SUMMARY
Male behaviors require both testosterone and estrogen. Circulating testosterone activates the androgen receptor (AR) and is also converted into estrogen in the brain via aromatase. This conversion is the primary source of estrogen to the male brain. It is unclear whether testosterone and estrogen signaling interact to masculinize neural circuits. Using a genetic approach, we show extensive sexual dimorphism in the number and projections of aromatase expressing neurons. The masculinization of these cells is independent of AR but can be induced by either testosterone or estrogen, indicating a role for aromatase in sexual differentiation of these neurons. We provide evidence suggesting that aromatase is also important in activating male aggression and urine marking as these behaviors can be elicited by testosterone in males mutant for AR. Taken together with additional findings, our results suggest that aromatization of testosterone into estrogen is important for the development and activation of neural circuits that control male territorial behaviors.
INTRODUCTION
All sexually reproducing species exhibit gender dimorphisms in behavior. Such sex differences can be observed in various displays, including in mating, aggression, territorial marking, and parental care. Behavioral dimorphisms can be observed in socially naive animals suggesting that sexual differentiation of the underlying neural circuits is tightly controlled by internal physiological regulators. In vertebrates, gonadal steroid hormones play a central role in the development and function of these neural circuits (Arnold et al., 2003; Goy and McEwen, 1980; Morris et al., 2004). Both testosterone and estrogen are required for male behaviors in many vertebrates, including mammals. It remains to be determined how these two hormonal pathways intersect to control dimorphic behaviors in males (Juntti et al., 2008).
Testosterone is required for male behaviors in most vertebrates, including mice and humans. Testosterone mediates its effects by activating AR and male mice mutant for this receptor do not display sexual behavior or aggression (Ohno et al., 1974). Testosterone is essential in newborn and adult male mice for the display of sex specific behaviors such as aggression (Finney and Erpino, 1976; Peters et al., 1972; Wallis and Luttge, 1975). This testicular hormone is thought to masculinize neural circuits in neonatal rodents, and to act upon these pathways in adult males to permit the display of dimorphic behaviors (Phoenix et al., 1959).
Estrogen is also essential for male behaviors. The requirement for estrogen to masculinize behavior seems counter-intuitive as this ovarian hormone is essentially undetectable in the male circulation. All estrogenic steroids are synthesized in vivo from testosterone or related androgens in a reaction catalyzed by aromatase. Aromatase expressing cells in the brain convert circulating testosterone into estrogen, and it is this local estrogen that is thought to control dimorphic behaviors in males (Figure 1A) (MacLusky and Naftolin, 1981; Naftolin and Ryan, 1975). Consistent with a requirement for estrogen in male behaviors, aromatase activity is essential for male behaviors. Mice mutant for aromatase exhibit a profound reduction in male sexual behavior and aggression (Honda et al., 1998; Toda et al., 2001). Similar to testosterone, estrogen is essential in neonates and adults for the display of dimorphic behaviors in males (Finney and Erpino, 1976; McCarthy, 2008; Scordalakes and Rissman, 2004; Toda et al., 2001; Wallis and Luttge, 1975). Estrogen mediates many of its effects by signaling through the estrogen receptors ERα and ERβ, which exhibit overlapping expression patterns, and regulate masculinization of the brain and behavior in a complex, redundant manner (Bodo et al., 2006; Ogawa et al., 1999; Ogawa et al., 2000; Ogawa et al., 2004; Perez et al., 2003; Rissman et al., 1997). The role of a third estrogen receptor, GPR30, in male behaviors is presently unknown (Revankar et al., 2005).
Figure 1. Visualizing aromatase expressing neurons in the mouse brain.
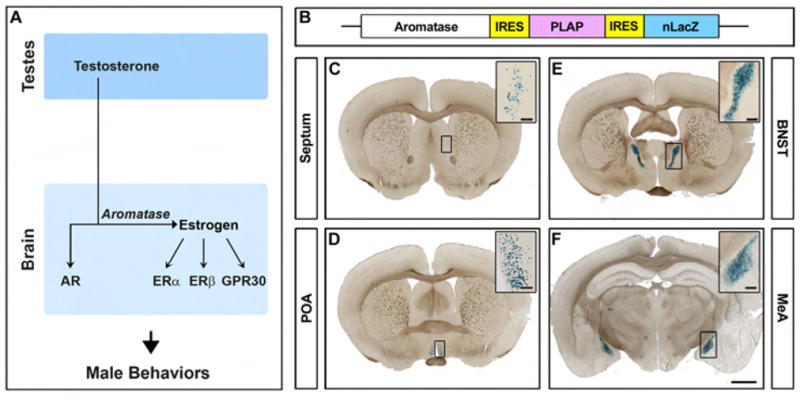
(A) Sex steroid hormone control of male behaviors. (B) Schematic of genetic modification of the aromatase locus. (C–F) Coronal sections through the forebrain of an adult male homozygous for the aromatase-IPIN allele stained for βgal activity. Aromatase is expressed in a sparse manner in discrete regions including the lateral septum (C), preoptic area (POA) (D), bed nucleus of the stria terminalis (BNST) (E), and medial amygdala (MeA) (F). Scale bar equals 2.5 mm. Inset scale bars equal 200 μm (C, D) and 50 μm (E, F).
The dual requirement for testosterone and estrogen signaling in male behaviors suggests that these two pathways may interact genetically to control these dimorphic displays. One potential site of interaction is the control of aromatase expression. We have therefore sought to determine whether aromatase expression is regulated by testosterone or estrogen signaling. To visualize aromatase expression at cellular resolution, we have genetically modified the aromatase locus such that all cells expressing this enzyme co-express two reporters, nuclear targeted lacZ and placental alkaline phosphatase, thereby labeling the cell bodies and projections of aromatase positive neurons.
We find extensive, previously uncharacterized sexual dimorphisms in both the number and projections of neurons expressing aromatase. The masculinization of these neural pathways is independent of AR but can be induced in neonatal females by testosterone or estrogen, indicating that aromatase plays an important role in the sexual differentiation of these neurons. Moreover, testosterone activates male typical fighting and urine marking independent of AR, demonstrating that the differentiation and function of the neural circuits underlying these behaviors are governed by testosterone, at least in part, after its conversion into estrogen. Finally, our results show that adult gonadal hormones of either sex can support male territorial marking and fighting provided estrogen has neonatally masculinized the underlying neural circuitry.
