Abstract
CXCR4 receptors have been implicated in tumorigenesis and proliferation, making it a potential target for colorectal cancer therapy. Expression of this chemokine receptor on cellular surfaces appears to promote metastasis by directly stimulating tumor cell migration and invasion. The receptor/ligand, CXCR4/SDF-1α, pair are critically important to angiogenesis and vascular remodeling which supports cancer proliferation. Our work has shown that a novel apoptotic peptide of HIV-1, Nef-M1, can act as a CXCR4 antagonist, inducing apoptosis in CXCR4 containing cells. Four colorectal tumor cell lines (HT-29, LS174t, SW480, WiDr), were evaluated for their response to Nef-M1 peptide via in vivo and in vitro. The presence of CXCR4 receptors on tumor cells was determined using immunohistochemical and RT-PCR analyses. Solid xenografts derived from tumor cell lines grown in SCID mice, were evaluated for the persistence of the receptor. Xenografts propagated in SCID mice from each of the four cell lines demonstrated high levels of receptor expression as well. The effects of Nef-M1 in vivo via splenic injected mice and subsequent hepatic metastasis also demonstrated dramatic reduction of primary tumor growth in the spleen and secondary invasion of the liver. We concluded that Nef-M1 peptide, through physical interaction(s) with CXCR4, drives apoptotic reduction in in vivo primary tumor growth and metastasis.
Keywords: Colorectal cancer, Nef-M1, HIV-1, CXCR4, Metastasis
Introduction
Colorectal cancer (CRC) has remained the third most frequent cause of cancer related deaths among American men and women. The most common cause of death from colon cancer is hepatic metastasis. There have been no major advancements in the effective treatment of metastatic colorectal cancer in the last 40 years since the advent of 5-fluorouracil. Only recently has 5-fluorouracil, leucovorin and oxaliplatin shown some limited success. Hepatic metastasis is a key role to mortality of colorectal cancers (1, 2). Cells move from the primary tumor site, enter circulation and settle within hepatic tissues where they then propogate. Metastasis involves a complex series of steps, which involves chemotaxis, angiogenesis, and tumorigenesis. CXCR4 has been implicated as one of those chemokine receptors involved in tumorigenesis and metastasis (1, 3, 4).
The SDF-1α-chemokine ligand and the seven transmembrane G-protein-coupled receptor-CXCR4, (5), is a unique pair in that SDF-1α is the only host ligand for this receptor (3, 4). This becomes extremely important because identifying an agent that interrupts this binding may have profound therapeutic effectiveness. The ligand itself is expressed by stromal cells which includes endothelial cells. The pair induces strong chemotactic efficacy for leukocytes in vitro, and is a highly potent chemoattractant in vivo (6, 7, 8). Studies have been shown in vivo, that deficiency in the receptor and ligand causes phenotypic changes mediated by disruption of embryonic cell migration (6–12). Furthermore the receptor CXCR4 has been shown to be a coreceptor for HIV-1 and in our laboratory we have shown that it is through this receptor that the apoptosis occurs in CRC (13). The CXCR4 receptor is highly expressed in solid human cancers including pancreas, ovary, breast, prostate (14, 15), colon, and the rectum (16). The chemokine receptor/ligand pair have been shown to be critically involved in a growth factor regulated signaling system in endothelial cells that mediates steps in vascular remodeling and subsequent support of tumors (17).
The nef gene of Human Immunodeficiency Virus (HIV) encodes a 27–34 kd myristoylated protein, which is expressed early after establishment of the provirus in host cells (13,18). We have shown that soluble HIV-1 Nef protein, alone, is a potent inducer of apoptosis in a number of cell types through the chemokine co-receptor CXCR4. We have localized the apoptotic motifs in the HIV-1 Nef protein as previously described (13, 18). We hypothesize that HIV-1 Nef apoptotic peptide not only slows the rate of growth of primary human CRC xenografts but will also inhibits hepatic metastasis in SCID mice.
Materials and Methods
Proteins and Antibodies
Nef-Motif-1 (Nef-M1 or M1) and Nef sMotif-1 (Nef-sM1 or sM1, the scrambled amino acid sequence of Nef-M1) were obtained from Sigma Genosys (Houston, TX). The following antibodies were used: monoclonal mouse anti-human fusin clone 12G5, mIgG2a (CXCR4) (Research and Diagnostics Inc., Flanders, NJ); anti-mouse flourescein isothiocynate, mIgG (H+L) made in goat (Pierce Biotechnology, Rockford IL); monoclonal anti-mouse Texas Red, mIgG (Vector Laboratories, Burlingame, CA).
