In postindustrial societies, overeating, inactivity, and obesity have emerged as new challenges in public health.1,2 Considerable effort is now being devoted to determining the pathophysiologic consequences of overeating. Several lines of evidence suggest that caloric intake influences the rate of aging and the onset of associated diseases in animals and, possibly, humans.3–5
The observation that laboratory rats not only live longer but also have fewer age-associated diseases when their food intake is restricted dates back to the 1930s.3–7 Numerous subsequent studies have found that when the ad libitum food intake of mice and rats was reduced by 30 to 60 percent, the average life span and the maximal life span (the mean survival of the longest-lived decile) increased by similar amounts.3 In contrast, rats with nearly unrestricted caloric intake (92 percent of the average unrestricted intake) that were kept lean with exercise and weighed about 40 percent less than sedentary control rats with the same caloric intake had an increase in the average life span but not in the maximal life span.8 In all these studies, the life-extending benefits of caloric restriction depended on the prevention of malnutrition and a reduction in overall caloric intake rather than any particular nutrient.3–5
Because caloric restriction can markedly prolong the life span, it is being widely studied to determine the mechanisms of aging. An increasing body of evidence suggests that cumulative oxidative damage to macromolecules such as protein, lipids, and DNA has a major role in aging. Caloric restriction attenuates both the degree of oxidative damage and the associated decline in function.7 We will review evidence that caloric restriction prolongs life in laboratory animals, evokes an array of responses, including a decrease in oxidative stress and damage, and may retard the aging process in humans.
CALORIC INTAKE, LONGEVITY, AND DISEASE IN LABORATORY ANIMALS
In contrast to the average life span, which can be prolonged by improving environmental conditions, the maximal life span is thought to be increased by actually decreasing the rate of aging.9 The average life span of humans has increased markedly since prehistoric times as a result of gains in public health and health care, whereas the maximal life span has remained largely unchanged.9
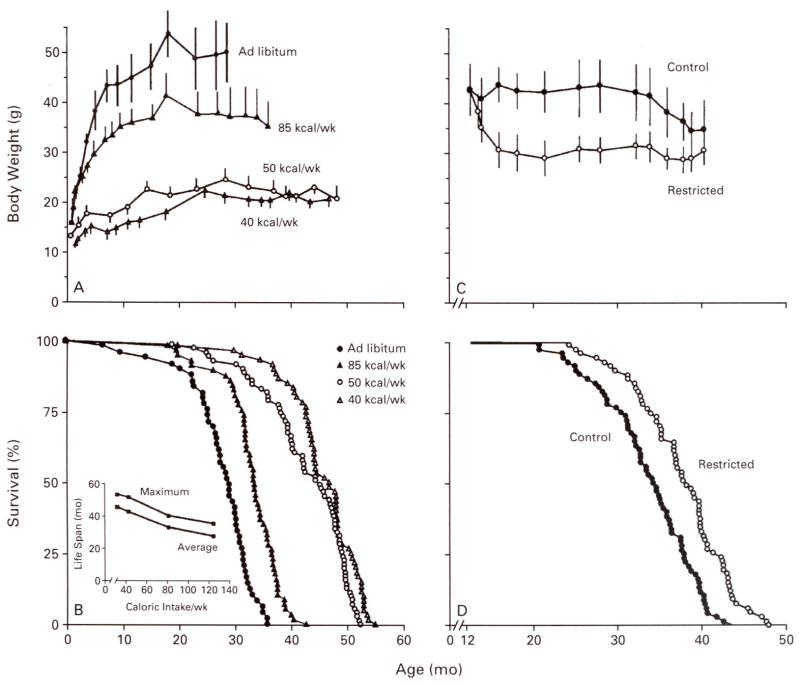
Figure 1A and 1B shows the inverse linear relation between caloric intake and life span in mice.10 Among groups of mice fed different amounts of calories starting at one month of age, the degree of caloric restriction was directly related to the reduction in body weight and the increase in average and maximal life spans. When caloric intake was restricted in middle-aged mice, the life span was also extended, albeit to a lesser degree (Fig. 1C and 1D),11 contravening the hypothesis that caloric restriction extends the life span by prolonging the developmental period.
Figure 1. Effect of Caloric Restriction, Initiated at 1 Month or 12 Months of Age, on Body Weight and Life Span in Mice.
