Abstract
Background and Purpose
Ischemic lesion recurrence on diffusion-weighted imaging (DWI-LR) is a frequently observed phenomenon after acute ischemic stroke. However, no study has elucidated the impact of DWI-LR on functional outcome.
Methods
Among a consecutive series of patients who presented with focal symptoms or signs compatible with stroke within 48 hours from the onset over a 50-month period, those who had relevant ischemic lesions on initial DWI and underwent follow-up DWI within 14 days after the onset were enrolled in this study. As outcome variables, the scores on the modified Rankin Disability Scale (mRDS) at 3 months and 1 year were measured prospectively and dichotomized into good (0-2) vs. poor (3-6). When calculating odds ratios (ORs), adjustment was performed for age, previous stroke, initial score on the NIH Stroke Scale, stroke subtype, and IV thrombolysis.
Results
Among those 786 patients finally enrolled in this study, 221 (28.1%) had DWI-LR. For a poor outcome at 3 months, the crude ORs of any, symptomatic, and asymptomatic DWI-LR were 2.70 [95% confidence interval (CI), 1.96 to 3.72], 10.03 (95% CI, 4.39 to 22.96), and 2.04 (95% CI, 1.44 to 2.88), respectively. With adjustment, the OR of symptomatic DWI-LR was 6.44 (95% CI, 2.50 to 16.57), whereas those of any and asymptomatic DWI-LR lost their statistical significance: 1.44 (95% CI, 0.94 to 2.20) and 1.04 (95% CI, 0.65 to 1.65), respectively. Analyzing with the 1-year outcome produced similar results.
Conclusions
This study shows that symptomatic early lesion recurrence can affect functional outcome after acute ischemic stroke, whereas an asymptomatic one may not.
Keywords: diffusion MRI, cerebral infarction, recurrence, modified Rankin Disability Scale
Introduction
Ischemic lesion recurrence on diffusion-weighted imaging (DWI-LR) is a relatively common phenomenon in the acute stage of ischemic stroke, with an incidence of 30.4% within the first week1 and about 40% at 1-3 months.2-4 The risk of subsequent clinical events is more than threefold higher in patients with DWI-LR within 1 week after the index stroke than in those without DWI-LR.4 Based on these results, DWI-LR was proposed as a potential "surrogate endpoint", which is defined as a measurement that can substitute for a true clinical endpoint in clinical trials.4 However, criteria for valid surrogate endpoints require that the surrogate must be a correlate of clinical outcome and fully capture the net effects of treatment on clinical outcome.5
Early symptomatic recurrence after index stroke was reported to aggravate the case fatality rate, disability, and prognosis of stroke.6-8 Although most cases of DWI-LR are clinically silent, their pathogenesis is assumed to be identical to that of clinical recurrence of stroke.3 The association of DWI-LR with functional outcome may be similar to that of symptomatic recurrence, although this remains to be proven. The aim of this study was to determine the impact of early DWI-LR on functional outcome after acute ischemic stroke as measured by the score on the modified Rankin Disability Scale (mRDS).
Methods
Subjects
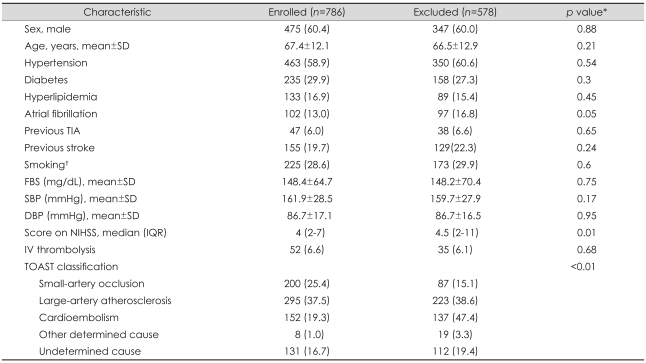
This was a retrospective analysis of ischemic stroke and transient ischemic attack patients admitted to the stroke center of Seoul National University Bundang Hospital between January 2004 and February 2008. Based on the prospective stroke registry,9 we selected patients 1) who presented with focal neurologic signs or symptoms compatible with stroke, 2) were hospitalized within 48 hours from the onset, and 3) had relevant ischemic lesions on DWI. During the study period, a consecutive series of 1,364 patients were hospitalized due to stroke within 48 hours from the onset and had relevant ischemic lesions on initial DWI. Among them, 578 patients were excluded for the following reasons: 1) no follow-up DWI within 14 days after stroke onset (n=393), 2) not assessed for functional outcome on the mRDS at 3 months (n=20), or 3) having procedures that could cause ischemic lesions on follow-up DWI iatrogenically (i.e., diagnostic cerebral angiography, endovascular interventions of cerebral arteries, coronary revascularization, heart surgery, and carotid endarterectomy) (n=165).10 A total of 786 patients were finally enrolled in this study. Most of the baseline characteristics did not differ significantly between those who were enrolled (n=786) and those who were excluded (n=578) (Table 1); excluded patients had more-severe neurologic deficits and a higher frequency of cardioembolic stroke.
