Abstract
Objectives: To assess whether the levonorgestrel intrauterine system could provide a conservative alternative to hysterectomy in the treatment of excessive uterine bleeding.
Design: Open randomised multicentre study with two parallel groups: a levonorgestrel intrauterine system group and a control group.
Setting: Gynaecology departments of three hospitals in Finland.
Subjects: Fifty six women aged 33-49 years scheduled to undergo hysterectomy for treatment of excessive uterine bleeding.
Interventions: Women were randomised either to continue with their current medical treatment or to have a levonorgestrel intrauterine system inserted.
Main outcome measure: Proportion of women cancelling their decision to undergo hysterectomy.
Results: At 6 months, 64.3% (95% confidence interval 44.1 to 81.4%) of the women in the levonorgestrel intrauterine system group and 14.3% (4.0 to 32.7%) in the control group had cancelled their decision to undergo hysterectomy (P<0.001).
Conclusions: The use of the levonorgestrel intrauterine system is a good conservative alternative to hysterectomy in the treatment of menorrhagia and should be considered before hysterectomy or other invasive treatments.
Introduction
Menorrhagia is a major reason for hysterectomy among fertile women.1 Abnormal uterine bleeding is a common reason for consulting general practitioners.2 Until recently, medical treatment has been disappointing,3,4 and various surgical alternatives in the form of endometrial ablation have been developed.5–7 The role of these surgical alternatives in the treatment of menorrhagia is not currently clear.8
The progestin levonorgestrel, released from an intrauterine system at a rate of 20 μg/24 hours, suppresses endometrial growth. The glands of the endometrium become atrophic and the epithelium inactive.9 This system, originally developed for contraception,10,11 has been shown to decrease the amount and duration of normal menstrual flow.12 The results of a non-comparative study showed a reduction of menstrual blood loss of 86% in menorrhagic women in only 3 months and a further reduction to 97% 12 months after insertion of the device.13 Comparison of the levonorgestrel intrauterine system with the non-steroidal anti-inflammatory drug flurbiprofen and tranexamic acid showed that the device decreased the measured volume of menstrual blood loss in comparison with control cycle measurements by 82% at 3 months and by 96% at 12 months, while the flurbiprofen and tranexamic acid treatments decreased menstrual blood loss by only 21% and 44%, respectively.14
Many women scheduled for hysterectomy as the final treatment for menorrhagia might still prefer a conservative alternative. We invited women who had already made a decision to undergo hysterectomy to participate in a randomised study comparing the levonorgestrel intrauterine system with their current medical treatment. Our primary aim was to assess after 6 months whether the device could provide a conservative alternative to hysterectomy in the treatment of excessive uterine bleeding or dysmenorrhoea, or both.
Patients and methods
Patients
From hospital waiting lists we recruited women who had spontaneous cycles and who were scheduled to undergo hysterectomy for treatment of excessive uterine bleeding with or without dysmenorrhoea. Women were excluded from the study if they had one fibroid larger than 3 cm in diameter or more than three uterine fibroids as assessed by ultrasonography, a history or current clinical evidence or suspicion of malignancy or active liver disease, adnexal tumours or cysts, or pelvic inflammatory disease within the previous 12 months. If the women were prepared to accept yet another conservative attempt at treatment they were enrolled into the study.
Study design and treatment
The study was an open phase III randomised multicentre study with two parallel groups: a levonorgestrel intrauterine system group and a control group. The study was conducted in three hospitals in Finland: the City Maternity Hospital and the University Hospital, Helsinki, and the Central Hospital of Middle Finland, Jyväskylä.
The study was conducted according to the principles of the Declaration of Helsinki. Copies of the protocol and the informed consent form were submitted to and approved by the ethics committees of the participating study centres before we started the study.
The women were randomly allocated to the levonorgestrel intrauterine system group or the control group by using a randomisation table, the randomisation being balanced in blocks of four. Concealment was secured by using sealed envelopes.