RESULTS
Aromatase is expressed in a sparse manner in the mouse brain
To define where testosterone may be converted into estrogen in the mouse brain, we sought to characterize the expression of aromatase at cellular resolution. We used homologous recombination in ES cells to insert an IRES-PLAP-IRES-nuclear LacZ reporter cassette into the 3′ UTR of the aromatase locus (Figure 1B). The use of IRES elements permits faithful expression of PLAP and nuclear β-galactosidase (βgal) in cells transcribing the targeted allele (Shah et al., 2004). This strategy maintains the expression and function of aromatase, thereby permitting the examination of neural pathways expressing this enzyme in otherwise wildtype (WT) animals. In sharp contrast to aromatase−/− animals, mice homozygous for the aromatase-IRES-PLAP-IRES-nuclear LacZ (aromatase-IPIN) allele are fertile and behaviorally similar to their WT littermates, and they have normal levels of serum testosterone and estrogen (Supplemental Text).
Analysis of βgal activity in the brain reveals small pools of cells that account for less than 0.5% of neurons in the brain (Figure 1C–F). We observe βgal labeled cells in discrete locations, including the posteromedial component of the bed nucleus of the stria terminalis (BNST), posterodorsal component of the medial amygdala (MeA), preoptic hypothalamus (POA), and lateral septum. The expression of βgal mirrors the distribution of aromatase mRNA as revealed by in situ hybridization (Figure S1) and RT-PCR (Harada and Yamada, 1992). The absence of sensitive, specific antibodies to aromatase precludes colocalization studies with βgal in single cells. While in situ hybridization (ISH) reveals low levels of aromatase mRNA with poor signal:noise, the activity of our reporters is robust and offers superior cellular resolution (Figure S2). In addition, labeling the brain for PLAP activity reveals the soma and projection fibers of neurons expressing aromatase (Figure S3).
Aromatase expressing neurons display extensive sexual dimorphism
A comparison of aromatase positive cells in adult male and female mice bearing the aromatase-IPIN allele reveals several previously undescribed sexual dimorphisms. We find sex differences in both the number and projection patterns of aromatase expressing neurons. We find more aromatase positive cells in the BNST and MeA in males compared to females (Figure 2A–D, P). These two regions have previously been shown to regulate sexual and aggressive behaviors (Albert and Walsh, 1984; Kondo et al., 1998; Liu et al., 1997). We also observe sexual dimorphism in cell number in the caudal hypothalamus, where we observe more aromatase expressing cells in females compared to males (Figure 2E, F, P). The presence of more aromatase positive neurons in the female caudal hypothalamus is surprising as there is little testosterone in the female circulation that could serve as a substrate for conversion to estrogen. In any case, most βgal positive cells (> 95%; n ≥ 3) in these dimorphic clusters express the pan-neuronal marker NeuN, indicating that they are neurons. A similar proportion of NeuN positive cells in the BNST and MeA expresses βgal in both sexes, indicating an absolute increase in the total number of neurons in these regions in males (Supplemental Text).
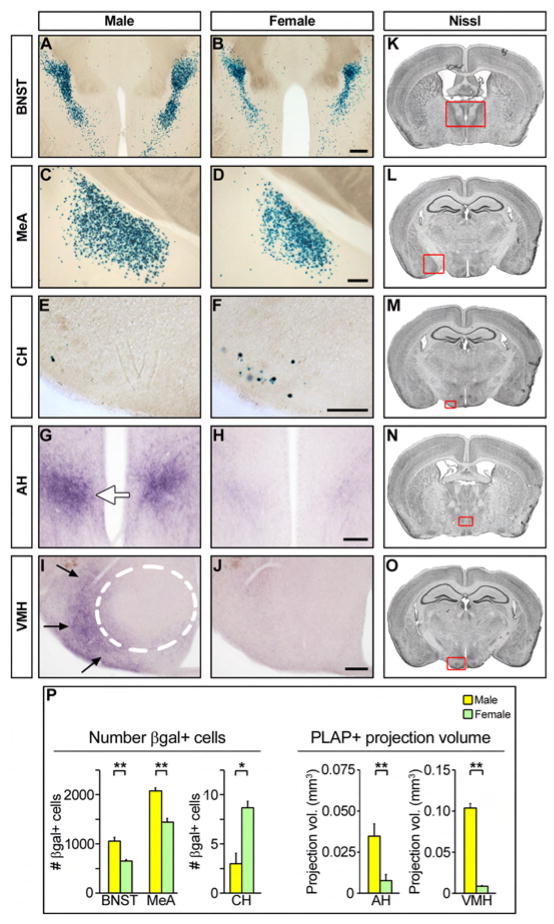
Figure 2. Extensive sexual dimorphism in aromatase expressing neural pathways.
(A–J) Coronal sections through the adult brain of male and female mice harboring the aromatase-IPIN allele stained for βgal (A–F) or PLAP activity (G–J). There are more aromatase+ cells in the male BNST and MeA, and in the female caudal hypothalamus (CH). PLAP+ fibers occupy a larger volume in the male AH (white arrow) and in the region (arrows) surrounding the VMH (dashed outline). (K–O) Nissl stained sections depicting locations of the BNST, MeA, CH, AH, and VMH. (P) Quantitation of sexual dimorphism in cell number (βgal+) and fiber tracts (PLAP+) of neurons expressing aromatase. Mean ± SEM; n ≥ 3; * p ≤ 0.015, ** p ≤ 0.005. Scale bars equal 500 μm (A, B) and 250 μm (C–J).
Labeling for PLAP activity also revealed sex differences in the fibers of aromatase expressing neurons. Consistent with the dimorphisms in cell number, PLAP labeling reveals a richer plexus of fibers in the BNST and MeA in males and in the caudal hypothalamus in females (Figure S3). We also observe previously undescribed dimorphic processes in the anterior hypothalamic region and the ventromedial hypothalamus (VMH), with PLAP labeled fibers occupying a larger volume in males compared to females (Figure 2G–J, P). In contrast to the poorly characterized anterior hypothalamic region, the VMH is known to regulate feeding and sexual behaviors (Musatov et al., 2006; Musatov et al., 2007). Within the limits of detection, the dimorphisms in PLAP activity are unlikely to reflect differences in aromatase expression levels as extended staining did not reveal additional fibers in the female brain. Both the anterior and ventromedial hypothalamic regions contain a few βgal positive cells in both sexes (Supplemental Text), indicating that the dimorphisms may reflect an increase in arborization of local neurons or projections from distant aromatase expressing neurons. There are more synapses on VMH neurons in males (Matsumoto and Arai, 1986), and our findings suggest that aromatase positive neurons may contribute to this dimorphic innervation. The pattern of PLAP labeled fibers surrounding the VMH resembles afferent input to this structure from the BNST, MeA, POA, and septum (Canteras et al., 1995; Choi et al., 2005; Dong and Swanson, 2004; Millhouse, 1973; Simerly and Swanson, 1988; Varoqueaux and Poulain, 1999). Each of these afferent regions expresses aromatase (Figures 1, S1), and may contribute to the dimorphic PLAP labeling in the VMH. Taken together, our studies using genetically encoded reporters reveal previously undescribed sex differences in aromatase expressing neural pathways.