Table 1.
Comparison of baseline characteristics between enrolled and excluded subjects
Values are number of patients (%) except where indicated.
*p values were calculated by Pearson's chi-square test, Student's t-test, or Mann-Whitney U test, †Current smoker or ceased smoking within 5 years prior to admission.
SD: standard deviation, TIA: transient ischemic attack, FBS: fasting blood sugar, SBP: systolic blood pressure, DBP: diastolic blood pressure, IQR: interquartile range, NIHSS: NIH Stroke Scale, TOAST: Trial of Org 10172 in Acute Stroke Treatment.
MRI protocols and diffusion-weighted imaging assessments
According to our stroke center's protocol, all eligible patients with acute ischemic stroke were scheduled for an MRI scan at presentation and for a follow-up scan usually within 1 week of hospitalization. The median interval from initial DWI to the final follow-up DWI was 4 days (interquartile range, 2 to 5 days). The MRI was conducted at 1.5 T (Intera, Philips Medical Systems, Best, the Netherlands) with a sensitivity-encoding head coil. DWI was performed using single-shot spin-echo echo-planar imaging with the following parameters: matrix=128×128, slice thickness=5 mm, TR=4,000-5,000 ms, TE=56-65 ms, and b=1,000 s/mm2.
All MRI scans that were performed within 14 days from stroke onset were reviewed by two neurologists (WJ Kim and Y Ko). DWI-LR was judged by them independently and blind to clinical information (kappa=0.83). Discrepancies were resolved by consensus. DWI-LR was defined as the presence of a newly developed ischemic lesion on follow-up DWI outside the region of the acutely symptomatic (index) lesions.1 Enlargement of the initial ischemic lesion was not regarded as a new ischemic lesion.1 New lesions were determined by slice-to-slice comparison of DWI scans across time points.
Clinical assessment
Clinical data were obtained from the stroke registry9 primarily and with additional review of the medical records if necessary. Among those lesions satisfying the MRI-based definition of DWI-LR, symptomatic DWI-LR was identified as the presence of newly developed neurological deficits that were clearly different from those of the index stroke.11 Lesions due to systemic causes of clinical deterioration (e.g., hypoxia, hypotension, hypo- or hyperglycemia, or infection) were not considered symptomatic. New lesions compatible with the MRI-based definition of DWI-LR but not identified as symptomatic were designated as asymptomatic DWI-LR. All patients were on antiplatelets or anticoagulants to prevent stroke recurrence, and also received treatment with other measures appropriate for secondary prevention of stroke (e.g., statins, antihypertensives, and hypoglycemics) based on current stroke guidelines.12,13
Stroke subtypes were categorized according to the classification of the Trial of Org 10172 in Acute Stroke Treatment (TOAST):14 1) large-artery atherosclerosis, 2) cardioembolism, 3) small-artery occlusion, 4) stroke of other determined cause, or 5) stroke of undetermined cause. We modified the criterion for lesion size of small-vessel occlusion to be less than 20 mm based on a report that an acute ischemic lesion is generally larger in DWI than in CT or conventional MRI.15
At 3 months and 1 year after stroke onset, the functional status of each patient was determined through telephone interview by a trained nurse (MH Yang) using the mRDS16 as part of a program monitoring the quality of inpatient stroke care. The mRDS score ranges from 0 to 6, with higher scores indicating greater disability (6 indicates death). For the analysis, the mRDS score was dichotomized into "good outcome" (0 to 2; patient is independent) vs. "poor outcome" (3 to 6; patient is dependent or dead).17
Power calculation
Before acquiring imaging data and refining the clinical data set by reviewing medical records, the power of this study was calculated based on the following assumptions. First, the frequency of DWI-LR was assumed to be 34% based on a previous report.1 Second, poor outcome was estimated as 30% in the no-DWI-LR group, based on 33.7% of those in the original cohort having a poor outcome (mRDS score ≥3); note that information on the mRDS score was available for 1,512 of 1,602 patients at 3 months. Third, the postulated odds ratio (OR) values were 1.5, 2.0, and 3.0, which for the final 786 subjects gave calculated powers of 72%, 99%, and 99%, respectively. All calculations were performed using nQuery Advisor (version 6.1, Statistical Solutions, Cork, Ireland).