Cell Culture
Four colorectal tumor cell lines were cultivated: HT-29, LS-174t, SW-480, and WiDr. Each cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cryo-preserved. MDA-MB-468 (468) and MDA-MB-231 (MDA-MB-231), derived from human breast adenocarcinoma were obtained through ATCC. These two cell lines served as our positive (MDA-MB-231) and negative (MDA-MB-468) controls for CXCR4 expression by immunohistochemistry (IHC). All cell lines were cultured in 5% CO2 at 37° C in RPMI 1640 supplemented with L-glutamine (Cellgro, Fisher Scientific, USA), 10% fetal bovine serum (Biowhittaker-Cambrex Walkersville, MD) and penicillin/streptomycin (Biowhittaker-Lonza, Walkersville, MD). Cell cultures were grown to confluencey. Cells were then harvested, repassaged, and injected into mice according to protocol.
Animals and Tumor Growth
Severe Combined Immunodeficiency (SCID) mice were obtained from Taconic Form (Taconic, NY) at approximately one month of age. After one week of quarantine, mice were inoculated with one million cells of each of the aforementioned cell lines subcutaneously to establish primary tumors. Another series of mice were given splenic injections with the same cell count (1×106 cells/0.1 cc). Briefly, splenic injections were done by having the spleens extracorporeally injected under direct vision and then replacing the spleen in its usual anatomical location. The abdominal wall and skin were closed with absorbable suture. All food, water, and bedding were sterilized by autoclaving. The animals received standard rodent chow and water, and resided in isolated micro-filtered cages in rooms designated for immune compromised mice. The mice were checked daily to assess health status and tumor growth. Body weight, nutritional intake, general activity level, and ruffling of mice fur served as our indicators of health status. Surgical procedures were done using disposable gowns, sterile gloves, and a laminar flow hood. Gloved hands were wet with liquid sterilant (Expor, Alcide Co. Norwalk, CT) before making direct contact with the mice.
Nef Injections
SCID mice were injected intraperitoneally at one week post cell implantation with either the active Nef peptide (M1), the scrambled amino sequence of Nef–M1(sM1) peptide, or were untreated (C). Nef peptides were diluted in phosphate buffer saline (PBS) at a concentration of 2 μg per 0.1ml. Injections were done biweekly for four weeks for all treatment groups. Additionally colorectal and breast cancer cell lines were evaluated by IHC, and RT-PCR analysis to determine their expression of CXCR4. The treatment protocol is shown in Table 1.
Table 1.
Inoculations using human colorectal cancer cell lines
| Descriptions | # of Mice |
|---|---|
| Subcutaneous injections for primary tumor Line) | 4 total growth (One Mouse for Each Cell |
| Splenic injections generating hepatic mets | 11 total |
| HT-29 | 4 |
| WiDr | 3 |
| LS-174t | 2 (1 died) |
| SW-480 | 2 (1 died) |
| Nef-M1 Effects on WiDr Splenic Injected Mice | 12 total |
| Nef peptide (M1) injections (N) | 4 |
| Untreated (control w/buffer) (C) | 4 |
| Scrambled Nef peptide (S) | 4 (1 died) |
| Total Mice | 27 |
Mice were sacrificed after four weeks of treatment. Each mouse was wet down with sterilant and carefully dissected to remove the entire spleens and livers. The tissue samples were measured and the experimental conditions of each recorded (i.e. untreated(C), sM1 (S), or Nef-M1 treated (N)). Pictures were taken using a Kodak DC290 zoom digital camera (Figures 4 and 5).
Figure 4. Primary splenic tumors.

Splenic tumors of mice treated with Nef-M1 (N), Control (C), and scramble peptide (S) bi-weekly for one month following spleens inoculation with CRC cells. Pictures were taken using a Kodak DC290 zoom digital camera.
