Abstract
INTRODUCTION:
Several meta-analyses have examined the efficacy of smoking cessation therapies in the general population. However, little is known about the efficacy of these therapies in cardiac patients. Therefore, a meta-analysis of randomized controlled trials (RCTs) was performed to determine the efficacy of behavioural therapy and pharmacotherapy for smoking cessation in cardiac patients.
METHODS:
The medical literature was systematically reviewed to identify smoking cessation RCTs in cardiac patients. Only RCTs that reported smoking abstinence at six or 12 months were included. Smoking abstinence was examined based on the ‘most rigorous criterion’, defined as the most conservative outcome reported in any given RCT.
RESULTS:
Eleven behavioural therapy RCTs that enrolled 2105 patients and four pharmacotherapy RCTs that enrolled 1542 patients were identified. RCTs differed in the type of behavioural therapy administered as well as the total length and duration of the intervention. RCTs differed in the type of pharmacotherapy administered (one nicotine patch RCT, one nicotine gum RCT and two bupropion RCTs). Behavioural therapy was associated with a significantly higher proportion of smoking abstinence than usual care (OR 1.97 [95% CI 1.37 to 2.85]). Pharmacotherapies were more efficacious than placebo (pooled OR 1.72 [95% CI 1.15 to 2.57]).
CONCLUSIONS:
Both behavioural therapy and pharmacotherapy are more efficacious than usual care for smoking cessation in cardiac patients. The present meta-analysis highlights the need for head-to-head RCTs to identify which smoking cessation therapy is preferred in cardiac patients as well as RCTs examining the efficacy of combined behavioural and pharmacotherapies.
Keywords: Behavioural therapy, Cardiac patients, Smoking cessation, Smoking cessation pharmacotherapy
Abstract
INTRODUCTION :
Plusieurs méta-analyses se sont penchées sur l’efficacité des mesures antitabagiques dans la population générale. Par contre, on en connaît peu sur l’efficacité de ces stratégies chez les patients cardiaques. C’est pourquoi on a procédé à une méta-analyse des essais randomisés et contrôlés (ERC) afin de mesurer l’efficacité des approches antitabagiques comportementales et pharmacothérapeutiques chez les patients cardiaques.
MÉTHODES :
Les auteurs ont passé en revue systématiquement la littérature médicale afin de relever les ERC ayant porté sur l’abandon du tabagisme chez des patients cardiaques. Les auteurs n’ont retenu que les ERC qui faisaient état d’une abstinence d’une durée de six ou 12 mois. L’abstinence a été analysée en fonction du critère le plus rigoureux défini par le paramètre le plus conservateur signalé parmi tous les ERC.
RÉSULTATS :
Les auteurs ont retenu 11 ERC portant sur une approche comportementale qui regroupaient 2 105 patients et quatre ERC portant sur une approche pharmacothérapeutique qui regroupaient 1 542 patients. Les premiers différaient quant à l’approche comportementale utilisée et quant à la durée totale de l’intervention. Les seconds différaient quant au type de pharmacothérapie administrée (un, portait sur un timbre de nicotine, un, sur une gomme de nicotine et deux, sur le bupropion). L’approche comportementale a été associée à une proportion significativement plus élevée d’abstinence par rapport à l’approche habituelle (RC 1,97 [IC à 95 %, 1,37 à 2,85]). Les pharmacothérapies ont été plus efficaces que le placebo (RC regroupé 1,72 [IC à 95 %, 1,15 à 2,57]).
CONCLUSIONS :
Les approches comportementales et pharmacothérapeutiques favorisent plus efficacement l’abandon tabagique que les approches habituelles chez les patients cardiaques. La présente méta-analyse rappelle la nécessité de réaliser d’une part, des ERC comparatifs directs pour déterminer quelle approche antitabagique convient le mieux aux patients cardiaques et d’autre part, des ERC pour vérifier l’efficacité des approches comportementales et pharmacothérapeutiques utilisées concomitamment.