Statistical analyses
Continuous variables are expressed as mean±standard deviation or median (interquartile range) values, whereas categorical variables are presented as absolute values and percentages. We compared baseline characteristics according to the presence of DWI-LR. Student's t-test or the Mann-Whitney U test was used for continuous variables, and Pearson's chi-square test was used for categorical variables. To express the association of any, symptomatic, and asymptomatic DWI-LR with functional outcome, ORs and their 95% confidence intervals (CIs) were calculated using logistic regression with and without adjustment. Variables were selected for adjustment if 1) they showed p<0.2 in bivariate analyses with both functional outcome and DWI-LR, and 2) they were judged to be clinical relevant.
In order to check whether dichotomizing a polychotomous outcome variable influences the analysis results, we performed repeated logistic analysis with two other dichotomizations: mRDS scores of 0/1 vs. 2-6 and 0-3 vs. 4-6.
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA) and a probability value of p<0.05 was considered statistically significant.
Results
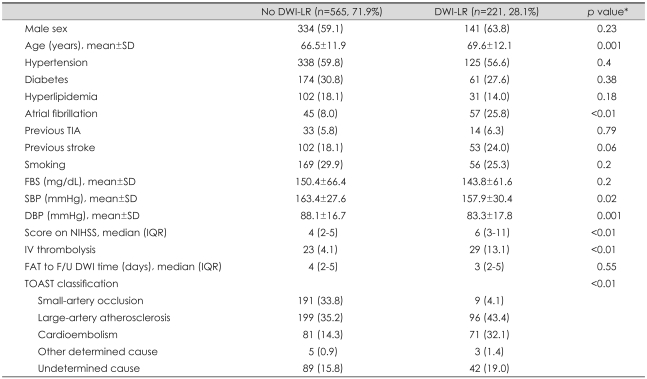
Among 786 patients who were finally enrolled in this study, 221 (28.1%) had any DWI-LR, with 180 (22.9%) having asymptomatic DWI-LR and 41 (5.2%) having symptomatic DWI-LR. Those in the DWI-LR group were older, had a higher prevalence of atrial fibrillation, presented with lower systolic blood pressure, lower diastolic blood pressure, and higher initial score on the NIH Stroke Scale (NIHSS), and received IV thrombolysis more frequently than those in the no-DWI-LR group (Table 2). DWI-LR occurred only rarely in those with small-artery occlusion (4.1%). Previous stroke tended to be more common in the DWI-LR group. With additional consideration of clinical relevance and the correlation with functional outcome, age, initial score on the NIHSS, TOAST classification, IV thrombolysis, and previous stroke were chosen as variables for adjustment.
Table 2.
Baseline characteristics by the presence of ischemic lesion recurrence on diffusion-weighted MRI (DWI) (DWI-LR)
*p values are calculated by Mann-Whithey U test, Student's t-test, or Pearson's chi-square test if appropriate.
SD: standard deviation, TIA: transient ischemic attack, FBS: fasting blood sugar, SBP: systolic blood pressure, DBP: diastolic blood pressure, IQR: interquartile range, NIHSS: NIH Stroke Scale, FAT: first abnormal time, F/U: follow-up, TOAST: Trial of Org 10172 in Acute Stroke Treatment.