Panels A and B show data from female C3B10F1 mice subjected to restricted caloric intake (40, 50, or 85 kcal per week) starting at one month of age.9 The maximal life span (inset, Panel B) is the mean survival for the longest-lived decile of each group. Panels C and D show data from male B10C3F1 mice subjected to restricted caloric intake (90 kcal per week) as compared with control mice (160 kcal per week), starting at 12 months of age.10 Caloric restriction at both ages extended both average and maximal life span. Each symbol in the survival curves (Panels B and D) represents one mouse. The bars in Panels A and C represent standard deviations. (Data in panels A and B are from Weindruch et al.10; data in panels C and D are from Weindruch and Walford.11)
In a study in which the body weight of genetically obese (ob/ob) C57BL/6J mice was kept at a normal level (approximately 35 g) by caloric restriction, the maximal life span of the animals increased by about 50 percent, despite the fact that their body fat (48 percent), although less than that in unmanipulated ob/ob mice (67 percent),12 was still more than twice that in genetically normal control mice (22 percent). The ob/ob mice with restricted caloric intake lived longer than the genetically normal controls and about as long as the genetically normal mice with restricted caloric intake and 13 percent body fat. In this study, the level of food consumption, not the degree of adiposity, was the key factor in prolonging life.
Caloric restriction also extends the life span in species as diverse as protozoans, water fleas, spiders, and guppies.3 In chickens, ad libitum feeding increases the incidence of diseases and reduces the life span.13 These studies in animals indicate that caloric intake above an optimal level shortens the life span.
In laboratory rodents, caloric restriction delays the onset of age-associated diseases such as cancer (including lymphomas and breast and prostate cancers), nephropathy, and cataracts.3–5 The onset of diabetes, hypertension, and hyperlipidemia is also delayed in rodents with restricted caloric intake.3 Caloric restriction virtually prevents the development of autoimmune diseases in several susceptible strains of mice. For example, in a strain susceptible to a lupus-like nephropathy, animals fed ad libitum contract the disease and usually die at around 12 months of age, whereas those whose caloric intake is restricted are much less likely to contract the disease, and they live for about 20 months.14
Some responses to caloric restriction are quite rapid. For example, in rats, blood glucose concentrations drop by approximately 20 percent after only five days of restricted caloric intake, and plasma insulin concentrations decrease by approximately 50 percent after three weeks.15 The steady-state concentrations of carbonyl (a marker of oxidative damage to proteins) in the brains of mice fed calorically restricted diets for one year were low but increased within three to six weeks after the introduction of an ad libitum regimen.16 The acute changes in carbonyl content generally paralleled changes in body weight after the switch in feeding regimens. Such findings indicate that the effects of caloric restriction can occur quickly but may also diminish rapidly when the restriction ends. Thus, caloric restriction may have to be sustained to be beneficial.
These studies of caloric restriction in rodents have prompted similar investigations in nonhuman primates.17–20 The outcome of this research in terms of longevity will not be known for several more years, because monkeys have relatively long life spans (approximately 40 years for rhesus monkeys, the most widely used model), and the studies were begun fairly recently. However, preliminary data suggest that the physiologic changes in monkeys in response to caloric restriction are similar to those in rodents: circulating insulin and glucose concentrations are decreased, insulin sensitivity improves, and body temperature is lowered.21,22 However, even if the long-term results of these studies show that caloric restriction prolongs life in primates, additional research will be needed before the findings can be applied to humans. In particular, it will be important to determine the fitness of rodents and primates with restricted caloric intake in order to assess their responses to experimentally induced stressors such as infection, hypothermia and hyperthermia, dehydration, and vigorous exercise.
THE ROLE OF OXIDATIVE STRESS
A major challenge is to identify the specific mechanism or mechanisms that initiate the multitude of deleterious changes characteristic of aging and to determine how caloric restriction affects these processes. Caloric restriction depresses the rate of energy metabolism, which decreases body temperature. When housed at room temperature (20 to 22°C), mice with restricted caloric intake have body temperatures that cycle from about 37°C to 23°C to 27°C daily.23 Body temperature also drops in rats with restricted caloric intake, although to a lesser extent,24 and in feral rodents when food is scarce.25 A depression in body temperature indicates a reduction in the rate of oxygen consumption.26 The hypometabolic state in animals with restricted caloric intake is reflected by the approximately 50 percent decrease in serum triiodothyronine concentrations.3–5 Additional factors, such as brown-fat metabolism and the activity of the sympathetic nervous system, are also likely to be involved in the decreased metabolic rate associated with caloric restriction in animals, although the role of these influences has not yet been specifically demonstrated.
