Abstract
OBJECTIVE:
To assist in the diagnosis of retinopathy of prematurity (ROP) to facilitate treatment in a timely manner to help prevent blindness.
DATA SOURCES:
Systematic review using MEDLINE including the following key words, “retinopathy of prematurity”, “retrolental fibroplasia”, “blind”, “blindness”, “vision screening”, “cryotherapy”, “cryosurgery”, “laser” and “ablative therapy”. The bibliographies of the references found using the above techniques were scanned for references missed in the primary search.
DATA SELECTION:
Eight population-based studies examining the incidence and severity of ROP were identified. Other studies of ROP were included because they contributed to an understanding of the natural history, treatment or long term outcome of ROP.
DATA EXTRACTION:
Data was analyzed cumulatively from the population-based studies to determine the incidence of ROP. For the natural history, treatment and schedule of eye examinations, data was reported from individual studies.
DATA SYNTHESIS:
Infants at greatest risk of ROP were 1500 g or less at birth, or 30 weeks gestational age or younger. An inverse relationship existed between the incidence and severity of ROP and birth weight or gestational age. The age of onset of ROP was four to six weeks; however, a few newborns presented with an aggressive form of ROP called ’rush disease’ as early as three weeks of age. For those requiring treatment for ROP, the maximum severity was about 11 weeks of age. Long term follow-up for refractive errors was more effective between six and 12 months and again at four years.
CONCLUSION:
Very premature or very low birth weight infants are at highest risk of ROP. Based upon published information, an optimal screening schedule is recommended and a long term follow-up strategy is provided.
Keywords: Blindness, Retinopathy of prematurity, Rush disease
Abstract
OBJECTIF :
Participer au diagnostic de fibroplasie rétrocristallinienne (FRC) afin de faciliter un traitement rapide favorisant la prévention de la cécité.
SOURCE DES DONNÉES :
Une étude systématique sur MEDLINE à l’aide des mots-clés suivants : retinopathy of prematurity, retrolental fibroplasia, blind, blindness, vision screening, cryotherapy, cryosurgery, laser et ablative therapy. On a numérisé les références bibliographiques obtenues par la technique précédente afin de déceler celles qui avaient été omises pendant la recherche originale.
SÉLECTION DES DONNÉES :
On a retenu huit études démographiques portant sur l’incidence et la gravité de la FRC. D’autres études de la FRC ont été incluses parce qu’elles contribuent à mieux comprendre l’évolution naturelle, le traitement ou l’issue à long terme de la FRC.
EXTRACTION DES DONNÉES :
Les données ont été analysées de manière cumulative à partir d’études démographiques pour déterminer l’incidence de FRC. Pour ce qui est de l’évolution naturelle, du traitement et du calendrier des examens oculaires, les données proviennent d’études individuelles.
SYNTHÈSE DES DONNÉES :
Les nourrissons les plus vulnérables à la FRC pesaient 1 500 g ou moins à la naissance ou avaient 30 semaines ou moins d’âge gestationnel. L’incidence et la gravité de la FRC sont inversement proportionnelles au poids à la naissance ou à l’âge gestationnel. La FRC se manifeste entre l’âge de quatre et six semaines, mais quelques nouveaunés présentent une forme agressive de FRC, la «maladie précipitée», dès l’âge de trois semaines. Pour ceux qui ont besoin d’un traitement contre la FRC, la gravité maximale s’observe à l’âge d’environ 11 semaines. Le suivi à long terme du pouvoir réfractif est plus efficace entre six et douze mois, puis à quatre ans.
CONCLUSION :
Les nourrissons très prématurés ou de très petit poids à la naissance sont les plus vulnérables à la FRC. Selon les renseignements publiés, on recommande un calendrier de dépistage optimal et on propose une stratégie de suivi à long terme.
Retinopathy of prematurity (ROP), formerly called retrolental fibroplasia, a disorder that can cause blindness in premature infants, was first described in 1942 by Terry (1). The outcome of a multicentre, randomized controlled trial of ablative therapy using cryotherapy for ROP (2) has brought hope of avoiding blindness from this disorder. This trial showed that 43% of untreated eyes with severe retinal involvement developed an unfavourable ophthalmological outcome compared with 22% of eyes managed with ablative treatment using cryotherapy. It is, therefore, essential that those who care for preterm infants know whom and when to screen for ROP. The purpose of this paper is to examine available evidence critically in order to answer the following questions:
Who is at high risk of developing ROP?
Who should examine the eyes of preterm infants to identify ROP?
When should ophthalmological examinations be performed?
When should treatment be administered for ROP?
When should long term follow-up of those with ROP be performed?
Although most of the information in this review is directed at neonatologists and ophthalmologists, the information about follow-up after discharge from the neonatal intensive care unit is of importance to paediatricians and family physicians.
Definition of terms: For the purposes of this review, the following definitions of terms are used.
Plus disease: Presence of ROP in which there is dilation and tortuosity of vessels in the posterior part of the retina.