Sexual differentiation of aromatase expressing neurons is independent of AR
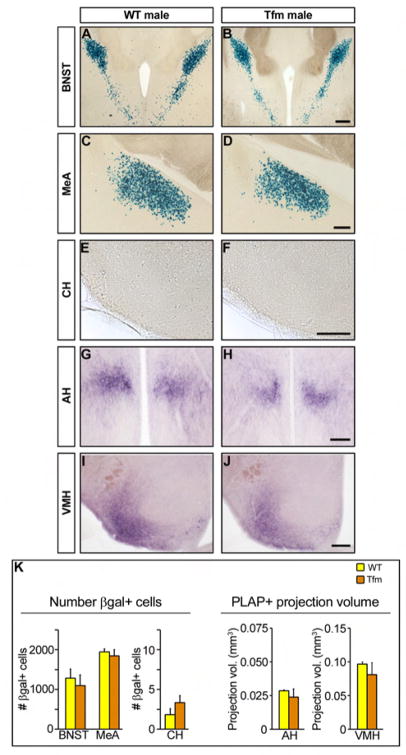
We tested whether masculinization of aromatase positive neural pathways requires the activity of androgens such as testosterone signaling through AR. We crossed mice carrying the aromatase-IPIN allele to animals harboring the tfm allele, a loss-of-function allele of the X-linked AR (Charest et al., 1991). We find male typical differentiation of aromatase positive neurons in Tfm mutant males. The number of βgal positive neurons in the BNST, MeA, and caudal hypothalamus is similar between Tfm and WT males (Figure 3A–F, K). Staining for PLAP activity labels male typical projection patterns in the VMH and the anterior hypothalamic area of Tfm mutant males (Figure 3G–K). These findings demonstrate that masculinization of the number and projections of aromatase expressing neurons is largely independent of testosterone signaling through AR.
Figure 3. Masculinization of aromatase expressing neurons is independent of AR.
(A–J) Coronal sections through the adult brain of males bearing the aromatase-IPIN allele and a WT or a loss-of-function allele (tfm) of AR stained for βgal (A–F) or PLAP activity (G–J). (K) There is no significant difference in the number of βgal+ cells or in the volume occupied by PLAP+ fibers between WT and Tfm males. Mean ± SEM; n ≥ 3; p ≥ 0.26. Scale bars equal 500 μm (A, B) and 250 μm (C–J).
Estrogen masculinizes aromatase expressing neurons
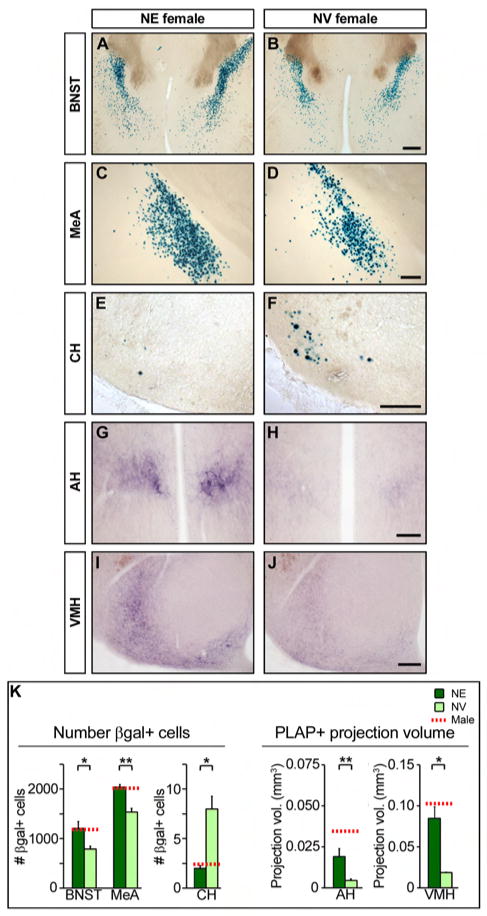
We next sought to determine the influence of estrogen in establishing the sexual dimorphisms in aromatase positive neurons. The complexity and redundancy within estrogen signaling pathways makes it difficult to test the relevance of individual ERs in establishing dimorphisms in aromatase expression. We therefore asked if estrogen is sufficient to masculinize aromatase expression in females. As estrogen influences sexual differentiation of neural pathways in the early neonatal period in male rodents (McCarthy, 2008), we treated female pups bearing the aromatase-IPIN allele with estrogen at P1 (postnatal day 1, the day of birth), P8, and P15. A cohort of control females was administered vehicle (NV) at these timepoints. The dosage and injection schedule were chosen as being the most effective at masculinizing aromatase positive neurons. We find that in females administered estrogen neonatally (NE), aromatase expressing neural pathways appear indistinguishable from those observed in WT males (Figure 4). These data demonstrate that neonatally administered estrogen can masculinize both the cell number and projection patterns of aromatase expressing neurons. Our results show that the masculinization of aromatase positive neurons is independent of AR (Figure 3) and can be induced in females by neonatal estrogen (Figure 4). In neonates, the ovaries are quiescent whereas the testes generate a surge in circulating testosterone immediately after birth (McCarthy, 2008). We therefore hypothesized that aromatization of testosterone into estrogen masculinizes aromatase positive neurons during this period. To test this hypothesis, we provided testosterone to neonatal females. We find that testosterone administration is equivalent to estrogen supplementation in masculinizing aromatase expressing neural pathways (Figure S6A), suggesting that testosterone induces male differentiation of these neurons after its conversion into estrogen in vivo.
Figure 4. Estrogen masculinizes aromatase expressing neural pathways.
(A–J) Coronal sections through the brain of adult NE and NV females bearing the aromatase-IPIN allele stained for βgal (A–F) or PLAP activity (G–J). (K) Increase in βgal+ cell number and volume occupied by PLAP+ fibers in NE females. Horizontal dashed lines represent the mean values in males as determined from the data in Figures 2 and 3. Mean ± SEM; n ≥ 3; * p ≤ 0.028, ** p ≤ 0.003; these p values were obtained for the comparisons between NE and NV females. Scale bars equal 500 μm (A, B) and 250 μm (C–J).