The levonorgestrel intrauterine system (Mirena) was inserted into the uterine cavity after menstruation according to the insertion instructions. The patients in the control group continued their existing medical treatment for excessive uterine bleeding or symptoms of dysmenorrhoea, or both. There was no wash out period of medication for bleeding or dysmenorrhoea at screening. Enrolment started on 15 November 1991 and finished at the end of 1993.
The primary measure of efficacy was the woman’s decision at 6 months, at discontinuation, or when the hysterectomy became available as to whether she would prefer her current treatment or hysterectomy. If she chose to continue the current treatment she was asked again at 12 months. Finally, at the end of 1995 all the patients’ records were checked to see how many women had undergone hysterectomy.
The degree of disturbance caused by their menstrual bleeding or pain, or both, on general wellbeing, work performance, physical activity, sexual activity, and general leisure time activity was assessed by using a visual analogue scale at screening, at 6 months, and at 12 months or at discontinuation. The visual analogue scale consisted of a horizontal line of 10 cm. The left end was indicated as “not disturbing,” the right end as “very disturbing.” The patients were asked to mark with a cross the point on the line that most closely indicated the effects of uterine bleeding or menstrual pain on normal life, without distinguishing between these two.15
The women were asked to mark in a menstrual diary their days of menstrual bleeding and spotting. The latter was defined as a bloody discharge not necessitating the use of pads or tampons. We calculated the distributions in centiles of the total number of days of bleeding and spotting in each month and each trimester using the menstrual diary analyser, a computer program developed for this purpose by JS.16
Statistical analysis
Power analysis was made before the study for the main measure of efficacy—that is, women preferring current treatment to hysterectomy at 6 months. We expected that 40% of patients in the levonorgestrel intrauterine system group and 10% in the control group would cancel the hysterectomy. The sample size required for the comparison of two binomial proportions of a two sided test at 5% level, when the power is 95, was 52 per group when π1=0.1 and π2=0.4. Taking into account the possibility of discontinuation, we planned to enrol 60 patients per group.
The study was started in two clinics. The rate of enrolment, however, was much slower than expected and a third clinic was therefore included. From the original recruitment time of 1 year the period was extended to 26 months. During that period the waiting time for hysterectomy shortened from over 12 months in each hospital to under 6 months in two hospitals. As the ethics committees had requested that women should be offered the operation once it became available, recruitment to a 6 month study became complicated. By this time, 28 patients were recruited per group, giving a power of 70%.
The main measure of efficacy—willingness to continue the current treatment instead of hysterectomy at 6 months—was analysed according to the principle of intention to treat by using the Mantel-Haenszel test. Comparison of visual analogue scale distributions between the groups at screening, differences between screening and 6 month results, and differences in the number of days of bleeding and spotting were analysed by using the Mann-Whitney U test. All statistical analyses were carried out with sas software package version 6.08. A P value <0.05 was considered significant. The tests were performed as two tailed tests.
Results
A total of 56 women were randomised, 28 to the levonorgestrel intrauterine system group and 28 to the control group. Three women cancelled participation before enrolment, two in the control group and one in the levonorgestrel group. The mean (SD) ages were 42.7 (3.4) and 41.7 (4.5) years in the levonorgestrel intrauterine system and control groups, respectively.
Eighteen out of 28 women (64.3%; 95% confidence interval 44.1% to 81.4%) in the levonorgestrel group and four out of 28 women (14.3%; 4.0% to 32.7%) in the control group cancelled hysterectomy at 6 months (P<0.001).
In the control group two women wished to continue their current drug treatment (prostaglandin synthesis inhibitors) and two decided to switch to the levonorgestrel intrauterine system. The proportion of women who decided to cancel hysterectomy was significantly higher in the levonorgestrel intrauterine system group (P<0.001) by 6 months.
At 12 months 12 women (57%) in the levonorgestrel group had discontinued the treatment. They all underwent hysterectomy. Table 1 gives the reasons for discontinuation and the histological diagnoses at hysterectomy.
Table 1.