Immunocytochemistry and Histological Staining
All cells lines were individually grown and fixed on collagen (25ng/ml) coated coverslips until ≈70% confluent. Coverslips were then washed twice with 1 ml PBS. After initial washes, 1 ml of PBS plus 10% goat serum (Gibco, Invitrogen, Carlsbad, CA) were added and allowed to sit for one hour at room temperature. The cocktail was removed and PBS with goat serum added along with primary antibody (1:250μl) and allowed to sit overnight at 4°C. The next day this solution was removed and the cells were rinsed three times with PBS. Secondary antibody (1:200 μl) with PBS plus 10% Goat Serum was added for one hour at room temperature. The cells were again rinsed with PBS and subsequently fixed with 4% paraformaldehyde (Sigma-Aldrich, Milwaukee, WI) for an hour. Coverslips were rinsed and allowed to air dry. The coverslips were mounted on slides using a drop of Vectashield.. Cells were visualized by epiflurorescence on a computer controlled microscope system (Zeiss microscope, Carl Zeiss, Thornwood, NY). Microscopic images were processed using a charged coupled device (CCD) camera, MC 100 SPOT, 60910 (Photonis Science, East Susses, U.K.). Further image processing was conducted utilizing the Image Pros Plus 2.0 software (Media Cybernetics, Silver Spring MD), and formatted using Adobe Illustrator 8.0 software.
Surgical procedures
All surgical procedures and surgical specimen retrieval were conducted under the guidelines and approvals of Morehouse School of Medicine, IACUC and IRB committees.
RT-PCR
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on tumor xenografts derived from cell lines injected in mice. mRNA was isolated using TRI-zol reagent (Invitrogen) from tumor cells removed from surgical specimens and xenografts from cell lines grown subcutaneously. Using the SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase Kit (Invitrogen, Cat # 1257-026), 5 μg of total RNA was primed reversed transcribed into cDNA in 50 μl reaction mixtures at 55° C for 30 min and was followed immediately by pre-denaturation consisting of one cycle at 94° C for 2 min. PCR amplification using the One-Step for 35 cycles of denaturing at 94° C for 15 sec, annealing at 60° C for 30 sec, and extension at 68° C for 2 min, followed by the final extension at 68° C for 5 minutes. The following primers were used for CXCR4:
1. hCXCR4-1 (F): 5′ATGAAACTTGGGGCGAGGAC-3′. (R): 5′CGGTGTAGTTATCTAGAAGTG-3′ (product size: 1097-bp). 2. hCXCR4-2 (F): 5′-ATGTCCATTCC TTTGCCTCT-3′. (R): 5′-AAAGCATAGAGGATGGGG TT-3′ (product size: 922-bp). 3. hCXCR4-3 (F): 5′-TA CCTGGCCATCGTCCACGC-3′. (R): 5′-TCCAA ACACGAGRGCATACC-3′ (product size: 508-bp).
*F-forward; *R-reverse. The primers were then loaded onto a 1.5% agarose gel for electrophoresis using 6x Promega dye. Markers were along 100 base pairing.
Results
CXCR4 Expression in CRC Cell Cultures and Xenografts
To evaluate the expression of CXCR4 receptor in colorectal cancer (CRC) cell lines, four cell lines were tested for the receptors by immunohistochemistry and histostaining. Using a specific antibody for CXCR4 as noted previously, we were able to identify CXCR4 receptors in each tumor cell line (Figure 1). This is in comparison with the MDA-MB-468 breast cell line (negative control), and MDA-MB-231 breast cell line (positive control). Each CRC cell line had varying degrees of expression on their surface and/or in the cytoplasm of the cells. No statistical analysis was applicable; therefore we relied on the gradation of immunofluorescent staining in the cell cultures. In Figure 2, RT-PCR analysis shows comparable results of mRNA levels consistent with positive expression in CRC cell lines and CRC xenografts derived from those cells, as compared with the positive control of MDA-MB-231 cell line and tumor tissue. The WiDr cell line had some loss of expression in the tumor. The immunohistochemistry was substantiated with RT-PCR data conducted with each of the same primary tumors formed. Fluorescent “quantification” is a future aim for observing CXCR4 expression to determine significance.
Figure 1. CXCR4 protein expression in colorectal cells was analyzed by immunocytochemistry.