Rush disease: A rapidly progressive form of ROP with a poor prognosis.
Threshold disease: At least five contiguous or eight cumulative clock hours of stage 3 ROP in zone 1 or 2 when ‘plus disease’ is present.
METHODS
A computerized search of MEDLINE from January 1966 to December 1997 was performed for the following key words: “retinopathy of prematurity”, “retrolental fibroplasia”, “blind”, “blindness”, “vision screening”, “cryotherapy”, “cryosurgery”, “laser” and “ablative therapy”. No language restrictions were applied when conducting this review. However, all population-based studies were found in English language publications. To obtain as complete a data set of references as possible, the bibliographies of the references found using the above techniques were scanned for references missed in the primary search.
To define a group at high risk of ROP, only population-based cohort studies of high risk infants who were screened from six to seven weeks postnatal age until the peripheral retina was fully vascularized were included. Although the 1984 International Classification of Retinopathy of Prematurity (3) has made the diagnosis of ROP more consistent, articles were not excluded if they failed to use this classification system.
To answer the question of when to start screening for ROP, studies were included in this review if they used early ophthalmological evaluations of infants at risk of ROP. Because there are limited data in the literature of ‘rush disease’, experience from case reports or other anecdotal experience was also included.
To study the frequency of eye screens, studies that included a group at high risk for ROP and provided details of ophthalmological examination schedules were included. Studies were included if ophthalmological examinations were initiated no later than at six to seven weeks postnatal age and follow-up examinations occurred at least every two weeks until the retina was fully vascularized or the infant required treatment for ROP. The data on the natural history did not all come from population-based studies.
Finally, the question of ablative therapy for ROP was addressed (2,4,5), and research priorities were identified.
RESULTS
Who is at high risk of developing ROP?
Answer: Infants born at 30 weeks’ gestation and younger or those weighing 1500 g or less at birth are at high risk.
Twenty-one population-based studies satisfying the search criteria were found (6–26). Nine studies (14–22) did not describe ROP by stage and were not considered further, and another four studies were found to include duplicate data (23–26), leaving eight population-based studies for inclusion (Table 1). Several other studies (27–39) were not included because they were not population-based. Of the eight population-based studies, one was from an exclusively urban population (7), while the others were a mix of rural and urban populations.
TABLE 1:
Basic characteristics of eight population-based studies of retinopathy of prematurity (ROP)
| First author (reference) | Entry criteria for ROP* screening | Era of screening | Country or region of origin | Follow-up rate |
|---|---|---|---|---|
| Saigal et al 1984 (12) | Birth weight 501 to 1000 g | 1977 to 1980 | Ontario, Canada | 92% |
| Ng et al 1988 (10) | Birth weight 1700 g or less and survived three weeks or longer | 1985 to 1987 | Three health regions, Great Britain | 93% |
| Darlow 1988 (11) | Birth weight 500 to 1499 g and survived | 1986 | New Zealand | 93% |
| Fledelius 1990 (9) | Birth weight 2 kg or less, or gestation 34 weeks or less and “risk factors not specified” | 1982 to 1987 | Frederiksborg County, Denmark | Unknown |
| Holmström et al 1993 (6) | Birth weight less than 1500 g and survived eight weeks or longer | 1988 to 1990 | Stockholm County, Sweden | 88% |
| Barnekow and Stigmar 1993 (8) | Gestation less than 33 weeks or birth weight 1500 g or less | Unknown | Region of southern Sweden | 90% |
| Maly 1993 (7) | Gestation less than 33 weeks or birth weight less than 1500 g | 1986 to 1990 | Malmö, Sweden | 90% |
| Haugen and Markestad 1997 (13) | Birth weight less than 1500 g | 1989 to 1993 | Two counties in western Norway | 85% |
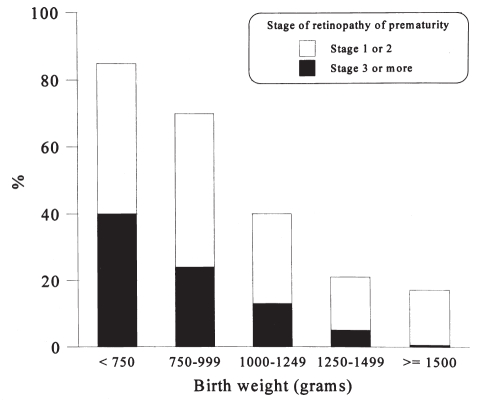
Figure 1 which was derived cumulatively (n=698) from four studies that reported ROP by stage in 250 g birth weight groups (6,8–10), graphically depicts the incidence of ROP and shows an inverse relationship between birth weight and the incidence of ROP. Table 2 summarizes the incidence of severe ROP (stage 3 or more) in survivors (n=2005) by birth weight (6,8–12). Figure 1 and Table 2 differ because not all studies reported data by 250 g birth weight groups; fewer studies are included in Figure 1.
Figure 1).