Almost 30% of all coronary artery disease (CAD)-related deaths in North America are attributable to cigarette smoking (1). Cigarette smoking promotes atherosclerosis and is associated with an increased risk of angina, myocardial infarction (MI), peripheral vascular disease, stroke and sudden death (1,2). One year after smoking cessation, the risk of CAD in the general population decreases to one-half that of smokers (3). Fifteen years after smoking cessation, the risk of CAD is the same as that of nonsmokers (3). A variety of diverse smoking cessation therapies exist including, among others, behavioural therapies (eg, telephone, group or individual counselling) and pharmacotherapies (eg, nicotine replacement therapy [NRT] and bupropion) (4–9). Randomized controlled trials (RCTs) have demonstrated that these smoking cessation therapies are efficacious in the general population. However, it is less clear whether these therapies are efficacious in cardiac patients. Cardiac patients are at increased risk for cardiac events and therefore, may receive the greatest benefit from efficacious smoking cessation therapies. Furthermore, previous studies have demonstrated that cardiac patients have a greater motivation to quit than otherwise healthy smokers (10). For this reason, we performed a meta-analysis to examine the efficacy of smoking cessation behavioural therapy and pharmacotherapy in cardiac patients.
METHODS
Search strategy
The present meta-analysis was performed in accordance with the guidelines recommended by the Quality of Reporting of Meta-analyses (QUORUM) statement (11). The literature was systematically reviewed to identify RCTs that examined behavioural or pharmacological therapies (including both NRTs and non-NRTs) for smoking cessation in cardiac patients. NRTs included nicotine gum, nicotine inhaler, nicotine nasal spray, nicotine tablet and transdermal nicotine, and non-NRTs included bupropion. The English language medical literature was searched using the MEDLINE, EMBASE and PsycINFO databases and the following key terms: ‘smoking cessation’, ‘smoking intervention’, ‘cardiac patients’, ‘myocardial infarction’, ‘coronary artery disease’, ‘cardiovascular disease’, ‘behavioral therapy’, ‘nicotine replacement therapy’, ‘smoking pharmacotherapy’, ‘smoking cessation aids’, ‘nicotine patch’, ‘nicotine gum’, ‘bupropion’, ‘nicotine inhaler’ and ‘clinical trials’. The search was limited to RCTs published between 1970 and August 2007. References cited in identified RCTs were reviewed for additional relevant RCTs. RCTs only published as abstracts were not considered.
Study inclusion and exclusion criteria
Cardiac patients were defined as any patient with cardiovascular disease (CVD), MI, angina, congestive heart failure, arrhythmia or CAD, or any patient who had undergone a cardiac procedure such as coronary artery bypass graft surgery or percutaneous coronary intervention. RCTs examining the efficacy of smoking cessation therapy in any type of cardiac patient were included. This relatively broad inclusion criterion was used because of the small number of smoking cessation RCTs conducted in this patient population. Studies were included even when efficacy was not the primary outcome (eg, if safety was the primary outcome measure and efficacy was a secondary outcome). RCTs that reported either point prevalence or continuous abstinence at six or 12 months were included. The meta-analysis was limited to RCTs that compared behavioural therapy with usual care and to double-blind, placebo-controlled pharmacotherapy RCTs.
The literature search was conducted by one author, and data abstraction was performed by two independent reviewers. Disagreements were resolved by a third reviewer. It is estimated that the two independent reviewers disagreed on less than 5% of data points. Authors of included studies were contacted when necessary to resolve ambiguities and provide additional information.
Classification of outcomes
Smoking abstinence was defined as continuous abstinence from cigarette smoking or point prevalence of abstinence. Continuous abstinence was defined as no smoking between the initial target quit date and the six- or 12-month follow-up time points. Point prevalence was defined as no smoking over a given time period, usually during the seven days preceding the follow-up appointment. Smoking abstinence was examined with respect to the ‘most rigorous criterion’ of abstinence reported, defined as the most conservative outcome reported in any given RCT, based on the following ranking: 1 – continuous abstinence at 12 months; 2 – continuous abstinence at six months; 3 – point prevalence at 12 months; and 4 – point prevalence at six months. This outcome measure has been used previously (7). Outcomes were assessed using an intention-to-treat analysis. All patients (excluding those who had died before follow-up) who were randomly assigned but were unavailable at follow-up were classified as smokers.