Estrogen promotes cell survival in the neonatal BNST and MeA
We wished to uncover the mechanism whereby estrogen drives sexual differentiation of aromatase positive neurons. An equivalent number of cells expresses aromatase in both sexes in the BNST and MeA at P1, whereas there are more βgal positive cells in these regions in males compared to females at P14 (Figure 5A–E, and data not shown). Neurons in the BNST and MeA are born prenatally in rodents (al-Shamma and De Vries, 1996; Bayer, 1980), and we therefore asked if the sexual dimorphisms in these regions resulted from sex specific apoptosis. We labeled apoptotic nuclei in the neonatal BNST and MeA with the TUNEL assay. We find more TUNEL positive cells in the BNST in females compared to males at several timepoints from P1 -P10, a finding consistent with previous work (Gotsiridze et al., 2007). Moreover, we observe a similar increase in TUNEL positive cells in the MeA in females compared to males at these timepoints (Figure 5F–I). The most significant sex difference in cell death was observed at P4 in the MeA and P7 in the BNST, with a ~2 fold increase in apoptotic nuclei in females compared to males (Figure 5J). As the conditions for TUNEL preclude co-labeling for βgal, we immunolabeled sections through the BNST and MeA for βgal and effectors of apoptosis (using a cocktail of antibodies to activated Caspase-3, 9 and Apaf-1) in P7 mice bearing the aromatase-IPIN allele. In accord with results of the TUNEL assay, these effectors of apoptosis label significantly more cells in females compared to males in the BNST and MeA (≥2 fold in each region; n=3; p < 0.001). We find many apoptotic cells expressing βgal in both sexes, with significantly more double-labeled cells present in females compared to males (% apoptotic cells expressing βgal in BNST: females, 50 ± 1; males, 26 ± 2; n = 3; p < 0.001. % apoptotic cells expressing βgal in MeA: females, 54 ± 2; males, 35 ± 3; n = 3; p < 0.01).
Figure 5. Estrogen promotes cell survival in the neonatal BNST and MeA.
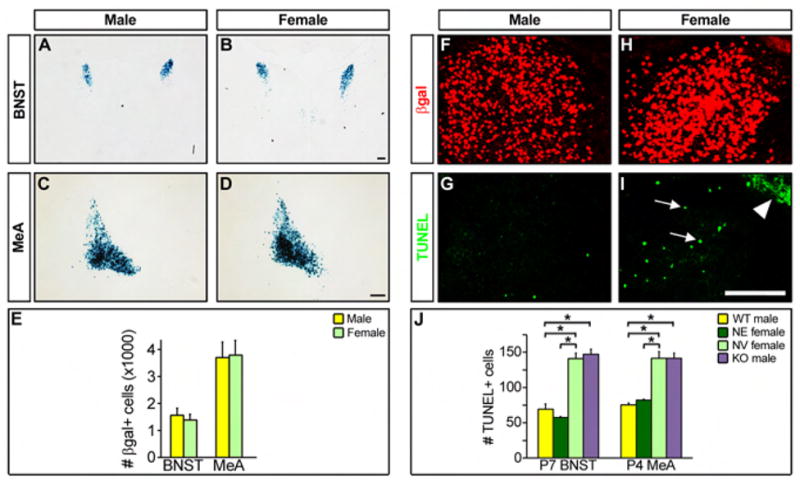
(A–D) Coronal sections through the brain of P1 male and female mice bearing the aromatase-IPIN allele stained for βgal activity. (E) No sex difference in the number of βgal+ cells at P1. Mean ± SEM; n = 3; p ≥ 0.63. (F–I) Adjacent coronal sections through the MeA of P4 male and female mice bearing the aromatase-IPIN allele immunolabeled for βgal or stained for apoptosis with TUNEL (arrows). Arrowhead (I) indicates autofluorescent material. (J) There are more apoptotic cells in the BNST and MeA in control (NV) females and aromatase−/− (KO) males compared to control (WT) males and estrogen treated (NE) females. Mean ± SEM; n = 3; * p ≤ 0.004. Scale bars equal 250 μm (A–D) and 100 μm (F–I).
As neonatally administered estrogen is sufficient to masculinize aromatase positive neurons in the female BNST and MeA, we asked whether such supplementation promoted the survival of cells fated to die in these regions. Neonatal estrogen treatment promotes cell survival such that the number of TUNEL positive nuclei in the BNST and MeA is indistinguishable between NE females and WT males (Figure 5J). Such cell survival promoting effects of estrogen are reminiscent of the known neuroprotective effects of this hormone (Arai et al., 1996). Is estrogen synthesis essential for cell survival in the BNST and MeA? To address this issue, we analyzed cell death in male mice null for aromatase (Honda et al., 1998). In these males, the number of apoptotic figures is comparable to that observed in WT females, and significantly different from WT males (Figure 5J). In summary, we observe more cell death in the BNST and MeA in females than in males, with an increase in the survival of aromatase expressing cells in males compared to females. Further, administration of estrogen to female pups reduces cell death whereas abrogation of estrogen synthesis increases apoptosis in males, demonstrating that estrogen is necessary and sufficient to promote cell survival in the BNST and MeA.
Neonatal estrogen exposure masculinizes territorial but not sexual behavior
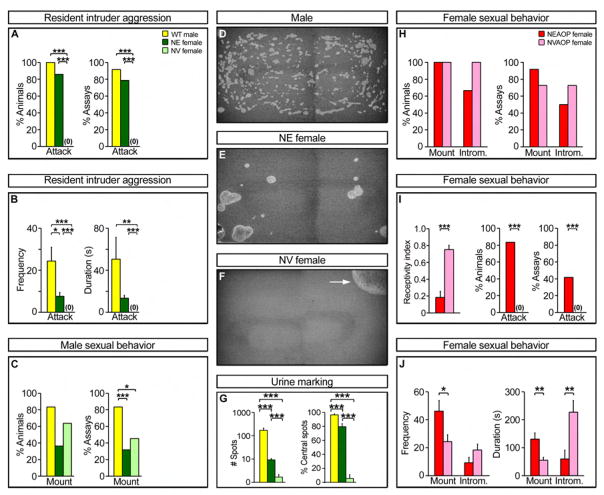
We tested adult NE females bearing the aromatase-IPIN allele to determine whether sex specific behaviors were also masculinized. Males reliably mate with females at high frequency whereas females exhibit male pattern sexual behavior at a low frequency towards females (Baum et al., 1974; Jyotika et al., 2007; Spors and Sobel, 2007). NE and NV females were individually housed as adults and presented with a WT female in estrus. Some resident females, regardless of hormone treatment, mated with the intruder, showing no apparent differences in mounting or pelvic thrusts, which are indicative of intromission (penetration) in males. In sharp contrast to resident males, both NE and NV females mated with intruders in fewer assays, consistent with the notion that neonatal estrogen does not alter the low frequency of male sexual behavior in females (Figure 6C) (Burns-Cusato et al., 2004; Vale et al., 1973).