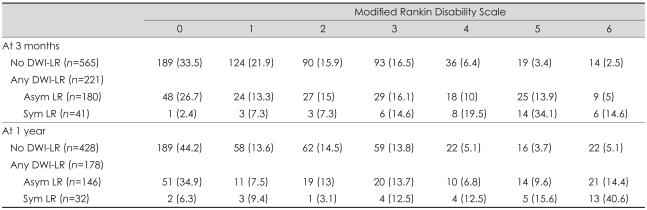
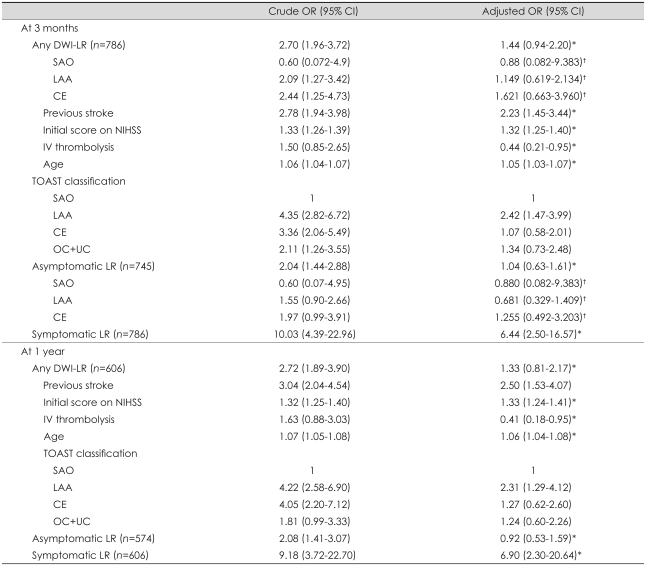
The functional outcome was worse in the DWI-LR group than in the no-DWI-LR group (Table 3). At 3 months the outcome was poor (mRDS score of 3-6) in 28.7% and 52.0% of subjects in the no-DWI-LR and DWI-LR groups, respectively, and the ORs of any DWI-LR before and after adjustment were 2.70 (95% CI, 1.96 to 3.72) and 1.44 (95% CI, 0.94 to 2.20), respectively (Table 4). We analyzed the association between asymptomatic DWI-LR and functional outcome by removing 41 symptomatic recurrent stroke patients from the data set. On crude analysis the contribution of asymptomatic DWI-LR to functional outcome remained significant (Table 4), but the significance disappeared after adjustment (adjusted OR, 1.04; 95% CI, 0.63 to 1.61). Symptomatic DWI-LR was associated with poor outcome (adjusted OR, 6.44; 95% CI, 2.50 to 16.57).
Table 3.
Functional outcome (mRDS score) at 3 months and 1 year by DWI-LR
Values are number of patients (%).
Asym LR: asymptomatic DWI-LR, Sym LR: symptomatic DWI-LR, mRDS: modified Rankin disability scale, DWI-LR: lesion recurrence on diffusion-weighted imaging.
Table 4.
Odds ratios (ORs) for disability at 3 months and 1 year
*Adjusted for previous stroke, initial score on NIHSS, IV thrombolysis, age, and TOAST classification, †Adjusted for previous stroke, initial score on NIHSS, IV thrombolysis, and age.
DWI-LR: lesion recurrence on diffusion-weighted imaging, SAO: small-artery occlusion, LAA: large-artery atherosclerosis, CE: cardioembolism, NIHSS: NIH Stroke Scale, OC: other determined cause, UC: undetermined cause.
The results were not changed in additional analyses with 1-year outcome or stratification by TOAST (Table 3 and 4). Repeated analyses with different dichotomization methods for outcome variable (mRDS scores of 0/1 vs. 2-6 and 0-3 vs. 4-6) also did not show a significant association between asymptomatic DWI-LR and functional outcome after adjustment (results are not presented here).
Discussion
To the best of our knowledge, this is the first study of the impact of early asymptomatic ischemic lesion recurrence on functional outcome of acute ischemic stroke. There are previous reports on the clinical importance of early lesion recurrence with respect to predicting late lesion recurrence3 and subsequent clinical events,4 and even lesion recurrence has been suggested as a surrogate endpoint of stroke prevention trials.1,3,4,18 However, the present study shows that asymptomatic ischemic lesion recurrence may not influence clinical outcome at 3 months, at least in terms of functional disability.