Total length of the intervention refers to the total amount of time that behavioural therapy was administered. Duration of the intervention refers to the total amount of time over which the intervention spanned.
Statistical analysis
Using random-effects models, two meta-analyses were conducted. The first analysis combined data from behavioural therapy RCTs and the second pooled data from pharmacotherapy RCTs. There was an insufficient number of RCTs for each type of behavioural therapy (eg, telephone, individual or group counselling) or pharmacotherapy (eg, bupropion, transdermal nicotine patch or nicotine gum) to conduct meaningful analyses by type of intervention. All analyses were conducted using Review Manager 4.2.8 (The Cochrane Collaboration, United Kingdom).
RESULTS
Behavioural therapy
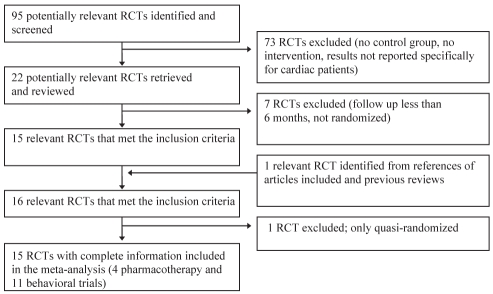
Eleven RCTs comparing the efficacy of behavioural therapy with that of usual care in cardiac patients were identified (Figure 1 and Table 1). These RCTs enrolled a total of 2105 patients. Six additional behavioural studies were identified, but were excluded because patients were not randomly assigned or follow-up was insufficient (12–17). The 11 behavioural RCTs retained involved a broad range of cardiac patients (Table 1). Usual care was defined differently in each study, but most often involved verbal advice from a physician or nurse to quit smoking (1,18–24).
Figure 1).
Flow diagram of randomized controlled trials (RCTs) included in the meta-analysis
TABLE 1.
Trials examining smoking cessation behavioural therapy in cardiac patients
| Study, year | Cardiac population | Sample (n) | Intervention | Time of follow-up (months) | Sessions (n), length of each session | Total length of intervention, duration | Biochemical validation |
|---|---|---|---|---|---|---|---|
| Hajek et al (18), 2002 | MI or CABG | 540 | Booklet, quiz, declare quitting, meet others for support | 12 | 1×, 20–30 min | 20–30 min, 20–30 min | Expired CO and saliva cotinine |
| Ockene et al (1), 1992 | Coronary arteriography | 267 | Inpatient counselling and individual outpatient counselling. Self-help materials: manuals and relaxation tapes. Maintenance training. Telephone calls at weeks 1 and 3 for all patients. A call at 3 months for patients who reported quitting, and calls at months 2 and 4 for patients who reported smoking relapse | 12 | NR, NR | 73 min, 3–4 months | Saliva cotinine |
| DeBusk et al (19), 1994 | AMI | 252* | RN teach to monitor health habits, set goals, use feedback, individual session if relapse, self-efficacy, relapse prevention manual, relaxation tape. NRT option if relapse after discharge. Phone calls at 2 days, 1 week and every month for 5 months | 12 | 1×, 2 h | 2 h, 5 months | Plasma cotinine and expired CO |
| Quist-Paulsen and Gallefoss (20), 2003 | MI or unstable angina or CABG | 240 | Fear arousal/relapse prevention booklet by cardiac RN, telephone calls over 5 months | 12 | 3×, NR | 147 min, 5 months | Urinary cotinine |
| Feeney et al (21), 2001 | AMI | 198 | Stanford Heart Attack Staying Free program (cardiologist quit advice, high-risk situations for relapse identification manual, develop plan to manage these situations, coping strategy counselling for unconfident patients. Audiotapes for home). RN calls at 4 weeks, and 2, 3, 6 and 12 months | 12 | NR, NR | NR, 12 months | Urinary cotinine |