Incidence of retinopathy of prematurity based upon data cumulatively derived from surviving infants
TABLE 2:
Incidence of retinopathy of prematurity, stage 3 or higher, in surviving infants by birth weight
| First author (reference) | 1000 g or less | Birth weight grouping 100 to 11500 g | More than 1500 g |
|---|---|---|---|
| Saigal et al 1984 (12) | 15/117 (13%) | ||
| Ng et al 1988 (10) | 14/70 (20%) | 7/261 (3%) | 0/174 (0%) |
| Darlow 1988 (11) | 11/84 (13%) | 1/229 (<1%) | |
| Fledelius 1990 (9) | 2/20 (10%) | 9/104 (9%) | 3/287 (1%) |
| Holmström et al 1993 (6) | 25/74 (34%) | 27/186 (14%) | |
| Barnekow and Stigmar 1993 (8) | 5/23 (22%) | 1/100 (1%) | 0/69 (0%) |
| Haugen and Markestad 1997 (13) | 6/66 (9%) | 1/141 (<1%) | |
| Total | 76/454 | 46/1021 | 3/530 |
| Range | 9% to 34% | <1% to 14% | 0% to 1% |
Both Figure 1 and Table 2 indicate that infants with more than a 1500 g birth weight have a very low risk of developing ROP, particularly stage 3 or greater retinopathy that occurs in less 1% of these infants. These infants therefore would be expected to be at very low risk for blindness from ROP. Furthermore, the British Columbia Health Surveillance Registry of all births in the province between 1952 and 1986 (21) reported a rate of blindness due to ROP in British Columbia in infants greater than 1500 g birth weight of only two per 100,000 live births. Most of the infants with blindness in this group were born between 1952 and 1954, during the early epidemic of ROP. Ng et al (10) showed that none of 485 survivors who weighed more than 1500 g at birth became blind while Fledelius (9) noted that, of the three of 287 who developed stage 3 or more ROP, only one became blind. However, in a nationwide registry of visual impairment from 1971 to 1988 in Denmark (40) where registration is compulsory to ensure educational and social benefits, 13 of 139 infants who were blind due to ROP weighed more than 1500 g at birth, and 20 of 107 were 31 weeks gestational age or older. Unfortunately, the total number of births greater than 1500 g birth weight or 31 weeks gestational age or older was not reported in this publication. Therefore, the relative risk for heavier or more mature infants cannot be determined with certainty.
None of the eight population-based reports (6–13) identified factors that led to the development of ROP in the heavier or more mature infant. Of the smaller infants, Holmström et al (25) noted a statistical association between lower gestational age, lower birth weight or maternal essential hypertension and the incidence of ROP. The one heavier infant in Fledelius’ study (9) who became blind was a 1595 g birth weight, 29 week infant who, according to Fledelius, had rush disease. The possibility of genetic susceptibility to ROP, for example, among aboriginal people in Alaska (41) or among people from Asia (42), may explain some of the risk. Arrøe and Peitersen (37), reporting a hospital-based population of infants 32 weeks or younger, noted that ROP, when corrected for birth weight or gestational age, showed independent statistically significant associations with early intubation, hypotension, persistent ductus arteriosus and necrotising enterocolitis. Cats and Tan (27) conducted a case controlled series of 56 infants with ROP matched for sex, birth weight and gestational age with 56 controls, and found that septicemia and a greater number of blood transfusions were associated with a higher incidence of ROP. Oxygen administration has been noted to be associated with retrolental fibroplasia (43); however, use of the transcutaneous oxygen monitoring has failed to reduce the incidence of ROP (44). Other theoretical predisposing factors of ROP in infants who weigh 1500 g or more at birth include being large for gestational age, such as infants of diabetic mothers or infants with hydrops fetalis, and infants who are of low gestational age and may have increased needs for oxygen.
It might seem more rational to identify infants at risk of ROP based upon gestational age (Table 3). Two factors, however, limit the use of gestational age as the only screening criterion: first, four of the population-based studies included infants in their screening programs based solely on birth weight (Table 1) and second, only two of the studies (6,7), both from Sweden, gave a description of the method of assigning gestation. The study from the county of Stockholm used maternal dating (6) while that from the city of Malmö (7) used antenatal ultrasound dating. These two techniques may not give the same gestational age. It has been suggested that there is a consistently lower estimate of gestation by ultrasound than by maternal dating (45). In another study, it was shown that factors known to affect fetal size resulted in a systematically lower gestational age assessment (46). Despite these limitations, ROP seems to be more frequent in infants 30 weeks or younger.