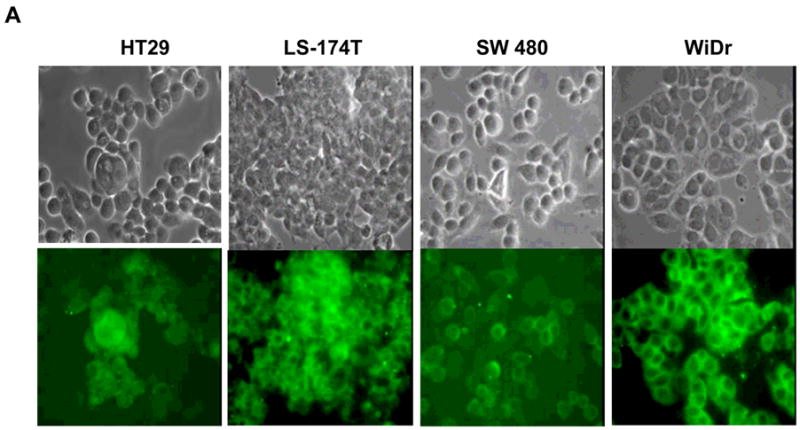
A. Colorectal cancer cell lines were evaluated for CXCR4 receptor expression. The top row is unstained cells and the bottom is IHC staining for CXCR4 using flourescein isothiocynatae (FITC) green. B. Breast cancer cells, MDA-MB-231 and MDA-MB- 468 were positive and negative controls, respectively. Magnification: 400x.
Figure 2. CXCR4 transcription was analyzed in colorectal tumor cell lines and primary tissue by Reverse Transcription Polymerase Chain Reaction (PCR).
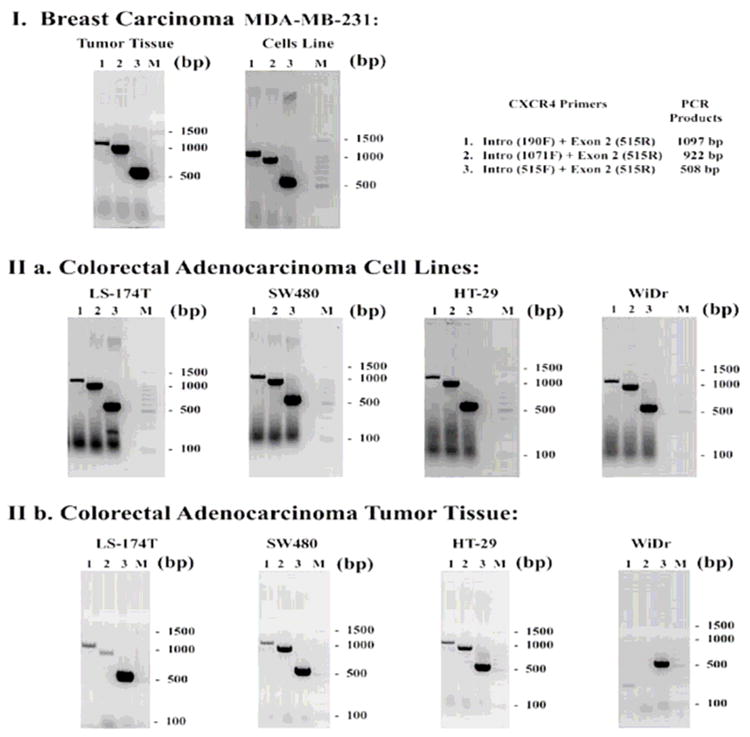
RNA was isolated from cells or from tumor tissue and reverse transcribed. The resultant PCR products were fractionated on a 1.5% agarose gel followed by ethidium bromide staining, and then analyzed by ABI Prism 3130 xI Sequence Detection System and 3130 xI Genetic Analyzer data collection software v 3.0. Lane M: 100 bp DNA ladder; lane 1: 1097 bp CXCR4 band; lane 2: 922 bp CXCR4 band; lane 3: 508 bp CXCR4 band. (I) As a positive control for RNA expression of CXCR4, a breast cancer cell line, MDA-MB-231, and in breast tumor tissue were analyzed. (II) Four colorectal adenocarcinoma cell lines (LS-174T, SW480, HT-29, WiDr) were analyzed for CXCR4 transcription. (III) Tumor xenografts grown subcutaneously from injected cells were also analyzed for CXCR4 transcription. The PCR products were designed to determine whether the CXCR4 transcript has deletions in it (e.g., IIb, WiDr appears to be a truncated transcript).
Nef-M1 Effects on Primary Tumor and Metastasis in Splenic Injection Mice
The direct effects of the antagonist peptide on primary colon cancer and metastasis were evaluated. Tumor cells lines were injected into spleen of the SCID mice and treated according to protocol. Figure 3 illustrates the extent of metastatic disease that occurred within the liver during the four week period. There are large splenic primaries and florid hepatic metastasis. Figure 4 shows spleens following injection with CRC tumor cell line (WiDr). In the untreated (C) and sM1 (S) spleens, there is a striking difference in tumor growth when compared to SCID mice treated with the active Nef-M1 peptide (N). A small tumor was noted in the spleen of one of the Nef-M1 (N) treated group, but all others in the group were tumor free.