| Taylor et al (22), 1990 | AMI | 173 | RN-managed, relapse prevention focus, high-risk situations for relapse identification manual, audiotapes, telephone calls monthly for 4 months, individual counselling plus NRT if needed | 12 | 1×, NR | NR, 6 months | Expired CO and serum thiocyanate |
| Miller et al (24), 1997 | CVD | 136† | 30 min RN-administered inpatient counselling at bedside. Role-playing to develop relapse prevention plan. Video on smoking relapse. Relaxation audiotape. Four 10 min telephone calls at 2, 7, 21 and 90 days after discharge. RN counselling session at 3 months if relapse | 12 | 1–2×, 30 min | NR, 3 months | Saliva cotinine |
| Dornelas et al (27), 2000 | MI | 100 | Bedside cessation counselling by psychologist, telephone counselling at 1, 4, 8, 12, 16, 20 and 26 weeks, relapse prevention | 12 | 1×, 20 min | 20 min, 6 months | None |
| Rigotti et al (23), 1994 | CABG | 87 | Three RN-delivered behaviour modification program sessions with video and individual counselling | 12 | 3×, 20 min | 60 min,1 week | Saliva cotinine |
| Carlsson et al (25), 1997 | AMI | 67 | Quitting education program, both individually and in groups | 12 | 2–3×, NR | 90 min, 3 months | None |
| Engblom et al (26), 1992 | CABG | 45‡ | Part of a multifactorial rehabilitation program. Smoking habits evaluated by questionnaire. Information about operation and recovery, group discussion with doctor about risk factors for heart disease, nutritionist advice, supervised exercise training. Refresher course at 8 months postoperatively | 12 | NR, NR | NR, 8 months | None |
252 of 585 were smokers;
Sample is part of a larger sample of hospital patients, data from minimal intervention group not included;
Sample is part of a larger sample of patients with coronary artery disease who do not smoke. AMI Acute myocardial infarction; CABG Coronary artery bypass graft surgery; CO Carbon monoxide; CVD Cardiovascular disease; MI Myocardial infarction; NR Not reported; NRT Nicotine replacement therapy; RN Registered nurse
Behavioural RCTs also differed in the type of behavioural therapy administered (Table 1). In most RCTs, the therapy consisted of a major behavioural intervention that included stop-smoking advice or counselling. Most of the counselling administered was individual (one-on-one with a therapist or nurse); two studies used both individual and group therapy (25,26). One study also offered NRT to patients who relapsed (19). Follow-up telephone counselling was conducted in seven studies (1,19–22,24,27), varying in duration from three to 12 months, typically occurring on a monthly basis.
The length and number of behavioural therapy sessions varied between studies, ranging from one to three sessions. Therapy sessions lasted between 20 min and 150 min. The duration of the interventions varied widely, ranging from 20 min to 12 months. Studies also differed in the amount of clinical follow-up data collected, ranging from six months to five years of follow-up. Only one RCT reported point prevalence at six months (1), and nine of 11 RCTs reported continuous abstinence at 12 months (1,18–21,23,25–27) (Table 2). Smoking abstinence was biochemically validated in eight of 11 behavioural RCTs (1,18–24).
TABLE 2.