Several reasons can be suggested for the absence of an association between DWI-LR and mRDS at 3 months. An effect of asymptomatic DWI-LR on outcome could be mediated via an increased risk of further clinical events, or directly by the effect of silent ischemic lesions themselves. With respect to subsequent clinical events, 3 months of observation might have been too short for subsequent clinical events to occur and aggravate the clinical outcome in the DWI-LR group. In a previous study, recurrent ischemic stroke occurred in 11.4% of subjects in the early silent lesion recurrence group and in 5.8% of the no-recurrence group during a follow-up of 19.3±9.0 months.4 The higher proportion of subjects with smallartery occlusion in the present study compared to that study (25.4% vs. 17.4%) may be another reason. An effect of silent ischemic lesions on outcome may be too small to be detected by applying the mRDS to a sample of this size. The mRDS has been widely used for measuring functional disability after stroke. Whilst its validity and reliability have been confirmed,16,19 it might be not sufficiently sensitive to detect small differences. Neuropsychological deficits in the absence of clinical stroke after coronary bypass surgery20,21 and cognitive deficits associated with new infarcts on MRI, mostly asymptomatic, in an elderly cohort22 suggest that more-sophisticated methods-such as cognitive tests or measures of the quality of life-could reveal the impact of silent lesions on functional outcome. Furthermore, even when using the mRDS, increasing the sample size may only enable the detection of a modest contribution of DWI-LR to functional outcome, such as an OR of 1.5 or less. Further studies with larger samples and appropriate tools are warranted.
Lesion growth and lesion recurrence can be considered to be two major types of changes in ischemic lesions on DWI-MRI causing neurological deterioration after acute ischemic stroke. Evolution of ischemic lesions is very common,23 and ischemic lesion growth is strongly correlated with clinical outcome.24,25 Therefore, the negative correlation between asymptomatic lesion recurrence and clinical outcome can be explained by the impact on clinical outcome being greater for lesion growth than for lesion recurrence. However, lesion growth was not measured in this study.
The frequency of DWI-LR in this study was 28.1% and varied according to TOAST classification, from 46.7% in cardioembolic stroke to 4.5% in lacunar stroke. The reported incidence of early lesion recurrence has varied from 33.7% to 34.3%.1,3,4 The lower frequency of DWI-LR in our study may be attributable to the proportion of subjects with small-artery occlusion being higher in our study than in previous studies (25.4% vs. 12.1% and 12.5%).1,4
The rate of symptomatic DWI-LR was 5.2% in this study. Considering that we used all DWI scans that were performed within 14 days from symptom onset, this rate is similar to those of previous studies: the reported cumulative rates of clinical stroke recurrence were 2% within 1 week26 and 1.6-8% within 2 weeks.27-29 As expected, this study showed that symptomatic DWI-LR was strongly correlated with poor outcome in terms of functional disability at 3 months after stroke (crude and adjusted ORs, 10.03 and 6.44, respectively), which is similar to previous studies of the impact of early recurrence of stroke on functional disability.7,30,31
Specific evidence is necessary for a surrogate endpoint to be considered an adequate substitute for the real endpoint. A surrogate endpoint has biologic relevance in terms of 1) the relationship between the candidate surrogate and true outcome, 2) synchronized changes among the surrogate endpoint and functional status, 3) reflection of the pathophysiologic process, and 4) the association between the surrogate endpoint and true outcome.5 Because the present study showed that early asymptomatic ischemic lesion recurrence does not predict functional disability after stroke, DWI-LR is not an appropriate surrogate marker for use in clinical trials aimed at improving functional disability caused by stroke. Instead, ischemic lesion growth may be more helpful than lesion recurrence.
This study was subject to some limitations. First, this was a single-center, retrospective cohort study. Second, we excluded 40% of patients hospitalized during the study period. Although most baseline characteristics were comparable, the subjects who were excluded had more-severe neurological deficits, less-frequent small-artery occlusion, and more-frequent cardioembolic stroke. Third, follow-up DWI was performed at various intervals from stroke onset, although the interval did not differ between the DWI-LR-positive and DWI-LR-negative groups.
In conclusion, this study shows that symptomatic early lesion recurrence can affect functional outcome after acute ischemic stroke, whereas asymptomatic early lesion recurrence may not. However, the use of more sophisticated tools for detecting subtle changes due to asymptomatic recurrence with larger samples may produce different results.
Acknowledgements
This study was supported by grants of the Korea Health 21 R&D project, Ministry of Health and Welfare, Korea (A060171).