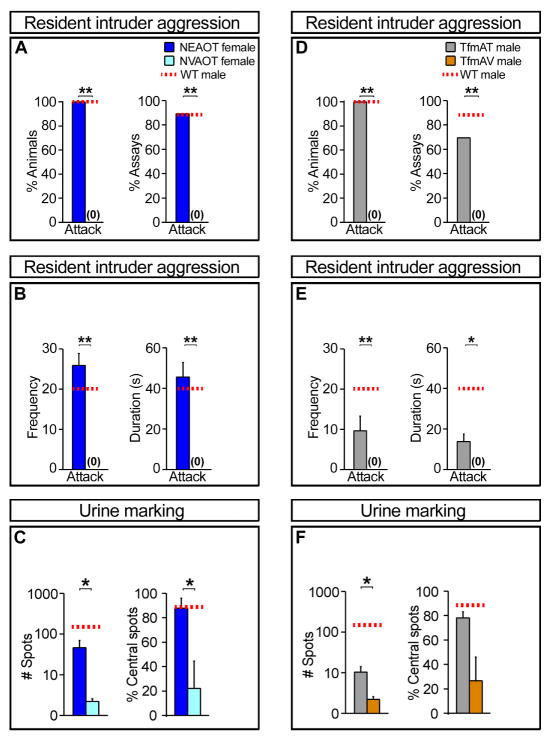
Figure 6. Estrogen masculinizes territorial but not sexual behavior.
(A, B) WT males and NE females attack intruders in resident intruder aggression tests. Resident males fight with greater frequency and for longer duration (B). (C) WT males and NE and NV females exhibit male sexual behavior towards estrous intruders. Males mate in more assays than females. (D–G) WT males and NE females scatter many urine drops, the majority of which are located away from the cage perimeter (% central spots). NV females deposit urine in one or a few large pools (arrow) near cage walls. (H) WT males mount and intromit (Introm.) both NEAOP and NVAOP females equivalently (n ≥ 6; p ≥ 0.121). (I) NEAOP females have a low receptivity index and attack resident males in many assays. (J) Resident males mount NEAOP females more frequently and for a longer duration but intromit for a shorter duration, consistent with lowered sexual receptivity. Mean ± SEM; n ≥ 6; * p ≤ 0.037, ** p ≤ 0.022, *** p ≤ 0.009.
We next examined NE females for male territorial behaviors. Individually housed male but not female residents attack male intruders (resident intruder aggression test) (Miczek et al., 2001). In striking contrast to NV females who never displayed aggression, we find that, like males, most NE female residents attack a male intruder (Figure 6A; Movies S1, S2). Similar to male residents, NE females initiated bouts of biting, chasing, wrestling, and tumbling. The aggression displayed by NE animals is not a response to mating attempts by the male: in most assays (73%; n = 11 assays) the fighting preceded any mating attempt, and most individual attacks (93%; n = 90 events) were not preceded by sexual behavior. As part of territorial defense, resident male mice mark their territory by scattering many urine drops across the cage floor, whereas females pool their urine at the cage perimeter (Desjardins et al., 1973; Kimura and Hagiwara, 1985). NE females deposit urine in a pattern resembling that of males (Figure 6D–G). Unlike NV females who pool urine, NE mice scatter significantly more urine drops, with a large fraction of such drops deposited away from the perimeter. Because neonatal testosterone exposure mimicked neonatal estrogen exposure in masculinizing aromatase-expressing circuits, we wished to test if neonatal testosterone would also mimic the effects of neonatal estrogen on territorial behaviors. Our data show that neonatal testosterone and estrogen are equivalent in eliciting male territorial behaviors in females (Figure S6B-D). Taken together, these results suggest that neonatal testosterone masculinizes territorial marking and aggression, at least in part, after its aromatization into estrogen in vivo.
We next asked whether NE females are sexually receptive to males. The standard test of female mating involves surgical removal of the ovaries (ovariectomy) followed by estrogen and progesterone injections to induce estrus on the day of testing (Beach, 1976). Accordingly, we hormonally primed ovariectomized females treated neonatally with estrogen (NEAOP) or vehicle (NVAOP), and presented them to male residents. Male mice vigorously mount females, but only receptive females permit such mounts to proceed to intromission. Thus, one measure of female receptivity is the ratio of intromissions to the total number of mounts (receptivity index). There was a large reduction in the receptivity index and the duration of intromissions in NEAOP females compared to NVAOP controls (Figure 6I, J), consistent with the notion that neonatal estrogen defeminizes sexual receptivity (Whalen and Nadler, 1963). Unlike NVAOP mice, NEAOP females actively rejected mounts, often attacking and chasing the male (Figure 6I). This reduction in sexual behavior does not reflect a lack of interest from males as these residents attempted to mate with NEAOP or NVAOP females in most assays (Figure 6H). Moreover, the low receptivity index of NEAOP females resulted from an absolute increase in the number of mounts (Figure 6J), demonstrating the males’ interest in mating with these mice. Thus, neonatal estrogen treatment appears to permanently defeminize sexual receptivity, even under estrus-inducing hormonal conditions.
Gonadal hormones are required to activate the display of sex specific behaviors such as aggression in adults (Beeman, 1947; Goy and McEwen, 1980; Morris et al., 2004). However, we observe male type fighting and urine marking in NE females in the absence of adult supplements of sex steroids. We hypothesized that male type fighting and urine marking may be activated by ovarian hormones. We directly tested this by ovariectomizing a cohort of adult NE animals: such females did not fight (0/5 females attacked males) or scatter urine (5/5 females pooled urine), demonstrating that the masculinized brain in NE animals utilizes ovarian hormones to activate these male behaviors. In summary, NE females exhibit a dissociation of sex typical behaviors: they do not mate like females or males but they display masculinized patterns of aggression and urine marking in the presence of ovarian hormones.
Neonatal estrogen exposure masculinizes the behavioral response to testosterone independent of AR
There are significant quantitative differences in fighting and urine marking between NE females and WT males (Figure 6B, G). We hypothesized that such differences reflect the low levels of circulating testosterone in NE mice. Testosterone titers are equivalent between NE and NV females, and > 10 fold lower than male titers. By contrast, all three groups of animals have similar, low baseline levels of estrogen, with periodic elevations of estrogen in the females that presumably accompany estrus (Supplemental Text). We masculinized circulating testosterone levels by providing this hormone to adult, ovariectomized females that were treated neonatally with estrogen (NEAOT) or vehicle (NVAOT) (Serum testosterone: males, 5.8 ± 1.4 nM; NEAOT females, 8.6 ± 3 nM; NVAOT females, 9.1 ± 4.3 nM; n = 4; p > 0.42). In resident intruder aggression tests, we find that NEAOT but not NVAOT females attack male intruders (Figure 7A; Movies S3, S4). Moreover, the pattern, frequency, and duration of attacks were similar between NEAOT and male residents (Figure 7B). The number and pattern of urine marks were also indistinguishable between NEAOT females and males (Figure 7C). These findings demonstrate that neonatal estrogen exposure masculinizes the response to testosterone in adults, and that male typical levels of testosterone augment the degree of male territorial displays without substantially altering the nature of these behaviors.