Results of randomized controlled trials examining smoking cessation behavioural therapy in cardiac patients
| Study, year | Cardiac population | Sample (n) |
6 months continuous abstinence |
12 months |
||||
|---|---|---|---|---|---|---|---|---|
|
Point prevalence |
Continuous abstinence |
|||||||
| Treatment (%) | Control (%) | Treatment (%) | Control (%) | Treatment (%) | Control (%) | |||
| Hajek et al (18), 2002 | MI or CABG | 540 | NR | NR | 39 | 43 | 37 | 41 |
| Ockene et al (1), 1992 | Coronary angiography | 267 | 45 | 34 | 53 | 42 | 35 | 28 |
| DeBusk et al (19), 1994 | AMI | 252 | 69* | 55 | NR | NR | 70* | 53 |
| Quist-Paulsen and Gallefoss (20), 2003 | MI or unstable angina or CABG | 240 | NR | NR | NR | NR | 57* | 37 |
| Feeney et al (21), 2001 | AMI | 198 | NR | NR | NR | NR | 39* | 2 |
| Taylor et al (22), 1990 | AMI | 173 | NR | NR | 61* | 32 | NR | NR |
| Miller et al (24), 1997 | CVD | 136† | NR | NR | 34* | 24 | NR | NR |
| Dornelas et al (27), 2000 | MI | 100 | 67* | 44 | NR | NR | 56* | 35 |
| Rigotti et al (23), 1994 | CABG | 87 | NR | NR | 61 | 54 | 51 | 51 |
| Carlsson et al (25), 1997 | AMI | 67 | NR | NR | NR | NR | 50 | 29 |
| Engblom et al (26), 1992 | CABG | 45 | NR | NR | NR | NR | 44* | 20 |
Statistically significant at P<0.05;
Sample is part of a larger sample of hospitalized patients, data from minimal intervention group not included and 6-month point prevalence was only examined in this study. AMI Acute myocardial infarction; CABG Coronary artery bypass graft surgery; CVD Cardiovascular disease; MI Myocardial infarction; NR Not reported
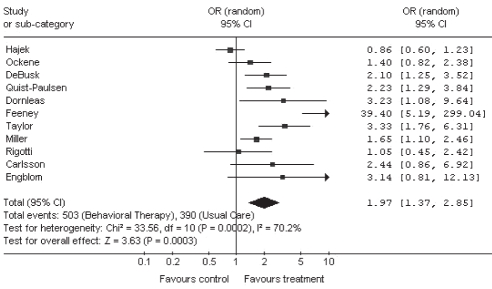
After pooling the results of the 11 RCTs using a random effects model, it was found that behavioural therapy was associated with substantially higher smoking abstinence than usual care (pooled OR 1.97 [95% CI 1.37 to 2.85]) (Figure 2). There were insufficient data to stratify the analyses by type of behavioural therapy (eg, telephone, individual or group counselling).
Figure 2).
Meta-analysis of randomized controlled trials (RCTs) examining the efficacy of smoking cessation behavioural therapy compared with usual care in cardiac patients. Smoking abstinence was examined with respect to the ‘most rigorous criterion’ of abstinence reported, defined as the most conservative outcome reported in any given RCT, based on the following ranking: 1 – continuous abstinence at 12 months; 2 – continuous abstinence at six months; 3 – point prevalence at 12 months; 4 – point prevalence at six months
Pharmacotherapy
Four RCTs examining the efficacy of smoking cessation pharmacotherapy in cardiac patients that met the present study’s inclusion criteria were identified (Figure 1 and Table 3). Each study used a different pharmacotherapy (nicotine gum, transdermal nicotine patch or bupropion), with the exception of bupropion, which was examined in two studies. Two smoking cessation pharmacotherapy RCTs were excluded because they did not have a minimum of six months of follow-up data (28,29).
TABLE 3.
Trials examining smoking cessation pharmacotherapy in cardiac patients
| Study, year | Cardiac population | Sample (n) | Intervention | Duration (weeks) | Time of follow-up (months) | Daily dose (mg) | Biochemical validation |
|---|---|---|---|---|---|---|---|
| Tonstad et al (30), 2003 | CVD (had to have at least one of the following conditions: MI >3 months previously, an interventional cardiac procedure, stable angina, PVD or CHF) | 626 | Bupropion | 7 | 12 | 300* | Expired CO |
| Joseph et al (32), 1996 | CVD (history of MI, CABG, angioplasty, stenosis >50%, angina, CHF, arrhythmia, PVD, CVD or cor pulmonale) | 584 | Nicotine patch | 10 | 6 | 21 for 6 weeks; 14 for 2 weeks; 7 for 2 weeks | Expired CO |