TABLE 3:
Retinopathy of prematurity, stage 3 or higher, in surviving infants by gestational age
| First author | 28 weeks or younger | Gestational age in weeks | Older than 32 weeks | |
|---|---|---|---|---|
| 29 to 30 weeks | 31 to 32 weeks | |||
| Darlow 1988 (11) | 11/124 (9%) | 1/112 (1%) | 0/50 (0%) | 0/27 (0%) |
| Maly 1993 (7) | 19/47 (40%) | |||
| Fledelius 1990 | 6/30 (20%) | 6/63 (10%) | 1/81 (1%) | 0/237 (0%) |
| Holmström et al 1993 (6) | 32/110 (29%) | 16/84 (19%) | 4/44 (9%) | 0/22 (0%) |
| Barnekow and Stigmar 1993 (8) | 6/52 (12%) | 0/49 (0%) | 0/85 (0%) | 0/7 (0%) |
| Total, | 74/363 | 23/308 | 5/260 | 0/293 |
| Range | 9% to 40% | 0% to 19% | 0% to 9% | 0 to 1% |
Who should examine the eyes of preterm infants to identify ROP?
Answer: An observer skilled in the recognition of ROP should conduct the examination.
Eye examination of the preterm infant requires a combination of knowledge of the International Classification of Retinopathy of Prematurity (3) and skills in conducting the examination. Unfortunately, some regions in Canada do not possess the expertise to identify ROP properly and are at a great distance from centres that possess this expertise. This presents special problems in identifying ROP in infants cared for in these regions. Proper provision must be made for appropriate ophthalmological assessments at all institutions caring for very low birth weight or very preterm infants.
When should eye assessments be performed?
Answer: The first examination should be performed at four to six weeks. Regular follow-up examinations should occur every two to four weeks if no ROP is identified. For those with ROP, more frequent examinations are necessary.
The issue of when to initiate examinations of the eyes is based upon the studies with early eye assessments. The Japanese were the first to report the rush form of ROP (47–49). Nissenkorn et al (50) later reported three infants with normal ophthalmological examinations at two weeks of age but who had stage 3 ROP at four to five weeks of age. All three were of 26 weeks gestational age, and weighed between 800 and 950 g at birth and represented 5% of cases of ROP. Acheson and Schulenburg (51) noted four of 137 (1.3%) infants from a hospital-based population with rush disease. Four infants in the Danish study by Fledelius (9) were observed to have rush disease, representing 4% of surviving newborns of younger than 31 weeks gestational age or 2.4% of very low birth weight infants. These four children were between 25 and 30 weeks gestational age at birth and had birth weights of 920 to 1595 g. All four developed stage 4 or 5 ROP and ultimately became blind. In the Danish study by Nødgaard et al (15), four of 141 (3%) very low birth weight infants developed ROP within six to nine weeks of birth and progressed to stage 4, representing 16% (four of 25) of all cases of ROP in their region. Although the rush form of ROP was not commented upon in Holmström et al’s study (6), the earliest onset of ROP was at three weeks of age. Finally, in a population-based study of infants born between 1981 and 1985 in East Midlands of England, Fielder and colleagues (26) noted that four of the 143 (3%) infants who developed ROP did so before 35 days postnatal age. Unfortunately, most publications failed to give denominators making it difficult to determine the incidence of rush disease with any certainty; however, it probably represents fewer than 5% of the infants developing ROP.
One publication resulting from the Randomized Trial of Cryotherapy included serial eye examinations of all infants weighing less than 1250 g at birth who were enrolled in the study (52). A precise age of onset could not be easily determined despite initial examinations at four to six weeks of age because 21.5% had already developed some degree of ROP by that age. The first examination occurred at an average age of five weeks of age or 33 weeks post-menstrual age. The age at which severe retinal involvement (threshold disease) was most commonly recognized was 37 weeks postmenstrual age or 11 weeks (range five to 21 weeks) postnatal age.
In the study by Fledelius (9), the mean ± SD postnatal age of onset of ROP was 6.3±1.4 weeks. Postmenstrual age was 36.0±2.3 weeks at onset of disease (range 31 to 42 weeks). Holmström et al (23) found a similar age of onset, 7.9 weeks (range three to 16) postnatal age or 36 weeks (range 31 to 43) weeks postmenstrual age. Fielder et al (42) noted 92% of those with ROP presented between 30 and 40 weeks postmenstrual age.
When should treatment be administered for ROP?
Answer: Treatment should be administered in the presence of stage 3 ROP with severe retinal involvement.
The multicentre trial of ablative treatment was designed to determine whether treating infants with birth weight less than 1251 g and severe retinal involvement reaching threshold disease with cryotherapy could prevent unfavourable ophthalmological outcome. The investigators defined an unfavourable outcome as a retinal fold involving the macula; a retinal detachment involving zone 1 of the posterior pole; or retrolental tissue or ‘mass’. Severe retinal involvement was called ‘threshold disease’ and was defined as at least five contiguous or eight cumulative clock hours of stage 3 ROP in zone 1 or 2 when plus disease was present. Infants with symmetrical severe retinal involvement had one eye randomly assigned to receive ablative treatment with cryotherapy and the other eye assigned to the untreated group. Infants in whom only one eye developed severe retinal involvement were randomly assigned to one of the two groups. An unfavourable outcome occurred in 36 of 153 eyes treated with ablative therapy compared with 67 of 147 untreated eyes (relative risk favouring the treated group 0.52, 95% confidence interval 0.37 to 0.72).