Reasons for discontinuation of use of levonorgestrel intrauterine device and histological diagnosis at hysterectomy
| Case No | Month of discontinuation | Reason for dissatisfaction | Histological diagnosis |
|---|---|---|---|
| 1 | 3 | Weight gain, headache, operation offered from waiting list | Fibroids |
| 2 | 6 | Prolonged bleeding and spotting | Adenomyosis |
| 3 | 6 | Prolonged bleeding, pain | Fibroids, adenomyosis |
| 4 | 6 | Excessive bleeding | None |
| 5 | 6 | Wanted hysterectomy | Adenomyosis |
| 6 | 6 | Spotting, pain | Fibroids |
| 7 | 6 | Personal reasons | Adenomyosis |
| 8 | 7 | Pain | Adhesions |
| 9 | 8 | Spotting | None |
| 10 | 9 | Depression, acne | None |
| 11 | 9 | Prolonged bleeding, spotting | Several fibroids, adenomyosis |
| 12 | 12 | Prolonged | Chronic endometritis |
By the end of 1995, 13 of 27 women (48%) continued using the levonorgestrel intrauterine system, with an average follow up time of 3 years (range 23-49 months).
Evaluation by visual analogue scale of the quality of life showed no difference between the groups at screening (table 2). Visual analogue scale scores had not changed at 6 months in the control group whereas they were significantly improved in each category in the study group (P⩽0.002). The beneficial effect was maintained in the levonorgestrel group at 12 months.
Table 2.
Menstrual disturbance scores by group. Figures are medians (Wilcoxon based 95% confidence intervals)
| Variable |
At screening*
|
At 6 months†
|
Levonorgestrel at 12 months | |||
|---|---|---|---|---|---|---|
| Control group | Levonorgestrel | Control group | Levonorgestrel | |||
| General wellbeing | 87 (77 to 92) | 90 (74 to 94) | 79 (64 to 87) | 24 (14 to 40) | 10 (4 to 29) | |
| Work performance | 75 (61 to 80) | 79 (62 to 89) | 76 (54 to 87) | 20 (5 to 35) | 6 (3 to 11) | |
| Physical activity | 78 (64 to 92) | 88 (64 to 95) | 78 (55 to 88) | 27 (9 to 38) | 10 (3 to 28) | |
| Sex life | 66 (52 to 80) | 68 (49 to 86) | 66 (51 to 85) | 36 (17 to 49) | 8 (3 to 28) | |
| Leisure time activty | 74 (64 to 85) | 76 (54 to 86) | 74 (54 to 86) | 11 (5 to 27) | 6 (3 to 29) | |
At screening there were no significant differences between groups.
At 6 months all differences between groups were significant (P=0.002 for sex life and P<0.001 for all other variables).
The distribution by centiles of the total number of days of bleeding during the first 6 months in the control group and during 12 months in the levonorgestrel group is presented in figure 1. The median number of days of bleeding remained at a constant level of 4-5 days per month in the control group, while in the levonorgestrel group it decreased from 7 in the first month to 3 in the sixth month and 2 in the 12th month. Differences in the number of days of bleeding between the groups did not reach significance.
Figure 1.
Number of days of bleeding per month in control group and in levonorgestrel intrauterine system group. Points are medians with 25th and 75th centiles and 5th and 95th centiles
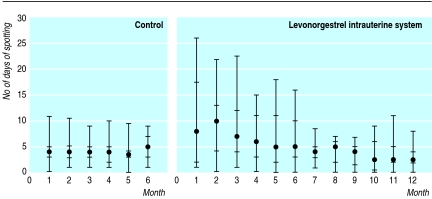
The distribution of the number of days of spotting in both groups is presented in figure 2. In the control group the median number of days of spotting remained at a nearly constant level of about 4 days a month. In the levonorgestrel group the median number of days of spotting was at its highest level, 10 days a month, during the second month. Thereafter it declined to 5 in the sixth month and 2.5 in the 12th month. The number of days of spotting was significantly lower in the control group in the first 3 months (P=0.001) and also in the second 3 months (P=0.016). No serious adverse events were seen during the study.