Early in this century, Rubner27 postulated an inverse relation between metabolic rate and life span on the basis of a comparison among mammalian species. Later studies in cold-blooded animals, in which the rate of metabolism can be manipulated by altering the ambient temperature, confirmed the inverse relation between metabolic rate and longevity. These findings were the basis of Pearl’s “rate of living” theory of aging.28 More recently, the rate of living (or metabolic rate) has been linked to the rate of production of partially reduced oxygen species,7,29 which are normal byproducts of oxygen metabolism.30
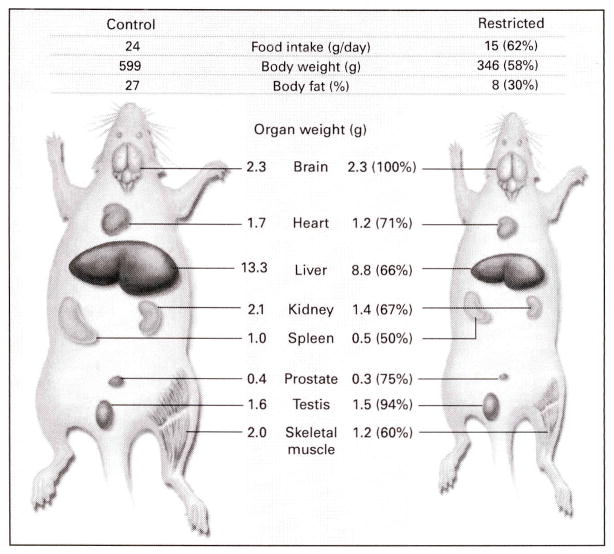
Two studies31–32 but not a third33 found that the metabolic rate, usually expressed as oxygen consumption per unit of lean body mass, was depressed in rats and nonhuman primates after long-term caloric restriction. It is important to note that body composition and organ weights in animals with restricted caloric intake differ from those in control animals. Rats with restricted caloric intake generally have 70 percent less body fat than controls and a proportionally smaller heart, liver, kidney, prostate, spleen, and mass of skeletal muscle,3 whereas the weights of the brain and testes are similar to those in controls (Fig. 2). Caloric restriction also greatly reduces weights and cell numbers in lymphoid tissues.3 Because rates of oxygen consumption differ among organs, metabolic rates of control and caloric restriction based on total lean body mass may obscure organ-specific variations. Differences in the metabolic fuels used by control animals and those with restricted caloric intake also affect the amount of oxygen needed for energy metabolism. For example, a shift toward protein catabolism in mice with restricted caloric intake is indicated by the fivefold increase in the activity of hepatic carbamyl phosphate synthetase I.34 This mitochondrial enzyme, which has a rate-limiting role in the biosynthesis of urea, catalyzes the condensation of ammonia and bicarbonate, produced during protein catabolism, to carbamyl phosphate.
Figure 2. Effect of Caloric Restriction on Body Composition and Organ Weights in Rats.
Data are from studies of 24-month-old male Sprague–Dawley rats either fed a control diet (80 percent of the average ad libitum intake) or subjected to caloric restriction (approximately 50 percent of the ad libitum intake) starting at 1 month of age. The numbers in parentheses are percentages of control values. The reduction in the weights of organs with caloric restriction varies widely. (Data kindly provided by Dr. Kevin Keenan, Merck, West Point, Pa.)
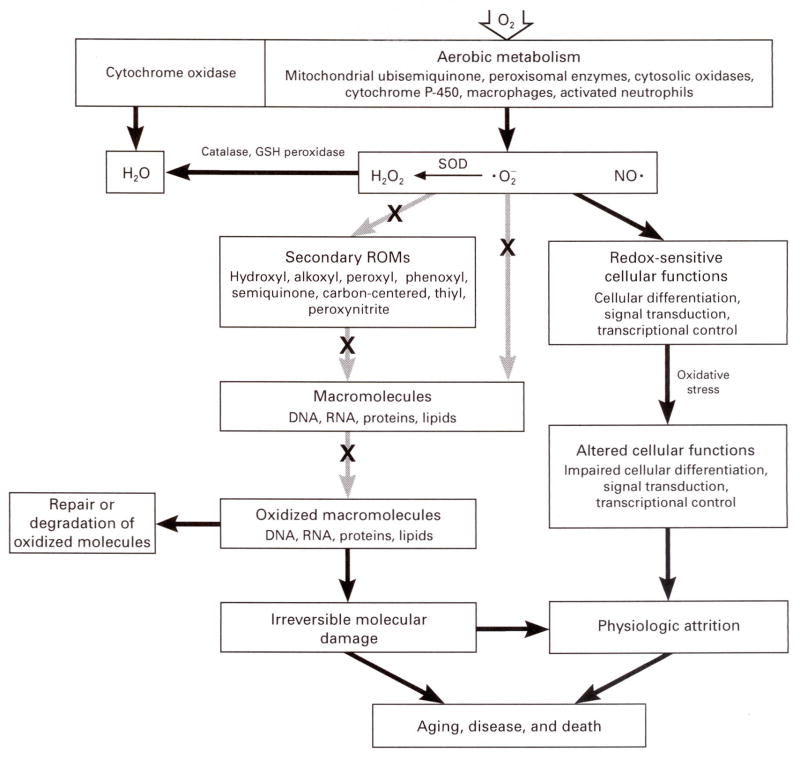
The link between oxygen consumption and aging is now widely believed to involve reactive oxygen metabolites, the byproducts of oxygen metabolism (Fig. 3). Approximately 2 to 3 percent of oxygen used by cells is chemically reduced by additions of single electrons, which sequentially generate superoxide anion ( ) and hydrogen peroxide. The latter readily permeates cellular membranes and can enter virtually all cellular compartments.30 The scission of hydrogen peroxide, catalyzed by free or loosely bound ferrous cation (Fe2+) or another reduced transition metal, forms one of the most reactive molecules known, the hydroxyl free radical (·OH). This moiety is believed to be the predominant agent of damage to macromolecules such as proteins, DNA, and