Laser photocoagulation has also been used as ablative therapy and to date four randomized controlled trials have been conducted comparing laser photocoagulation with cryotherapy (53–56). Preliminary results suggest the two techniques give similar results but more work needs to be done to clarify the indications for each technique. It is not the purpose of this publication to evaluate the various techniques of ablative therapy for severe retinal involvement from ROP.
Some ophthalmologists believe that severe retinal involvement may regress and thus avoid the need for surgery. If this decision is made, it is absolutely essential to examine the eyes more frequently, usually once daily until regression occurs.
When should long term follow-up for ROP begin?
Answer: Long term follow-up should begin within six to 12 months postcorrected gestational age and at age four years.
As part of the ablative treatment with cryotherapy for ROP, Dobson et al (4) showed that eyes of infants with less severe retinal involvement generally have intact visual acuity, although later complications including strabismus, nystagmus, myopia, refractive errors, retinal hemorrhage, amblyopia or retinal detachment have been noted in infants even when there is little or no ROP at routine screening (4,5,39). Screening such infants within one year and again around four years of age for these potential complications and to test for visual acuity is recommended. Dobson et al (4) also showed that 50% of those eyes with severe (threshold) retinal involvement had visual acuity so low that acuity testing could not be performed and the remainder had significantly lower acuity than those with less severe or no retinal disease. Some of these infants could be identified with poor visual acuity as early as one year of age.
Unresolved issues
Research priorities need to focus primarily on an effective means of prevention. In a systematic review (57), only restriction of oxygen and vitamin A supplementation have shown any reduction in the incidence of ROP, while neither vitamin E nor indomethacin used prophylactically have shown reductions in the incidence of ROP. Other areas requiring systematic reviews include the use of surfactants or postnatal steroids.
The current treatment for ROP is ablative therapy, with either cryotherapy or laser photocoagulation for severe retinal involvement. Further randomized controlled trials comparing the two techniques will need to be conducted to determine whether one technique is superior.
A Canadian surveillance system is recommended because it would provide more information about risk factors and the long term outcome of ROP.
Finally, it is clear from reviewing the literature that there are different recommendations regarding when to start and how often to screen for ROP. Further clarification of the timing of the initial and subsequent eye examinations in the neonatal intensive care unit is needed.
RECOMMENDATIONS
Based upon the data presented above, the following recommendations can be made.
Risk groups
When accurately known, a gestational age of 30 weeks or less should be used to identify newborns requiring screening for ROP (Table 3). Because gestational age is not always known and is more prone to bias (45,46) than birth weight, those infants 1500 g birth weight or less should also be screened (Figure 1, Table 2). A combination of gestational age and birth weight should identify almost all infants at risk of ROP with severe retinal involvement. For example, in Fledelius’ study (9), the one blind infant weighing more than 1500 g at birth was 29 weeks gestational age, and of the two others who developed severe myopia, one was younger than 31 weeks and the other was 35 to 36 weeks. Other infants may need to be examined on the basis of individual patient problems. Occasionally in discussions between ophthalmologists and neonatologists, local problems may reveal infants in that region at increased risk for ROP and, hence, they may need to be added to the centre’s own screening program.
Eye examinations
Fundoscopic examination of the retina of preterm infants is a procedural skill requiring ongoing practice to develop and maintain. The examiner should be thoroughly familiar with the International Classification of Retinopathy of Prematurity (3). Ophthalmologists are, for the most part, the only group currently possessing this unique combination of skills.
Schedule of ophthalmological examinations
Based upon the data presented, it is appropriate to use corrected gestational age to schedule assessments. Such assessments might reasonably start around 31 to 32 weeks corrected gestational age. However, as discussed previously, gestational age is not always known, and post-menstrual age may be unavailable. Consequently, a more pragmatic approach is to use postnatal age starting with the first assessment around four to six weeks of age to identify the rush form of ROP. Repeat assessments should be scheduled at regular intervals, at least every two to four weeks, to identify severe potentially treatable ROP (52). Evaluations should continue until the retina is adequately vascularized, even if the newborn has been transferred or discharged from the high risk neonatal centre. In the event that ROP does occur, then more frequent examinations should be scheduled as required.
Management of severe retinal involvement
Infants with severe retinal involvement due to ROP should either be examined daily to see if the disease regresses or have immediate ablative therapy of the vascular retina.
Long term follow-up ophthalmological examinations
Because of the risk of reduced visual acuity in those with severe retinal involvement (threshold disease), an ophthalmological evaluation should occur by one year of age (4,5). Because moderate degrees of retinal involvement are also associated with some reduction in visual acuity at age three and a half to four years, an ophthalmological examination should be scheduled for four years of age.