Figure 2.
Number of days of spotting per month in control group and in levonorgestrel intrauterine system group. Points are medians with 25th and 75th centiles and 5th and 95th centiles
Discussion
The levonorgestrel intrauterine system has been approved for contraception in several European countries and also for treatment of menorrhagia in most of these countries. We tested this new medical treatment as a final alternative in women who had already chosen hysterectomy. Our data suggest that the levonorgestrel intrauterine system gives good short term results. Two thirds of the patients with the levonorgestrel device cancelled their hysterectomy at the 6 month follow up. Only 14% in the control group cancelled hysterectomy. In addition, half of these women chose the levonorgestrel device as their future treatment.
Improvement in quality of life
The quality of life of women suffering from menorrhagia is impaired in many respects.17 Excessive bleeding or pain, or both, may impose severe constraints on their professional, social, and family activities. There was no improvement in the menstrual disturbance score in the control group whereas it significantly improved in patients with the levonorgestrel intrauterine system in all aspects evaluated. This happened despite the fact that there was an initial increase in the number of days of spotting from a median of 8 days in the first month to a median of 10 days in the second month. An initial increase in the number of days of spotting for 3-6 months is well known when the levonorgestrel device is used for contraception.16,18 Our results indicate that the improvement in the quality of life of levonorgestrel intrauterine system users is maintained as these improved scores were also seen at 12 months.
Because of the nature of the device it would be impossible to carry out a blind comparative study between it and other medical treatments. As this was an open study and the subjects were well aware of their treatment a possible placebo effect cannot be excluded. The levonorgestrel system has been on the market in Finland since 1990, and its reputation as regards reduction of menstrual bleeding has spread. The short term results with the current study design are also subject to bias because of the potential disappointment of the control group as regards continuing with their current treatment. This does not seem to have affected the overall conclusions, however, as only another three (11%) of the levonorgestrel users discontinued between 6 and 12 months. One woman reported lower back pain and depression. The other two women reported a poor response to the treatment, and one of the two experienced fibroid growth during the treatment. In long term follow up to a mean of 3 years 48% of the patients treated with the levonorgestrel system were still using it.
Effective conservative alternative
The insertion of a levonorgestrel intrauterine system is a simple procedure. Although it is slightly larger than many other intrauterine systems, insertion is usually easy for most parous women. Surgical methods of treatment for menorrhagia, including endometrial ablation, are effective but invasive operations; need operating theatre facilities; and can be associated with considerable morbidity.
Key messages
Two thirds of women treated for menorrhagia with the levonorgestrel intrauterine system cancelled their decision to undergo hysterectomy
The median number of days of bleeding decreased in the levonorgestrel group from 7 in the first month to 3 in the sixth month and 2 in the 12th month
The variation between individual women in the number of days of bleeding and spotting is great in users of the levonorgestrel intrauterine system
The treatment of menorrhagia with the levonorgestrel intrauterine system improved general well being and work performance and physical, sexual, and leisure time activity
Treatment of menorrhagia with the levonorgestrel intrauterine system should be considered before hysterectomy is decided on
The results of our study suggest an important clinical implication. A woman considering hysterectomy because of menorrhagia or dysmenorrhoea, or both, should be offered the levonorgestrel intrauterine system before she comes to a final decision on hysterectomy. Many menorrhagic women wish to retain their potential fertility. In addition, the levonorgestrel intrauterine system acts as a contraceptive in contrast with prostaglandin synthesis inhibitors and inhibitors of fibrinolysis or procedures used for endometrial ablation. A reduction in the number of annual hysterectomies, even by less than half, would be a considerable achievement. Even greater reductions in rates of hysterectomy could be achieved if medical treatment with intrauterine levonorgestrel could be started at an earlier stage.
Acknowledgments
The levonorgestrel releasing intrauterine systems (Levonova/Mirena) were provided by Leiras Oy, Turku, Finland. Data analysis was also carried out by Leiras Oy. Dr Hannele Savonius is acknowledged for technical help during the study.