lipids.7,35,36 Oxidative damage to these molecules appears to be site-specific and dependent on hydrogen peroxide and a reduced transition metal. Hence, macromolecules containing transition metals, such as aconitase, may be prime targets of oxidative damage.37 Although cells contain an elaborate network of antioxidative defenses, reactive oxygen metabolites are detectable in them under normal conditions. Indeed, cells are probably under continuous oxidative stress because of an innate imbalance between the generation and inactivation of reactive oxygen metabolites.
Figure 3. Cumulative Oxidative Damage during Aging.
Reactive oxygen metabolites (ROMs) such as superoxide anion ( ), hydrogen peroxide (H2O2), and nitric oxide (NO·), are generated at several intracellular sites and either are inactivated by antioxidative defenses or give rise to a constellation of secondary ROMs. Interactions with ROMs may cause oxidative modifications in macromolecules, some of which may be repaired. The age-related accumulation of unrepaired, irreversible oxidative damage may be a causal factor in aging and certain diseases. ROMs have also been linked to differentiation and signal transduction. An X indicates the interdiction by nonenzymatic antioxidant defense mechanisms (e.g., glutathione [GSH], vitamin E, vitamin C, and urate). SOD denotes superoxide dismutase.
Reactive oxygen metabolites can cause enzymes to lose activity, induce mutations, and damage cell membranes.7,35,36 However, they also have useful functions, such as modulation of the cellular redox state, signal transduction, activation of gene-transcription factors, and apoptosis.38,39 Shifts in the level of oxidative stress probably affect all these cellular functions.
It is widely postulated that damage to macromolecules from reactive oxygen metabolites is a major cause of senescence.7,37,40 The irreversible decline of all organisms in the latter part of life may reflect the accumulation of oxidative stress, and the magnitude of the stress may account for variations in life span among species and among individuals of the same species. The extension of the life span through caloric restriction, hibernation, and in cold-blooded animals, lower body temperature may be attributable to the attenuation of oxidative stress.
In a variety of species, concentrations of oxidatively damaged proteins, DNA, and lipids in tissues increase with age.7,29,37 Oxidative damage to proteins (indicated by carbonylation, loss of sulfhydryl groups, and selective decreases in enzymatic activity) increases with age in both mammalian and insect tissues. Oxidative damage to DNA, often determined by the measurement of 8-hydroxydeoxyguanosine, also increases with age. The primary reasons for the age-associated increase in oxidative damage appear to be increased rates of production of superoxide anion and hydrogen peroxide in mitochondria and, to a lesser extent, decreased efficiency of defenses against antioxidative damage.7,29 A direct link between oxidative stress and aging was found in studies with transgenic strains of the fruit fly Drosophila melanogaster that overexpress copper-zinc superoxide dismutase and catalase.41 These enzymes eliminate superoxide anion and hydrogen peroxide, and thus provide the first line of defense against oxidative damage. The transgenic flies had a lower rate of oxidative damage to DNA and proteins and lived up to 34 percent longer than control flies.