Footnotes
FETUS AND NEWBORN COMMITTEE
Members: Drs Daniel Faucher, Royal Victoria Hospital, Montreal, Quebec; Douglas McMillan (chair), Foothills Hospital, Calgary, Alberta; Arne Ohlsson, Women’s College Hospital, Toronto, Ontario; Michael Vincer (principal author), IWK Grace Health Centre, Halifax, Nova Scotia; Robin Walker, Children’s Hospital of Eastern Ontario, Ottawa, Ontario; John Watts (director responsible), Children’s Hospital at Hamilton Health Sciences Corporation, Hamilton, Ontario
Liaisons: Ms Debbie Askin, St Boniface Hospital, Winnipeg, Manitoba (Neonatal Nursing); Drs Cheryl Levitt, McMaster University Medical Centre, Hamilton Health Sciences Corporation, Hamilton, Ontario (College of Family Physicians of Canada); William Oh, Rhode Island Hospital, Providence, Rhode Island (Committee on Fetus and Newborn, American Academy of Pediatrics); James Lemons, Riley Hospital for Children, Indianapolis, Indiana (Committee on Fetus and Newborn, American Academy of Pediatrics); Robert Liston, IWK Grace Health Centre, Halifax, Nova Scotia (Maternal-Fetal Medicine Committee, Society of Obstetricians and Gynaecologists of Canada); Catherine McCourt, Laboratory Centre for Disease Control, Health Canada, Ottawa, Ontario (Health Canada); Reg Sauve, Alberta Children’s Hospital, Calgary, Alberta (Section of Neonatal Perinatal Medicine, Canadian Paediatric Society)
REFERENCES
- 1.Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: I Preliminary report. Am J Ophthalmol. 1942;25:203. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicentre trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 1988;106:471–9. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 3.The committee for the classification of retinopathy of prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–4. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 4.Dobson V, Quinn GE, Summers CG, et al. Effect of acute-phase retinopathy of prematurity on grading acuity development in the very low birth weight infant. Invest Ophthalmol Vis Sci. 1994;35:4236–44. [PubMed] [Google Scholar]
- 5.Cryotherapy for retinopathy of prematurity cooperative group The natural ocular outcome of premature birth and retinopathy: Status at 1 year. Arch Ophthalmol. 1994;112:903–12. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- 6.Holmström G, el Azazi M, Jacobson L, et al. A population-based, prospective study of the development of ROP in prematurely born children in the Stockholm area of Sweden. Br J Ophthalmol. 1993;77:417–23. doi: 10.1136/bjo.77.7.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maly E. Frequency and natural history of retinopathy of prematurity (ROP): A prospective study in a Swedish city 1986–1990. Acta Ophthalmol. 1993;210(S):52–5. doi: 10.1111/j.1755-3768.1993.tb04153.x. [DOI] [PubMed] [Google Scholar]
- 8.Barnekow BB, Stigmar G. Retinopathy of prematurity in the southern part of Sweden. Acta Ophthalmol. 1993;210(S):48–51. doi: 10.1111/j.1755-3768.1993.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 9.Fledelius HC. Retinopathy of prematurity. Clinical findings in a Danish county 1982–1987. Acta Ophthalmol. 1990;68:209–13. doi: 10.1111/j.1755-3768.1990.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 10.Ng YK, Fielder AR, Shaw DE, Levene MI. Epidemiology of retinopathy of prematurity. Lancet. 1988;ii:1235–8. doi: 10.1016/s0140-6736(88)90820-3. [DOI] [PubMed] [Google Scholar]
- 11.Darlow BA. Incidence of retinopathy of prematurity in New Zealand. Arch Dis Child. 1988;63:1083–6. doi: 10.1136/adc.63.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saigal S, Rosenbaum P, Stoskopf B, et al. Outcome in infants 501–1000 gm birth weight delivered to residents of the McMaster Health Region. J Pediatr. 1984;105:969–76. doi: 10.1016/s0022-3476(84)80093-1. [DOI] [PubMed] [Google Scholar]
- 13.Haugen H, Markestad T. Incidence of retinopathy of prematurity (ROP) in the western part of Norway. A population-based retrospective study. Acta Ophthalmol Scand. 1997;75:305–7. doi: 10.1111/j.1600-0420.1997.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Fledelius HC. Retinopathy of prematurity in Frederiksborg county 1988–1990. Acta Ophthalmol. 1993;210(S):59–62. doi: 10.1111/j.1755-3768.1993.tb04155.x. [DOI] [PubMed] [Google Scholar]