Footnotes
Funding: This study was supported by Leiras Oy, Turku, Finland, in the form of a grant, drugs, and statistical analysis.
Conflict of interest: None.
References
- 1.Vessey MP, Villard-Mackintosh L, McPherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. Br J Obstet Gynaecol. 1992;99:402–407. doi: 10.1111/j.1471-0528.1992.tb13758.x. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of General Practitioners, Office of Population Censuses and Surveys, Department of Health and Social Security. 1981-1982 morbidity statistics from general practice. Third national study. London: HMSO, 1986. (Series MB5 No 1.)
- 3.Coulter A, Kelland J, Peto V, Rees MC. Treating menorrhagia in primary care. An overview of drug trials and a survey of prescribing practice. Int J Technol Assess Health Care. 1995;11:456–471. doi: 10.1017/s0266462300008679. [DOI] [PubMed] [Google Scholar]
- 4.Shaw RW. Assessment of medical treatments for menorrhagia. Br J Obstet Gynaecol. 1994;101(suppl 11):15–18. doi: 10.1111/j.1471-0528.1994.tb13690.x. [DOI] [PubMed] [Google Scholar]
- 5.Magos AL, Baumann R, Turnbull AC. Transcervical resection of endometrium in women with menorrhagia. BMJ. 1989;298:1209–1212. doi: 10.1136/bmj.298.6682.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paskowitz RA. “Rollerball” ablation of the endometrium. J Reprod Med. 1995;40:333–336. [PubMed] [Google Scholar]
- 7.Singer A, Almanza R, Gutierrez A, Haber G, Bolduc LR, Neuwirth R. Preliminary clinical experience with a thermal balloon endometrial ablation method to treat menorrhagia. Obstet Gynecol. 1994;83:732–734. [PubMed] [Google Scholar]
- 8.Lilford R J. Hysterectomy: will it pay the bills in 2007? BMJ 1997;314:160-1. [DOI] [PMC free article] [PubMed]
- 9.Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine systems. Int J Gynecol Pathol. 1986;5:235–241. doi: 10.1097/00004347-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson CG, Johansson EDB, Luukkainen T. A d-norgestrel-releasing IUD. Contraception. 1976;13:503–514. doi: 10.1016/s0010-7824(76)80036-4. [DOI] [PubMed] [Google Scholar]
- 11.Luukkainen T, Lähteenmäki P, Toivonen J. Levonorgestrel-releasing intrauterine system. Ann Med. 1990;22:85–90. doi: 10.3109/07853899009147248. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson CG. Comparative quantitation of menstrual blood loss with a D-norgestrel-releasing IUD and a Nova-T-Copper device. Contraception. 1977;15:379–387. doi: 10.1016/0010-7824(77)90001-4. [DOI] [PubMed] [Google Scholar]
- 13.Andersson K, Rybo G. Levonorgestrel-releasing intrauterine system in the treatment of menorrhagia. Br J Obstet Gynecol. 1990;97:690–694. doi: 10.1111/j.1471-0528.1990.tb16240.x. [DOI] [PubMed] [Google Scholar]
- 14.Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879–883. doi: 10.1016/s0002-9378(11)90533-x. [DOI] [PubMed] [Google Scholar]
- 15.Jaeschke R, Singer J, Guyatt GH. A comparison of seven-point and visual analogue scales. Cont Clin Trials. 1990;11:43–51. doi: 10.1016/0197-2456(90)90031-v. [DOI] [PubMed] [Google Scholar]
- 16.Suvisaari J, Lähteenmäki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel-releasing intrauterine system. Contraception. 1996;54:201–208. doi: 10.1016/s0010-7824(96)00189-8. [DOI] [PubMed] [Google Scholar]
- 17.Coulter A, Peto V, Jenkinson C. Quality of life and patient satisfaction following treatment for menorrhagia. Fam Pract. 1994;11:394–401. doi: 10.1093/fampra/11.4.394. [DOI] [PubMed] [Google Scholar]
- 18.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use. Contraception. 1994;49:56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]