Figure 7. Neonatal estrogen masculinizes the response to adult testosterone administration.
(A, B) NEAOT resident females attack intruder males equivalently to WT male residents. NVAOT females do not initiate attacks. (C) NEAOT females deposit many urine drops away from the cage perimeter, in a manner equivalent to WT males. (D, E) TfmAT residents attack intruder males in a manner similar to WT male residents. TfmAV residents do not initiate attacks. (F) TfmAT males deposit significantly more urine drops than TfmAV males. Horizontal dashed lines denote the mean values of these behavioral displays in WT males. Mean ± SEM; n ≥ 5; * p ≤ 0.031, ** p ≤ 0.002; these p values were obtained for the comparisons between NEAOT and NVAOT females, and TfmAT and TfmAV mutant males.
These data show that adult testosterone administration is sufficient to activate male territorial behaviors in NE females, consistent with a functional role for aromatase expressing neural pathways in these mice. It is possible, however, that neonatal estrogen treatment masculinizes AR positive pathways in the brain, which in turn respond to adult testosterone and activate male territorial displays. We therefore examined the behavioral response of adult AR mutant (Tfm) males to testosterone administration. At birth, AR mutants have normal titers of testosterone (Sato et al., 2004), thereby leading to the development of a male pattern of aromatase expressing neurons following local conversion into estrogen (Figure 3). However, these mutants subsequently develop testicular atrophy, resulting in extremely low levels of circulating testosterone in adult life (Sato et al., 2004). As adults, these mutants do not attack intruders and they pool urine at the cage perimeter (Ohno et al., 1974) (Scott Juntti and NMS, unpublished observations). We find that provision of testosterone to adult Tfm males (TfmAT) significantly increases the number of urine marks compared to mutants administered vehicle (TfmAV) (Figure 7F). The number of urine spots was lower compared to WT males, indicating that a male typical frequency of urine marking may require additional contributions from AR signaling. While TfmAT males deposited more urine spots in the cage center compared to TfmAV controls, this trend did not reach statistical significance, suggesting AR signaling is essential for this component of territorial marking. In contrast to this complex control of male urine marking, we find that TfmAT mice attack intruders in the resident intruder assay similar to WT residents. Indeed, all TfmAT males attacked the intruder in most assays, whereas none of the TfmAV residents initiated attacks (Figure 7D). Moreover, both the frequency and duration of the attacks were similar between WT and TfmAT males (Figure 7E). Taken together, these results provide evidence that testosterone elicits many components of male territorial behaviors in adult animals independent of AR.
DISCUSSION
The role of aromatase expressing neurons in controlling male behaviors
We have used genetic reporters to visualize aromatase expressing neural pathways. Our reporters reveal aromatase expression at cellular resolution in discrete pools in regions previously shown to express aromatase (Roselli et al., 1998; Wagner and Morrell, 1996). This small set of aromatase positive neurons is therefore likely to influence the diverse neural circuits that utilize estrogen signaling to control male behaviors. The sensitive nature of the reporters reveals previously unreported sex differences in the number and projections of aromatase positive neurons. Even within regions such as the BNST, only a subset of cells expresses aromatase, suggesting functional specialization within these large dimorphic neuronal pools (Hines et al., 1992; Morris et al., 2008; Shah et al., 2004). Indeed, most aromatase positive cells in the BNST (98% ± 0.5; n = 3) express AR, whereas only a subset of AR positive neurons co-labels with aromatase (35% ± 2; n = 3). The reciprocal connectivity between the aromatase expressing regions we have identified suggests the interesting possibility that aromatase positive neurons may form an interconnected network that regulates sexually dimorphic behaviors.
Aromatase expressing neurons are largely restricted to neural pathways implicated in sexual and aggressive behaviors. Our results show that aromatization of testosterone into estrogen plays an important role in activating male territorial behaviors. However, the behavioral relevance of the dimorphisms we observe in aromatase positive neurons remains to be determined. These neurons may serve as a dimorphic neuroendocrine source of estrogen or they may directly participate in the circuits that control male behaviors. It is unlikely that such dimorphisms are required solely to provide a dimorphic source of local estrogen as NE females exhibit male territorial displays in response to circulating ovarian hormones. Consistent with the notion that these cells may function within neural circuits that mediate dimorphic behaviors, many aromatase positive neurons also express ERα in the BNST and MeA (Figure S4 and data not shown). It will be important, in future studies, to understand the functional significance of the sex differences in cell number and connectivity that we have identified in this study.
Masculinization of aromatase positive neural pathways and territorial behavior is governed by aromatase
We find that neonatal estrogen exposure masculinizes aromatase expressing neurons and territorial behaviors in females. These results seem counter-intuitive as one would expect that estrogen produced by the neonatal ovaries should induce male typical differentiation in all WT females. In fact, the ovaries are quiescent in neonates (McCarthy, 2008). By contrast, males experience a neonatal surge in circulating testosterone, leading to a corresponding increase in estrogen in the brain via local aromatization (Amateau et al., 2004). Our provision of estrogen to female pups therefore exposes their brains to this hormone during a period when only males would experience a local rise in estrogen. Such plasticity of the female brain to the masculinizing effects of estrogen is transient as adult females do not have a male pattern of aromatase expression despite the spikes in estrogen within the ~4 day estrous cycle. Indeed, we find that estrogen administration to adult WT females does not masculinize aromatase positive neurons and behavior (Figure S5 and data not shown). In order to determine whether testosterone exposure could masculinize aromatase positive neural pathways and territorial behavior, we provided testosterone to neonatal females. We find that this manipulation masculinizes aromatase expressing neurons and territorial behaviors to levels similar to those observed in NE females (Figures 4, S6). This finding suggests that the masculinizing effects of neonatal estrogen treatment are unlikely to be a gain-of-function of estrogen signaling, but rather reflect, at least in part, the physiological conversion of testosterone into estrogen by aromatase. Such locally derived estrogen may also influence the differentiation of other, aromatase negative, neuronal pools, which may play an important role in the subsequent display of sex typical behaviors.