- 15.Nødgaard H, Andreasen H, Hansen H, et al. Risk factors associated with retinopathy of prematurity (ROP) in Northern Jutland, Denmark 1990–1993. Acta Ophthalmol Scand. 1996;74:306–10. doi: 10.1111/j.1600-0420.1996.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 16.Fledius HC. Retinopathy of prematurity in a Danish County: Trends over a 12-year period 1982–93. Acta Ophthalmol Scand. 1996;74:285–7. doi: 10.1111/j.1600-0420.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 17.Pennefather PM, Clarke MP, Strong NP, et al. Ocular outcome in children born before 32 weeks gestation. Eye. 1995;9(Supp):26–30. [PubMed] [Google Scholar]
- 18.Schollin J, Crafoord S, Stenström I, et al. Severe retinopathy of prematurity in infants from the southern regions of Sweden. Acta Pædiatr. 1994;83:408–11. doi: 10.1111/j.1651-2227.1994.tb18129.x. [DOI] [PubMed] [Google Scholar]
- 19.Gallo JE, Holmström G, Kugelberg U, et al. Regressed retinopathy of prematurity in children aged 5–10 years. Acta Ophthalmol. 1993;210(S):41–3. doi: 10.1111/j.1755-3768.1993.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 20.Gallo JE, Holmström G, Kugelberg U, et al. Regressed retinopathy of prematurity and its sequelae in children aged 5–10 years. Br J Ophthalmol. 1991;75:527–31. doi: 10.1136/bjo.75.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson DL, Sheps SB, Hong SH, et al. Retinopathy of prematurity-induced blindness: Birth weight specific survival and the new epidemic. Pediatrics. 1990;86:405–12. [PubMed] [Google Scholar]
- 22.Gibson DL, Sheps SB, Schechter MT, et al. Retinopathy of prematurity: A new epidemic? Pediatrics. 1989;83:486–92. [PubMed] [Google Scholar]
- 23.Holmström G, el Azazi M, Jacobson L, et al. Epidemiology of ROP in the Stockholm area of Sweden. Acta Ophthalmol. 1993;210(S):44–7. doi: 10.1111/j.1755-3768.1993.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 24.Darlow BA, Horwood LJ, Clemett RS. Retinopathy of prematurity: risk factors in a prospective population-based study. Paediatr Perinat Epidemiol. 1992;6:62–80. doi: 10.1111/j.1365-3016.1992.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 25.Holmström G, Thomassen P, Broberger U. Maternal risk factors for retinopathy of prematurity – a population-based study. Acta Obstet Gynecol. 1996;75:628–35. doi: 10.3109/00016349609054687. [DOI] [PubMed] [Google Scholar]
- 26.Fielder AR, Ng YK, Levene MI. Retinopathy of prematurity: Age of onset. Arch Dis Child. 1986;61:774–8. doi: 10.1136/adc.61.8.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cats BP, Tan KEWP. Retinopathy of prematurity: review of a four-year period. Br J Ophthalmol. 1985;69:500–3. doi: 10.1136/bjo.69.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn JT, Bancalari E, Bachynski BN, et al. Retinopathy of prematurity: Diagnosis, severity and natural history. Ophthalmol. 1987;94:620–9. [PubMed] [Google Scholar]
- 29.Phelps DL, Rosenbaum AL, Isenberg SJ, et al. Tocopherol efficacy and safety for preventing retinopathy of prematurity: A randomized controlled, double-masked trial. Pediatrics. 1987;79:489–500. [PubMed] [Google Scholar]
- 30.Darlow BA, Clemett RS. Retinopathy of prematurity: screening and optimal use of the ophthalmologist’s time. Aust NZ J Ophthalmol. 1990;18:41–6. doi: 10.1111/j.1442-9071.1990.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 31.Quinn GE, Johnson L, Otis C, et al. Incidence, severity and time course of retinopathy of prematurity in a randomized clinical trial of vitamin E prophylaxis. Doc Ophthalmol. 1990;74:223–8. doi: 10.1007/BF02482612. [DOI] [PubMed] [Google Scholar]
- 32.Acheson JF, Schulenburg WE. Surveillance for retinopathy of prematurity in practice: Experience from one neonatal intensive care unit. Eye. 1991;5:80–5. doi: 10.1038/eye.1991.16. [DOI] [PubMed] [Google Scholar]
- 33.Nissenkorn I, Ben Sira I, Kremer I, et al. Eleven years’ experience with retinopathy of prematurity: Visual results and contribution of cryoablation. Br J Ophthalmol. 1991;75:158–9. doi: 10.1136/bjo.75.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielder AR, Robinson J, Shaw DE, et al. Light and retinopathy of prematurity: Does retinal location offer a clue? Pediatrics. 1992;89:648–3. [PubMed] [Google Scholar]
- 35.Griesen G. Retinopathy of prematurity in three follow-up studies from Rigshospitalet during the period 1976–1987. Acta Ophthalmol. 1993;210(S):30–3. doi: 10.1111/j.1755-3768.1993.tb04147.x. [DOI] [PubMed] [Google Scholar]
- 36.Lappi M. Retinopathy of prematurity in southern Finland. Acta Ophthalmol. 1993;210(S):56–8. doi: 10.1111/j.1755-3768.1993.tb04154.x. [DOI] [PubMed] [Google Scholar]