Figure 3. Primary tumor growth and liver metastasis.
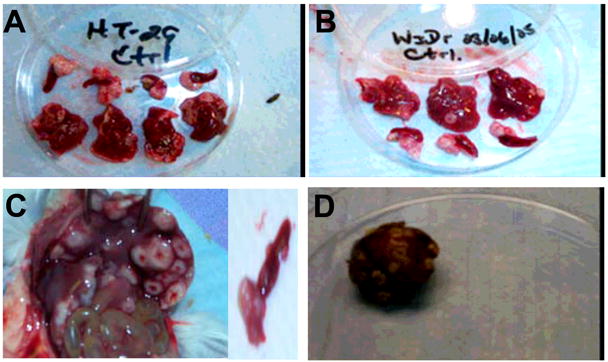
Spleens and livers excised from the SCID mice four weeks after spleens were inoculated with tumor cells (A-HT-29, B-WiDr, C-LS174t, D-SW480). Primary tumor growth was seen in the spleens of all mice. All mice were untreated. Pictures were taken using a Kodak DC290 zoom digital camera.
Control animals (buffer and sM1 treated) had higher morbidities: their eyes became glossy and pale in color, appetite and weight diminished, and movements were slowed, and pale tails suggested anemia. None of the M1 treated mice died before the end of the four week research period. The group of mice also maintained adequate eating habits and suffered little to no weight loss.
Discussion
To study the colorectal cancer (CRC) and the effects of the antagonist peptide, Nef-M1, as a potential therapeutic agent, we focused on the SCID mouse model. This model permits xenograft tumor growth and development in the subcutaneous tissue following cellular or tissue implantation (14). CRC grows locally in the subcutaneous tissue without peritoneal metastases. This allows monitoring of the effect of the Nef peptide on the primary growth of these tumors over an extended period time. This model also allows the formation of hepatic metastases from primary splenic injections. Therefore we can study the effects of the Nef-M1 peptide on hepatic metastases from human colorectal cancer.
The evidence from these studies with colorectal cell lines confirmed that these lines were positive for the CXCR4 receptor through immunofluorescence and RT-PCR. CXCR4 is vital for proper angiogenesis and metastasis. Using any of these cell lines, one can use targeted therapy with a CXCR4 antagonist, such as Nef-M1, to induce apoptosis and inhibit growth of CRC. Expression of CXCR4 on the cell surface promotes metastasis and acts directly on tumor cell migration and invasion. Because tumor cells are known to have an embryonic or dedifferentiated state, CXCR4 expression is higher among those cells than normal cells (3). The ability of a tumor to implant, grow, and metastasize is due to its ability to generate neovascularization through angiogenesis (19, 20). Evidence has shown a link between CXCR4 and vascular endothelial growth factor (VEGF), together being potent in driving vascularization (21, 22). Combined with the ability of VEGF to enhance CXCR4 expression and increase vessel density, a direct association with metastasis of CRC is expected. The ability of Nef-M1 to bind with CXCR4 and inhibit angiogenesis (unpublished results from our lab) enhances its ability to further inhibit tumor implantation and growth.
There are several agents currently being developed that specifically target the CXCR4 receptor (12, 17). By blocking the CXCR4 receptor from interacting with its natural ligand, inhibition of primary tumor growth and metastasis will occur. These other antagonists were originally created to combat HIV-1 in a similar fashion. However, those antagonists do not eliminate the cell, but rather physically compete with the SDF-1α ligand. Our work suggests that Nef-M1 peptide interacts with CXCR4 just like these other synthetic antagonists. However, it goes a step further and induces apoptosis within those tumor cells, thus destroying them all together.
In previous studies it was shown that when primary tumor xenografts (from surgical specimens) were treated with Nef-M1 for one month, growth was reduced up to 75% (5). Additionally, caspase assays of surgical specimen xenografts showed elevated levels of caspase-3 (5). Caspases are essential for driving the apoptosis process, and have been termed “executioner” proteins for their roles in the cell. Failure of the cell to induce the apoptosis program is one of the main contributions to tumor development and autoimmune diseases. By regulating and reducing (through apoptosis) tumor growth, complete excision of CRC becomes possible, and metastasis can be reduced dramatically or even eliminated altogether. Without any regulation, tumor cells continue to proliferate, which eventually leads to metastasis and death of the host. As shown in Figure 4, the primary splenic tumors known to induce hepatic metastasis can be significantly inhibited using Nef-M1 peptide (N).