Comparisons among mammals and insects with wide variations in longevity indicate that the species with longer life spans generate superoxide anion and hydrogen peroxide at lower rates,29,42 accrue less oxidative damage,43,44 and resist experimentally induced oxidative stress.45 Birds, which have high metabolic rates but are nonetheless quite long-lived, generate mitochondrial superoxide anion and hydrogen peroxide at low rates and have relatively high activities of superoxide dismutase and glutathione peroxidase.46
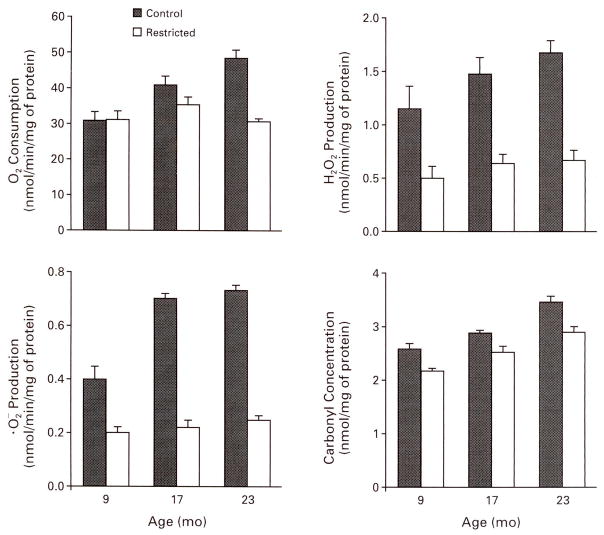
Caloric restriction decreases the steady-state concentrations of the products of oxidative damage to proteins, DNA, and lipids.47,48 This effect may involve decreased production of mitochondrial superoxide anion and hydrogen peroxide47 (Fig. 4) and increased antioxidative defenses.49 In mice, most of the age-associated increase in oxidative damage to DNA occurs in postmitotic tissues such as brain, heart, and skeletal muscle, and most of the attenuation of the damage by caloric restriction also occurs in these tissues.48 The functional consequences of oxidative damage to protein were demonstrated by a study in which the severity of oxidative damage in different regions of the brain in mice was correlated with age-related losses in cognitive and motor functions.50 Caloric restriction retarded these losses and lowered the level of oxidative damage to proteins in the pertinent brain regions.16
Figure 4. Effects of Age and Caloric Restriction on Rates of Mitochondrial Oxygen Consumption, Mitochondrial Superoxide Anion and Hydrogen Peroxide Production, and Concentrations of Protein Carbonyls in the Brain in Mice.
Data are for male C57BL/6NNia mice either fed ad libitum or subjected to a 40 percent reduction in caloric intake from four months of age. denotes superoxide anion, O2 oxygen, and H2O2 hydrogen peroxide. The bars represent standard errors. Data were adapted from Sohal et al.47
CALORIC INTAKE AND HEALTH
Our understanding of the consequences of long-term caloric restriction in nonobese people is relatively meager. Epidemiologic studies indicate that energy intake and the body-mass index (the weight in kilograms divided by the square of the height in meters) are directly related to mortality and the incidence of certain diseases, but these data suffer from the difficulty of assessing caloric intake in large populations.51
The relation between weight (body-mass index) and mortality is controversial. There are reports of no association, an inverse association, and a J-shaped or U-shaped curve (i.e., an association between the lowest and highest body-mass indexes and increased mortality). These discrepancies have been reviewed by Lee et al. for the Harvard Alumni Health Study52 and by Manson et al. for the Nurses’ Health Study.53 Both studies found that mortality from all causes was reduced in study participants with body-mass indexes that were 15 to 20 percent below the national average. These analyses accounted for cigarette smoking and illness-related weight loss. In both studies, the people in the group with the lowest body-mass index (less than 19.0 for women53 and less than 22.5 for men52) had about a 20 percent lower risk of death than those in the group with the next higher body-mass index. An investigation of the effect of a westernized diet on the health of Japanese people supports the association between caloric intake and health.54 In Okinawa, energy intake was found to be 17 percent lower in adults and 36 percent lower in children than the average energy intake in Japan, and death rates from cerebrovascular disease, cancer, and heart disease were 31 to 41 percent lower than the national average. A recent study in Sweden showed that a high body-mass index and high levels of total food consumption and energy intake were risk factors for prostate cancer.55,56 Epidemiologic data57 also suggest, although with some exceptions, that caloric intake is directly correlated with the incidence of colorectal, breast, and stomach cancers.
