed] [Google Scholar]
- McCarty CA, Lau AS, Valeri SM, Weisz JR. Parent-child interactions in relation to critical and emotionally overinvolved expressed emotion (EE): Is EE a proxy for Behavior? Journal of Abnormal Child Psychology. 2004;32:83–93. doi: 10.1023/b:jacp.0000007582.61879.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Hammen C, Brennan PA, Ullman JB. The impact of maternal depression on adolescent adjustment: The role of expressed emotion. Journal of Consulting and Clinical Psychology. 2003;71:935–944. doi: 10.1037/0022-006X.71.5.935. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS, Seligman MEP. Learned helplessness in children: A longitudinal study of depression, achievement, and explanatory style. Journal of Personality and Social Psychology. 1986;51:435–442. doi: 10.1037//0022-3514.51.2.435. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS, Seligman MEP. Predictors and consequences of childhood depressive symptoms: A 5-year longitudinal study. Journal of Abnormal Psychology. 1992;101:405–422. doi: 10.1037//0021-843x.101.3.405. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Houston K, Hawton K, Harrison PJ. Deliberate self-harm is associated with allelic variation in the tryptophan hydroxylase gene (TPH A779C), but not with polymorphisms in five other serotonergic genes. Psychological Medicine. 2003;33:775–783. doi: 10.1017/s0033291703007463. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R. HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Robinson NS, Garber J, Hilsman R. Cognitions and stress: Direct and moderating effects on depressive versus externalizing symptoms during the junior high school transition. Journal of Abnormal Psychology. 1995;104:453–463. doi: 10.1037//0021-843x.104.3.453. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Toth SL. Expressed emotion in multiple subsystems of the families of toddlers with depressed mothers. Development and Psychopathology. 2004;16:689–709. doi: 10.1017/s0954579404004730. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schwartz CE, Dorer DJ, Beardslee WR, Lavori PW, Keller MB. Maternal expressed emotion and parental affective disorder: Risk for childhood depressive disorder, substance abuse, or conduct disorder. Journal of Psychiatric Research. 1990;24:231–250. doi: 10.1016/0022-3956(90)90013-g. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Peterson C, Kaslow NJ, Tenenbaum RL, Alloy LB, Abramson LY. Attributional style and depressive symptoms among children. Journal of Abnormal Psychology. 1984;93:235–241. doi: 10.1037//0021-843x.93.2.235. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Hayden EP, Singh SM, Dougherty LR, Olino TM, Durbin CE, Klein DN. An examination of the association between the 5-HTT promoter region polymorphism and depressogenic attributional styles in childhood. Personality and Individual Differences. 2008;45:425–428. doi: 10.1016/j.paid.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort difference on the Children’s Depression Inventory: A meta-analysis. Journal of Abnormal Psychology. 2002;111:578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Hammond M. Maternal depression and its relationship to life stress, perceptions of child behavior problems, parenting behaviors, and child conduct problems. Journal of Abnormal Child Psychology. 1988;16:299–315. doi: 10.1007/BF00913802. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Jensen P. What research suggests for depressed women with children. Journal of Clinical Psychiatry. 2002;63:641–647. doi: 10.4088/jcp.v63n0717. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch K-P, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. 2nd ed. New York: Wiley; 1997. [Google Scholar]
- Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu X, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]