Supplement
Comparison of baseline characteristics by functional outcome at 3 months (n=786)
References
- 1.Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003;54:66–74. doi: 10.1002/ana.10592. [DOI] [PubMed] [Google Scholar]
- 2.Sylaja PN, Coutts SB, Subramaniam S, Hill MD, Eliasziw M, Demchuk AM. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology. 2007;68:415–419. doi: 10.1212/01.wnl.0000252938.76188.52. [DOI] [PubMed] [Google Scholar]
- 3.Kang DW, Latour LL, Chalela JA, Dambrosia JA, Warach S. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology. 2004;63:2261–2265. doi: 10.1212/01.wnl.0000147295.50029.67. [DOI] [PubMed] [Google Scholar]
- 4.Kang DW, Lattimore SU, Latour LL, Warach S. Silent ischemic lesion recurrence on magnetic resonance imaging predicts subsequent clinical vascular events. Arch Neurol. 2006;63:1730–1733. doi: 10.1001/archneur.63.12.1730. [DOI] [PubMed] [Google Scholar]
- 5.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 6.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. 2004;35:731–735. doi: 10.1161/01.STR.0000116183.50167.D9. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Foulkes MA, Mohr JP, Wolf PA, Hier DB, Price TR. Determinants of early recurrence of cerebral infarction. The Stroke Data Bank. Stroke. 1989;20:983–989. doi: 10.1161/01.str.20.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99:625–633. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 9.Lee BC, Roh JK Korean Stroke Registry. International experience in stroke registries: Korean Stroke Registry. Am J Prev Med. 2006;31(6 Suppl 2):S243–S245. doi: 10.1016/j.amepre.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Derdeyn CP. Diffusion-weighted imaging as a surrogate marker for stroke as a complication of cerebrovascular procedures and devices. AJNR Am J Neuroradiol. 2001;22:1234–1235. [PMC free article] [PubMed] [Google Scholar]
- 11.Moroney JT, Bagiella E, Paik MC, Sacco RL, Desmond DW. Risk factors for early recurrence after ischemic stroke: the role of stroke syndrome and subtype. Stroke. 1998;29:2118–2124. doi: 10.1161/01.str.29.10.2118. [DOI] [PubMed] [Google Scholar]
- 12.European Stroke Council, European Neurological Society and European Federation of Neurological Societies. European Stroke Initiative recommendations for stroke management. Cerebrovasc Dis. 2000;10:335–351. doi: 10.1159/000016089. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 17.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30:1538–1541. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 18.Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and TIA patients identified on MR imaging. Neurology. 2005;65:513–517. doi: 10.1212/01.wnl.0000169031.39264.ff. [DOI] [PubMed] [Google Scholar]
- 19.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 20.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–1399. doi: 10.1161/01.str.25.7.1393. [DOI] [PubMed] [Google Scholar]
- 21.Fearn SJ, Pole R, Wesnes K, Faragher EB, Hooper TL, McCollum CN. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg. 2001;121:1150–1160. doi: 10.1067/mtc.2001.114099. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O'Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, O'Brien MW, Tong DC, Moseley ME, Albers GW. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:613–617. doi: 10.1001/archneur.58.4.613. [DOI] [PubMed] [Google Scholar]
- 24.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39:2257–2263. doi: 10.1161/STROKEAHA.107.511535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merino JG, Latour LL, Todd JW, Luby M, Schellinger PD, Kang DW, et al. Lesion volume change after treatment with tissue plasminogen activator can discriminate clinical responders from nonresponders. Stroke. 2007;38:2919–2923. doi: 10.1161/STROKEAHA.107.485995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 27.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205–1210. doi: 10.1016/s0140-6736(00)02085-7. [DOI] [PubMed] [Google Scholar]
- 28.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 29.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 30.Toyoda K, Okada Y, Kobayashi S. Early recurrence of ischemic stroke in Japanese patients: the Japan standard stroke registry study. Cerebrovasc Dis. 2007;24:289–295. doi: 10.1159/000105682. [DOI] [PubMed] [Google Scholar]
- 31.Weimar C, Ziegler A, Konig IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. J Neurol. 2002;249:888–895. doi: 10.1007/s00415-002-0755-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of baseline characteristics by functional outcome at 3 months (n=786)