One of the main causes of treatment failure and death of patients dealing with cancer is metastasis to the secondary organs (liver and lungs). Just as primary tumor growth undergoes specific steps, cancer metastasis involves complex, highly organized, non-random, and organselective processes (1, 4). These processes are still not clearly understood, but what is known is that the tumor aggressiveness is higher during metastasis than the primary tumor growth. CXCR4 protein expression was shown to be increased and the levels of SDF-1α were also shown to be higher in the more aggressive metastatic lesions than in primary tumors.(23) Patients with high levels of CXCR4 were found to have more extensive metastasis to the lymph nodes as compared with patients that had low CXCR4 expression in the tumors (24). The SCID mice splenically injected with tumor cells and subsequently untreated were found to form primary tumors in the spleen that then went on to metastasize forming metastatic lesions in the livers. No mice died from the Nef-M1 treatment during the four weeks. Alternatively, the control mice became increasingly ill over time with internal hemorrhaging observed. In these controls, aggressive metastasis was observed, with numerous small tumor nodules on the liver. Thus, our preliminary data (unpublished) shows that the identified neovascularization within the xenografts is inhibited through the anti-angiogenic effects by the antagonist peptide, Nef-M1. Primary tumor growth and metastasis of CRC to secondary organs can be inhibited through the actions of Nef-M1. A well developed model for producing hepatic metastasis from human surgical CRC specimens have been developed and reported by one of the authors (25). This will further enable assessment of Nef-M1 ability to inhibit metastasis of human CRC from a diverse group of patients.
These findings suggest that the Nef-M1 peptide can be an effective and significant inhibitor to the growth of primary human colorectal cancers even after local invasion occurs. Consistent hepatic metastasis can be achieved using the SCID mouse model and injecting CRC cells into the spleen. Additionally it is evident that Nef-M1 peptide inhibits primary tumor growth in the viscera and subcutaneously. The future direction of this research includes quantitative analysis of actual reduction of hepatic metastasis. By using human surgical specimens to develop hepatic metastatic xenografts we will evaluate the therapeutic efficacy of Nef-M1.
Acknowledgments
This work in part supported by the NIH grant U54-CA118948. This work and the researchers were also partially supported by NIH/NIGMS/MBRS (grant 58268), and NIH/NCRR/RCMI (grant G12-RR03034). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant C06 RR18386 from NIH/NCRR
References
- 1.Nicolson GL. Paracrine and autocrine growth mechanisms in tumor metastasis to specific sites with particular emphasis on brain and lung metastasis. Cancer Metastasis Rev. 1993;12:325–43. doi: 10.1007/BF00665961. [DOI] [PubMed] [Google Scholar]
- 2.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 3.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–11. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 4.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–6. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Federsppiel B, Melhado IG, Duncan AM, et al. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seventransmembrane segment (7-TMS) receptor isolated from human spleen. Genomics. 1993;16:707–12. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 8.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 9.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 10.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 12.Murakami T, Nakajima T, Koyanagi Y, et al. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–93. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J Virol. 2004;78:11084–96. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bumpers HL, Huang MB, Powell M, et al. Effects of HIV-1 Nef, a cytotoxic viral protein, on the growth of primary colorectal cancer. Cancer Biol Ther. 2005;4:65–9. doi: 10.4161/cbt.4.1.1377. [DOI] [PubMed] [Google Scholar]
- 15.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–5. [PubMed] [Google Scholar]
- 16.Zeelenberg IS, Ruuls-Van SL, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. [PubMed] [Google Scholar]
- 17.Gupta SK, Pillarisetti K, Thomas RA, Aiyar N. Pharmacological evidence for complex and multiple site interaction of CXCR4 with SDF-1alpha: implications for development of selective CXCR4 antagonists. Immunol Lett. 2001;78:29–34. doi: 10.1016/s0165-2478(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 18.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J Virol. 2004;78:3099–109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ, Singh RK, Yoneda J, et al. Critical determinants of neoplastic angiogenesis. Cancer J. 2000;6 (Suppl 3):S225–36. [PubMed] [Google Scholar]
- 21.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 23.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89:462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:R144–50. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bumpers HL, Alosco TR, Wang HQ, Petrelli NJ, Hoover EL, Bankert RB. Consistent hepatic metastasis of human colorectal cancer in severe combined immunodeficient mice. J Surg Res. 1996;61:282–8. doi: 10.1006/jsre.1996.0117. [DOI] [PubMed] [Google Scholar]