The physiologic responses of nonobese men to caloric restriction appear to resemble those of laboratory animals. In a study of middle-aged men in the Netherlands, a 20 percent reduction in the habitual caloric intake for 10 weeks resulted in a 10 percent loss of body weight, reductions in diastolic and systolic blood pressure, increases in serum high-density lipoprotein cholesterol concentrations,58 reductions in serum triiodothyronine concentrations and the metabolic rate,59 and beneficial effects on fibrinolytic factors.60 However, these men did not have the reduction in urinary excretion of 8-hydroxy-deoxyguanosine61 that an earlier study showed was closely associated with the metabolic rate in women of normal body weight.62
A rapidly expanding body of data implicates oxidative stress in the development of Parkinson’s disease, Alzheimer’s disease,65 heart failure,64 and other diseases. As noted in animal studies, organs such as the brain and heart, in which the parenchyma consists of postmitotic cells, are particularly susceptible to oxidative damage. Thus, oxidative stress and damage may be causal factors in the attrition of senescence and various diseases associated with aging, and caloric restriction may attenuate the damage.
DISCUSSION
Dr. Jeffrey S. Flier: What is the evidence that caloric restriction decreases mutation rates and increases apoptosis?
Dr. Weindruch: There is limited evidence that caloric restriction decreases mutation rates of specific genes. In mice with restricted caloric intake, as compared with control mice, mutations of the gene for hypoxanthine phosphoribosyltransferase in lymphocytes are reduced by a factor of three to four.65 Also, oxidative modifications to DNA (which can be mutagenic) accrue with aging at a slower rate in rodents with restricted caloric intake than in controls. Increases in apoptosis that appear to facilitate the elimination of preneoplastic cells have been reported in hepatocytes from rodents with restricted caloric intake.66,67
Dr. Franklin H. Epstein: Since most laboratory rats have an interstitial nephritis, can the prolongation of life through caloric restriction be separated from the effect of the progression of renal disease? Is this also a concern in studies with mice?
Dr. Weindruch: Each strain of rat and mouse used in research on aging has characteristic late-life diseases, and nephropathy is a major cause of death in some of these strains. For example, renal disease is very common in male Fischer 344 rats freely fed casein-based diets but is less common in those fed diets with soy as the main protein.68 Yet many other rat and mouse strains that are far less prone to renal disease have marked increases in life span in response to caloric restriction. In short, the retardation of disease by caloric restriction in rodent models is not confined to an effect on nephropathy but instead encompasses a broad spectrum of diseases.3
Dr. Epstein: How might a high-calorie diet increase the production of free radicals?
Dr. Weindruch: Several factors may be involved. A likely scenario is that the additional availability of nutrients (substrates) to mitochondria increases the rate of auto-oxidation of mitochondrial respiratory-chain components that generate superoxide anion.
A Physician: If caloric restriction is found to have the same benefits in humans as in the animal models you’ve discussed, do you think that people can actually be persuaded to reduce their caloric intake by, say, 30 percent? Just a few days on such a diet would probably be enough for most people.
Dr. Weindruch: Many people would probably have a difficult time reducing their food intake to this degree for an extended period. However, some people might be motivated to do so. For example, people from cancer-prone families or with a family history of early-onset degenerative diseases associated with aging might be suitable candidates for long-term dietary restriction. Also, progress in appetite control may make caloric restriction easier.
Dr. Flier: Caloric restriction lowers the secretion of the fat-derived hormone leptin. Could any of the apparent benefits of caloric restriction be a consequence of reduced leptin activity?
Dr. Weindruch: It is conceivable that decreased leptin activity contributes to the effects of caloric restriction because of the diverse physiologic processes that leptin may regulate.69 Decreases in energy expenditure and insulin secretion have been postulated to contribute to the effects of caloric restriction, and leptin is postulated to regulate both systems.
A Physician: Free-living mice and rats don’t show the effects of age because they don’t live long enough. In a laboratory setting, with caloric restriction, aren’t you just letting the animals achieve their ideal, maximal life span? The freely fed animal may actually die at a young age rather than at a normal age.
Dr. Weindruch: There is no need to invoke the freely fed animal as a standard. The data show that the more you restrict a mouse’s caloric intake, the longer it lives. I think that our ignorance about what goes on in the wild is unimportant if we recognize that animals continue to live longer up to the point at which caloric restriction becomes frank starvation.
Acknowledgments
Supported by grants from the National Institute on Aging, the National Center for Research Resources (Primate Research Center Program), and the American Cancer Society.