- 37.Arrøe M, Peitersen B. Retinopathy of prematurity: Review of a seven-year period in a Danish neonatal intensive care unit. Acta Paediatr. 1994;83:501–5. doi: 10.1111/j.1651-2227.1994.tb13067.x. [DOI] [PubMed] [Google Scholar]
- 38.Seiberth V, Linderkamp O, Knorz MC, et al. Controlled clinical trial of light and retinopathy of prematurity. Am J Ophthalmol. 1994;118:492–5. doi: 10.1016/s0002-9394(14)75801-5. [DOI] [PubMed] [Google Scholar]
- 39.Fleck BW, Wright E, Dhillon B, et al. An audit of the 1995 Royal College of Ophthalmologist guidelines for screening for retinopathy of prematurity applied retrospectively in one regional neonatal intensive care unit. Eye. 1995;9(S):31–5. [PubMed] [Google Scholar]
- 40.Fledelius HV, Rosenberg T. Retinopathy of prematurity. Where to set screening limits? Recommendations based on two Danish surveys. Acta Paediatr Scand. 1990;79:906–10. doi: 10.1111/j.1651-2227.1990.tb11351.x. [DOI] [PubMed] [Google Scholar]
- 41.Arnold RW, Kesler K, Avila E. Susceptibility to retinopathy of prematurity in Alaskan Natives. J Pediatr Ophthalmol Strabismus. 1994;31:192–4. doi: 10.3928/0191-3913-19940501-12. [DOI] [PubMed] [Google Scholar]
- 42.Fielder AR, Shaw DE, Robinson J, et al. Natural history of retinopathy of prematurity: A prospective study. Eye. 1992;6:233–42. doi: 10.1038/eye.1992.46. [DOI] [PubMed] [Google Scholar]
- 43.Kinsey VE, Hemphill FM. Etiology of retrolental fibroplasia and preliminary report of cooperative study of retrolental fibroplasia. Trans Am Acad Ophthalmol Otolaryngol. 1955;55:15–24. [PubMed] [Google Scholar]
- 44.Flynn JY, Bancalari E, Bawol R, et al. Retinopathy of prematurity: A randomized, prospective trial of transcutaneous oxygen monitoring. Ophthalmol. 1987;94:630–8. doi: 10.1016/s0161-6420(87)33400-1. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg RL, Davis RO, Cutter GR, et al. Prematurity, postdates, and growth retardation: The influence of use of ultrasonography on reported gestational age. Am J Obstet Gynecol. 1989;160:462–70. doi: 10.1016/0002-9378(89)90473-0. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen TB, Wilcox AJ, Hedegaard M, et al. Bias in studies of preterm and postterm delivery due to ultrasound assessment of gestational age. Epidemiol. 1995;6:533–7. doi: 10.1097/00001648-199509000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Uemura Y. Current status of retrolental fibroplasia: Report of the Joint Committee for the study of retrolental fibroplasia in Japan. Jpn J Ophthalmol. 1977;21:366–78. [Google Scholar]
- 48.Majima A. Studies on retinopathy of prematurity. 1. Statistical analysis of factors related to the occurrence and progression in active phase. Jpn J Ophthalmol. 1977;21:404–20. [Google Scholar]
- 49.Yamagishin N, Nagata M. Survey of the cicatricial stages of retinopathy of prematurity after treatment with photocoagulation. Folia Ophthalmol Jpn. 1979;30:101–6. [Google Scholar]
- 50.Nissenkorn I, Kremer I, Gilad E, et al. ‘Rush’ type retinopathy of prematurity: Report of three cases. Br J Ophthalmol. 1987;71:559–62. doi: 10.1136/bjo.71.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acheson JF, Schulenburg WE. Surveillance for retinopathy of prematurity in practice: Experience from on neonatal intensive care unit. Eye. 1991;5:80–5. doi: 10.1038/eye.1991.16. [DOI] [PubMed] [Google Scholar]
- 52.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. Ophthalmol. 1991;98:1628–40. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 53.Iverson DA, Trese MT, Orgel IK, et al. Laser photocoagulation for threshold retinopathy of prematurity. Arch Ophthalmol. 1991;109:1342–3. doi: 10.1001/archopht.1991.01080100022007. [DOI] [PubMed] [Google Scholar]
- 54.McNamara JA, Tasman W, Brown GC, et al. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology. 1991;98:576–680. doi: 10.1016/s0161-6420(91)32247-4. [DOI] [PubMed] [Google Scholar]
- 55.McNamara JA, Tasman W, Vander JF, et al. Diode laser photocoagulation for retinopathy of prematurity: Preliminary results. Arch Ophthalmol. 1992;110:1714–6. doi: 10.1001/archopht.1992.01080240054029. [DOI] [PubMed] [Google Scholar]
- 56.Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. Ophthalmol. 1993;100:238–44. doi: 10.1016/s0161-6420(93)31664-7. [DOI] [PubMed] [Google Scholar]
- 57.Watts JL. Retinopathy of prematurity. In: Sinclair JC, Bracken M, editors. Effective Care of the Newborn Infant. Oxford: Oxford University Press; 1992. pp. 617–39. [Google Scholar]