Our results do not exclude the possibility that androgen and estrogen signaling masculinize aromatase expressing neurons in a redundant manner. Previous work in rats indicates that testosterone signaling can upregulate aromatase activity (Roselli et al., 1987). These biochemical studies are not incompatible with our data as the expression of βgal and PLAP report cell number and fiber projections of neurons expressing aromatase and not aromatase activity. Nevertheless, we find that restoring circulating testosterone titers to WT male levels in AR mutants is sufficient to elicit male typical aggression and some components of urine marking. Taken together, our results demonstrate that estrogen synthesis in neonatal and adult life is sufficient to masculinize aromatase expressing neurons and territorial behaviors independent of AR.
The cellular mechanism of estrogen action
The dimorphic projections in the anterior hypothalamus and VMH could arise from sex specific neurite outgrowth or retraction. However, both regions contain aromatase expressing neurons in the postnatal period, making it difficult to distinguish the sexually dimorphic fibers from the processes of local neurons. The small number of aromatase expressing cells in the caudal hypothalamus makes it difficult to elucidate the mechanism underlying the sex difference in this region. Increased survival of aromatase positive cells in the neonatal male BNST and MeA likely accounts for the dimorphism in cell number in these regions. Moreover, estrogen is necessary and sufficient to promote such cell survival in vivo. Most aromatase expressing cells in the neonatal BNST and MeA also express ERα (Figure S4). Both regions also express ERβ (Figure S4), suggesting that estrogen may mediate cell survival of aromatase positive cells by signaling through one or more classes of receptors in a cell autonomous manner. Irrespective of the mechanism underlying cell survival, our study demonstrates that estrogen ultimately acts on the very cells that synthesize this hormone to promote their sexual differentiation in a positive feedback manner.
Separable components of gender related behaviors
Several research groups have recently provided insight into the molecular mechanisms underlying sex specific behaviors in fruitflies (Manoli et al., 2005; Stockinger et al., 2005; Vrontou et al., 2006). These studies show that the repertoire of sexually dimorphic displays in Drosophila appears to be regulated in an unitary manner by Fruitless, a putative transcription factor. In contrast to flies, and similar to humans (Byne, 2006; Hines, 2006), we find that dimorphic behaviors can be dissociated in mice: neonatal estrogen exposure masculinizes territorial but not sexual behaviors. The male typical fighting displayed by females treated neonatally with estrogen is unlikely to result from altered gender discrimination as these mice direct their aggression, like WT males, exclusively towards male intruders. What controls male sexual behavior? Previous work has demonstrated that testosterone supplementation to adult female mice is sufficient to elicit male mating behavior (Edwards and Burge, 1971). Consistent with these studies, we find that the majority of NEAOT and NVAOT residents exhibit male sexual behavior towards estrus intruders (5/5 NEAOT and 4/5 NVAOT females mated with estrous mice; p = 0.29, Chi-squared test). Such mating attempts were displayed in ≥ 70% of assays by both cohorts of females, a frequency that is comparable to WT males. These findings further underscore the notion that the neural circuits that mediate sexual and territorial behaviors are regulated by distinct hormonal and temporal mechanisms.
Previous work has demonstrated that females treated neonatally with estrogen fight with males in resident intruder assays (Simon et al., 1984). Such studies coupled neonatal and adult hormonal interventions, making it difficult to understand the long term behavioral consequences of neonatal estrogen exposure. We find that females treated solely with neonatal estrogen display masculinized patterns of fighting and urine marking in the presence of sex hormones produced by the ovaries. Administration of testosterone to these females to mimic normal male circulating titers of this hormone increases these behavioral displays to approximate the levels observed in males. These results indicate that the adult hormonal profile produced by the testes may not be instructive for male territorial behaviors: hormones produced by the adult gonads of either sex support male patterns of fighting and territorial marking provided that neonatal estrogen has masculinized the underlying neural circuits.
Marking behavior defines a range within which the animal will defend resources and advertise its social and reproductive status (Ralls, 1971). Sex differences in territorial marking appear to be innate and mice display dimorphic urine marking patterns even in social isolation (Desjardins et al., 1973; Kimura and Hagiwara, 1985), providing an objective assessment of what appears to be an internal representation of sexual differentiation of the brain. Females treated neonatally with estrogen fight and mark territory like males, demonstrating masculinization of social and solitary sex typical behaviors.
Circulating testosterone and locally derived estrogen in the brain are critical for the expression of male behaviors. It has been difficult to determine the individual contributions of these two hormones to masculinization of the brain and behavior. Our gene targeting strategy has allowed us to identify at cellular resolution the small population of aromatase expressing neurons that can synthesize estrogen from testosterone. Testosterone appears to serve, at least in part, as a pro-hormone for estrogen for the male typical differentiation of aromatase positive neurons and for masculinization of territorial behaviors. The genetic marking of this discrete set of aromatase expressing neural pathways should ultimately permit us to functionally link them with distinct sex specific behavioral outcomes.
EXPERIMENTAL PROCEDURES
Generation of mice bearing a modified aromatase allele
The IRES-PLAP-IRES-nLacZ reporter was inserted into the 3′ UTR of the aromatase locus using previously described strategies (Supplemental Methods) (Shah et al., 2004). All experiments involving animals were in accordance with IACUC protocols at UCSF.
Hormone supplementation
We injected steroid hormones into all females within a litter to test the effects of these steroids on neuronal differentiation and behavior. We injected pups subcutaneously with 5 μg of 17β-estradiol benzoate (EB) (Sigma) or 100 μg of testosterone propionate (Sigma) dissolved in 50 μL of sterile sesame oil (Sigma) at P1, P8, and P15. Control females in other litters were injected with 50 μL vehicle at the same timepoints.
To generate NEAOT and NVAOT mice, we ovariectomized adult NE or NV females and allowed them to recover for three weeks. We injected 100 μg of testosterone propionate (TP) dissolved in 50 μL sesame oil subcutaneously on alternate days in these animals. Such animals were used for behavioral testing ≥ 3 weeks after initiating TP injections. The same injection regimen was used to generate TfmAT and TfmAV males. Hormone titers were assayed with kits from Cayman Chemicals (estradiol) and DRG International (testosterone).
Histology
Sexually naive, group housed, age matched mice were used in all histological studies. PLAP or βgal activity was visualized in 80 μm (adult) or 12 μm (neonate) thick brain sections obtained from mice homozygous for the aromatase-IPIN allele. Immunolabeling was performed on 65 μm (adult) or 20 μm (neonate) thick brain sections obtained from mice heterozygous for the aromatase-IPIN allele. We used previously described protocols to process these sections for histochemistry or immunolabeling (Shah et al., 2004). Message for aromatase, ERα and ERβ was localized by ISH as described in the Supplemental Methods. Sections of 16 μm thickness were processed for TUNEL according to the manufacturer’s instructions (Chemicon). In these studies, we processed in parallel at least one animal of each sex or experimental manipulation (WT and Tfm males; NE and NV females; NT and NV females) bearing the aromatase-IPIN allele and one control animal with an unmodified aromatase allele. Quantitation of cell numbers and fiber innervation was performed using unbiased stereology and other approaches (Supplemental Methods). All histological analysis was performed by an investigator blind to sex, age, genotype, and hormone treatment.