Contributor Information
Richard Weindruch, Department of Medicine and Veterans Affairs Geriatric Research, Education, and Clinical Center, University of Wisconsin, Madison;
Rajindar S. Sohal, Department of Biological Sciences, Southern Methodist University, Dallas
References
- 1.Bray GA. Obesity. In: Zicgler EE, Filer LJJ, editors. Present knowledge in nutrition. Washington, D.C: ILSI Press; 1996. pp. 19–32.1.1. [Google Scholar]
- 2.Gibbs WW. Gaining on fat. Sci Am. 1996;275:88–94. doi: 10.1038/scientificamerican0896-88. [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Ill: Charles C Thomas; 1988. [Google Scholar]
- 4.Fishbein L. Biological effects of dietary restriction. New York: Springer-Verlag; 1991. [Google Scholar]
- 5.Yu BP. Modulation of aging processes by dietary restriction. Boca Raton, Fla: CRC Press; 1994. [Google Scholar]
- 6.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 7.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloszy JO. Mortality rate and longevity of food restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 9.Comfort A. The biology of senescence. 3. New York: Elsevier; 1979. [Google Scholar]
- 10.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging by dietary restriction in mice: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 11.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–8. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–8. doi: 10.1073/pnas.81.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katanbaf MN, Dunnington EA, Siegel PB. Restricted feeding in early and late-feathering chickens. 1. Growth and physiological responses. Poultry Sci. 1989;68:344–51. doi: 10.3382/ps.0680344. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976;73:1279–83. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartee GD, Dean DJ. Glucose transport with brief dietary restriction: heterogenous responses in muscles. Am J Physiol. 1994;266:E946–E952. doi: 10.1152/ajpendo.1994.266.6.E946. [DOI] [PubMed] [Google Scholar]
- 16.Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–97. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 17.Ingram DK, Cutler RG, Weindruch R, et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- 18.Kemnitz JW, Weindruch R, Roccker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol A Biol Sci Med Sci. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- 19.Hansen BC, Ortmeyer HK, Bodkin NL. Prevention of obesity in middle-aged monkeys: food intake during body weight clamp. Obes Res. 1995;3(Suppl 2):199S–204S. doi: 10.1002/j.1550-8528.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 20.Cefalu WT, Wagner JD, Wang ZQ, et al. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): a potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52:B10–B19. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 21.Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 22.Lane MA, Baer DJ, Rumpler WV, et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–64. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koizumi A, Tsukada M, Wada Y, Masuda H, Weindruch R. Mitotic activity in mice is suppressed by energy restriction-induced torpor. J Nutr. 1992;122:1446–53. doi: 10.1093/jn/122.7.1446. [DOI] [PubMed] [Google Scholar]
- 24.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7:113–24. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- 25.Tucker VA. Oxygen consumption, thermal conductance, and torpor in the California pocket mouse Perognathus californicus. J Cell Physiol. 1965;65:393–403. doi: 10.1002/jcp.1030650313. [DOI] [PubMed] [Google Scholar]
- 26.Prosser CL. Temperature. In: Prosser CL, editor. Comparative animal physiology. 3. Philadelphia: W.B. Saunders; 1973. pp. 362–428. [Google Scholar]
- 27.Rubner M. Das problem der lebensdauer und seine beziehungen zu wachstum und ernahrung. Munich, Germany: Oldenbourg; 1908. [Google Scholar]
- 28.Pearl R. The rate of living, being an account of some experimental studies on the biology of life duration. New York: Alfred A. Knopf; 1928. [Google Scholar]
- 29.Sohal RS, Orr WC. Is oxidative stress a causal factor in aging? In: Esser K, Martin GM, editors. Molecular aspects of aging. Chichester, England: John Wiley; 1995. pp. 109–27. [Google Scholar]
- 30.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 31.Dulloo AG, Girardier L. 24 hour energy expenditure several months after weight loss in the underfed rat: evidence for a chronic increase in whole-body metabolic efficiency. Int J Obes Relat Metab Disord. 1993;17:115–23. [PubMed] [Google Scholar]
- 32.Ramsey JJ, Roecker EB, Weindruch R, Kemnitz JW. Energy expenditure in adult male rhesus monkeys during the first 30 mo of dietary restriction. Am J Physiol. 1997;272:E901–E907. doi: 10.1152/ajpendo.1997.272.5.E901. [DOI] [PubMed] [Google Scholar]
- 33.McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985;248:E488–E490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 34.Tillman JB, Dhahbi JM, Mote PL, Walford RL, Spindler SR. Dietary calorie restriction in mice induces carbamyl phosphate synthetase I gene transcription tissue specifically. J Biol Chem. 1996;271:3500–6. doi: 10.1074/jbc.271.7.3500. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 36.McCord JM. Superoxide radical: controversies, contradictions, and paradoxes. Proc Soc Exp Biol Med. 1995;209:112–7. doi: 10.3181/00379727-209-43885c. [DOI] [PubMed] [Google Scholar]
- 37.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–85. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 39.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–20. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 40.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–30. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 42.Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621–7. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 43.Sohal RS, Ku H-H, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun. 1993;196:7–11. doi: 10.1006/bbrc.1993.2208. [DOI] [PubMed] [Google Scholar]
- 44.Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal S, Sohal RS. Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species. Exp Gerontol. 1996;31:365–72. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]
- 46.Ku H-H, Sohal RS. Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential. Mech Ageing Dev. 1993;72:67–76. doi: 10.1016/0047-6374(93)90132-b. [DOI] [PubMed] [Google Scholar]
- 47.Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–33. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 48.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–24. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 49.Rao G, Xia E, Nadakavukaren MJ, Richardson A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr. 1990;120:602–9. doi: 10.1093/jn/120.6.602. [DOI] [PubMed] [Google Scholar]
- 50.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–9. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huss-Ashmore R. Issues in the measurement of energy intake for free-living human populations. Am J Hum Biol. 1996;8:159–67. doi: 10.1002/(SICI)1520-6300(1996)8:2<159::AID-AJHB3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 52.Lee IM, Manson JE, Hennekens CH, Paffenbarger RS., Jr Body weight and mortality: a 27-year follow-up of middle-aged men. JAMA. 1993;270:2823–8. doi: 10.1001/jama.270.23.2823. [DOI] [PubMed] [Google Scholar]
- 53.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 54.Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–17. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- 55.Grönberg H, Damber L, Damber JE. Total food consumption and body mass index in relation to prostate cancer risk: a case-control study in Sweden with prospectively collected exposure data. J Urol. 1996;155:969–74. [PubMed] [Google Scholar]
- 56.Andersson S-O, Wolk A, Bergstrom R, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68:716–22. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 57.Albanes D. Energy balance, body size, and cancer. Crit Rev Oncol Hematol. 1990;10:283–303. doi: 10.1016/1040-8428(90)90036-r. [DOI] [PubMed] [Google Scholar]
- 58.Velthuis-te Wierik EJ, van den Berg H, Schaafsma G, Hendriks HE, Brouwer A. Energy restriction, a useful intervention to retard human ageing? Results of a feasibility study. Eur J Clin Nutr. 1994;48:138–48. [PubMed] [Google Scholar]
- 59.Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. Int I Obes Relat Metab Disord. 1995;19:318–24. [PubMed] [Google Scholar]
- 60.Velthuis-te Wierik EJ, Meijer P, Kluft C, van den Berg H. Beneficial effect of a moderately energy-restricted diet on fibrinolytic factors in non-obese men. Metabolism. 1995;44:1548–52. doi: 10.1016/0026-0495(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 61.Loft S, Velthuis-te Wierik EJM, van den Berg H, Poulsen HE. Energy restriction and oxidative DNA damage in humans. Cancer Epidemiol Biomarkers Prev. 1995;4:515–9. [PubMed] [Google Scholar]
- 62.Loft S, Astrup A, Buemann B, Poulsen HE. Oxidative DNA damage correlates with oxygen consumption in humans. FASEB J. 1994;8:534–7. doi: 10.1096/fasebj.8.8.8181672. [DOI] [PubMed] [Google Scholar]
- 63.Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–6. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 64.Romero-Alvira D, Roche E, Placer L. Cardiomyopathies and oxidative stress. Med Hypotheses. 1996;47:137–44. doi: 10.1016/s0306-9877(96)90453-3. [DOI] [PubMed] [Google Scholar]
- 65.Dempsey JL, Pfeiffer M, Morley AA. Effect of dietary restriction on in vivo somatic mutation in mice. Mutat Res. 1993;291:141–5. doi: 10.1016/0165-1161(93)90153-q. [DOI] [PubMed] [Google Scholar]
- 66.Grasl-Kraupp B, Bursch W, Ruttkay-Nedecky B, Wagner A, Lauer B, Schulte-Hermann R. Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci U S A. 1994;91:9995–9. doi: 10.1073/pnas.91.21.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muskhelishvili L, Turturro A, Hart RW, James SJ. Pi-class glutathione-S-transferase-positive hepatocytes in aging B6C3F1 mice undergo apoptosis induced by dietary restriction. Am J Pathol. 1996;149:1585–91. [PMC free article] [PubMed] [Google Scholar]
- 68.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease-processes of Fischer rats. J Gerontol A Biol Sci Med Sci. 1988;43:B5–B12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- 69.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway — a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–32. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]