Behavioral Assays
We used 10 – 24 week old singly housed mice in behavioral tests, which were done ≥ 1 hour after lights were switched off. Mice were first tested for male sexual behavior in their home cage in a 30 minute assay with an estrous female. The residents were subsequently tested for territorial marking. Mice were allowed to explore a fresh cage lined with Whatman filter paper for one hour, and then returned to their homecage. The marking pattern was visualized with UV transillumination. Residents were subsequently tested for aggression directed towards a WT intruder male for 15 minutes. NE and NV females were then ovariectomized and tested for female sexual behavior after estrus induction in the homecage of a sexually experienced male for 30 minutes. Each animal was tested twice for sexual behavior and aggression, allowing us to analyze the total fraction of assays in which these behaviors were observed. We always exposed the experimental animals to mice they had previously not encountered, and individual assays were separated by ≥ 2–3 days. A separate cohort of NE and NV females was used to generate NEAOT and NVAOT mice. All tests were scored by an experimenter blind to the sex, genotype, and hormone treatment of mice, using a software package we developed in Matlab.
Statistical Analysis
We used the Chi-squared test to determine whether the proportion of experimental animals exhibiting a particular behavior was significantly different from control subjects. All other experimental comparisons were analyzed using both parametric (Student’s t test) and non-parametric (Kolmogorov-Smirnov, ks-test) tests of significance. All statistically significant results presented in the text (p < 0.05) using the Student’s t test were also determined to be statistically significant with the ks-test.
Supplementary Material
Acknowledgments
We thank C. Barberini for software programming; C. Cheung for technical assistance with ISH; D. Lubahn for providing us with aromatase–/+ animals; V. Mandiyan, L. Crothers, and C. Carey for technical assistance; P. Ohara for assistance with stereology; and R. Axel, T. Clandinin, H. Ingraham, D. Julius, S. Lomvardas, and Shah lab members for critical discussions and comments on the manuscript. This work was supported by Genentech Graduate Fellowships (MVW, JKC); National Science Foundation Graduate Research Fellowship (EJF); Achievement Rewards for College Scientists Scholarship (JKC); National Institutes of Health Institutional Training Grant (JT); National Institutes of Health (R01), Career Awards in the Biomedical Sciences, UCSF Program for Breakthrough Biomedical Research, Edward Mallinckrodt, Jr. Foundation, and McKnight Foundation for Neuroscience (NMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Shamma HA, De Vries GJ. Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. J Neurobiol. 1996;29:91–98. doi: 10.1002/(SICI)1097-4695(199601)29:1<91::AID-NEU7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: a comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Sodersten P, Vreeburg JT. Mounting and receptive behavior in the ovariectomized female rat: influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm Behav. 1974;5:175–190. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Beeman EA. The effect of male hormone on aggressive behavior in mice. Physiol Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Burns-Cusato M, Scordalakes EM, Rissman EF. Of mice and missing data: what we know (and need to learn) about male sexual behavior. Physiol Behav. 2004;83:217–232. doi: 10.1016/j.physbeh.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Byne W. Developmental endocrine influences on gender identity: implications for management of disorders of sex development. Mt Sinai J Med. 2006;73:950–959. [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- Finney HC, Erpino MJ. Synergistic effect of estradiol benzoate and dihydrotestosterone on aggression in mice. Horm Behav. 1976;7:391–400. doi: 10.1016/0018-506x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol. 2007;67:355–362. doi: 10.1002/dneu.20353. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. Cambridge, MA: MIT Press; 1980. [Google Scholar]
- Harada N, Yamada K. Ontogeny of aromatase messenger ribonucleic acid in mouse brain: fluorometrical quantitation by polymerase chain reaction. Endocrinology. 1992;131:2306–2312. doi: 10.1210/endo.131.5.1425429. [DOI] [PubMed] [Google Scholar]
- Hines M. Prenatal testosterone and gender-related behaviour. Eur J Endocrinol . 2006;155(Suppl 1):S115–121. doi: 10.1530/eje.1.02236. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviors. Horm Behav. 2008;53:627–637. doi: 10.1016/j.yhbeh.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: Effects of sex steroids. Horm Behav. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1998;91:215–222. [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Development of sexual dimorphism in synaptic organization in the ventromedial nucleus of the hypothalamus in rats. Neurosci Lett. 1986;68:165–168. doi: 10.1016/0304-3940(86)90135-7. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. Certain ventromedial hypothalamic afferents. Brain Res. 1973;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ. The metabolism of androgens in central neuroendocrine tissues. J Steroid Biochem. 1975;6:993–997. doi: 10.1016/0022-4731(75)90340-4. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci U S A. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Choleris E, Pfaff D. Genetic influences on aggressive behaviors and arousability in animals. Ann N Y Acad Sci. 2004;1036:257–266. doi: 10.1196/annals.1330.016. [DOI] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV. TfM mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3:235–242. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Bronson FH, Whitsett JM. Neonatal castration and intermale aggression in mice. Physiol Behav. 1972;8:265–268. doi: 10.1016/0031-9384(72)90371-x. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinol. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simon NG, Gandelman R, Gray JL. Endocrine induction of intermale aggression in mice: a comparison of hormonal regimens and their relationship to naturally occurring behavior. Physiol Behav. 1984;33:379–383. doi: 10.1016/0031-9384(84)90157-4. [DOI] [PubMed] [Google Scholar]
- Spors H, Sobel N. Male behavior by knockout. Neuron. 2007;55:689–693. doi: 10.1016/j.neuron.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Vale JR, Ray D, Vale CA. The interaction of genotype and exogenous neonatal androgen and estrogen: sex behavior in female mice. Dev Psychobiol. 1973;6:319–327. doi: 10.1002/dev.420060405. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Poulain P. Projections of the mediolateral part of the lateral septum to the hypothalamus, revealed by Fos expression and axonal tracing in rats. Anat Embryol (Berl) 1999;199:249–263. doi: 10.1007/s004290050226. [DOI] [PubMed] [Google Scholar]
- Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Maintenance of male sexual behavior by combined treatment with oestrogen and dihydrotestosterone in CD-1 mice. J Endocrinol. 1975;66:257–262. doi: 10.1677/joe.0.0660257. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Nadler RD. Suppression of the development of female mating behavior by estrogen administered in infancy. Science. 1963;141:273–274. doi: 10.1126/science.141.3577.273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.