| Rigotti et al (31), 2006 | Acute CVD (patients admitted with MI or unstable angina, CABG or other cardiovascular conditions with documented CAD) | 247 | Bupropion | 12 | 12 | 300* | Saliva cotinine |
| Campbell et al (36), 1991 | In-hospital CVD patients† | 85 | Nicotine gum | NR‡ | 12 | 2§ | Expired CO |
Taken as 150 mg twice daily;
Called heart disease in the study, not specified further. Patients with other smoking-related diseases were included (total n=219);
Median duration of gum use was 37 days;
Stronger gum (4 mg) was offered for up to 3 months to those still smoking. CABG Coronary artery bypass graft surgery; CHF Congestive heart failure; CO Carbon monoxide; CVD Cardiovascular disease; MI Myocardial infarction; NR Not reported; PVD Peripheral vascular disease
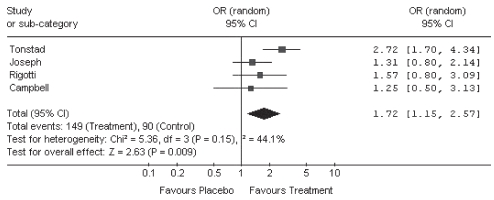
After pooling data from the four pharmacotherapy RCTs using a random effects model, it was determined that pharmacotherapy use was associated with significantly greater smoking abstinence compared with placebo among cardiac patients (OR 1.72 [95% CI 1.15 to 2.57]) (Figure 3). With only one RCT per pharmacotherapy, with the exception of bupropion, there were insufficient data to stratify results for NRTs by type of pharmacotherapy. In the two RCTs examining the efficacy of bupropion, bupropion was associated with higher rates of smoking abstinence in patients with CVD compared with placebo (OR 2.72 [95% CI 1.70 to 4.34]; OR 1.57 [95% CI 0.80 to 3.09]) (Figure 3 and Table 4). The RCTs examining nicotine patch (OR 1.31 [95% CI 0.80 to 2.14]) or nicotine gum (OR 1.25 [95% CI 0.50 to 3.13]) were too small to accurately estimate the ORs and were therefore inconclusive (Figure 3 and Table 4).
Figure 3).
Meta-analysis of randomized controlled trials (RCTs) examining the efficacy of smoking cessation pharmacotherapy compared with placebo in cardiac patients. Smoking abstinence was examined with respect to the ‘most rigorous criterion’ of abstinence reported, defined as the most conservative outcome reported in any given RCT, based on the following ranking: 1 – continuous abstinence at 12 months; 2 – continuous abstinence at six months; 3 – point prevalence at 12 months; 4 – point prevalence at six months
TABLE 4.
Results of randomized controlled trials examining smoking cessation pharmacotherapy in cardiac patients
| Study, year | Cardiac population | Sample (n) | Intervention |
6 months |
12 months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Point prevalence |
Continuous abstinence |
Point prevalence |
Continuous abstinence |
||||||||
| Treatment (%) | Control (%) | Treatment (%) | Control (%) | Treatment (%) | Control (%) | Treatment (%) | Control (%) | ||||
| Tonstad et al (30), 2003 | CVD (had to have at least one of the following conditions: MI >3 months previously, an interventional cardiac procedure, stable angina, PVD or CHF) | 626 | Bupropion | 34* | 12 | 27* | 11 | 27* | 12 | 22* | 9 |
| Joseph et al (32), 1996 | CVD (history of MI, CABG, angioplasty, stenosis >50%, angina, CHF, arrhythmia, PVD, CVD or cor pulmonale) | 584 | Nicotine patch | NR | NR | 14 | 11 | NR | NR | NR | NR |
| Rigotti et al (31), 2006 | Acute CVD (patients admitted with MI or unstable angina, CABG or other cardiovascular conditions with documented CAD) | 247 | Bupropion | NR | NR | NR | NR | 25 | 21 | 20 | 14 |
| Campbell et al (36), 1991 | In-hospital CVD patients† | 85 | Nicotine gum | NR | NR | NR | NR | NR | NR | 34 | 29 |
Statistically significant at P<0.05;
Referred to as heart disease, not defined in the study. Patients with other smoking-related diseases were included (total n=219). AMI Acute myocardial infarction; CABG Coronary artery bypass graft surgery; CAD Coronary artery disease; CHF Congestive heart failure; CVD Cardiovascular disease; MI Myocardial infarction; NR Not reported; PVD Peripheral vascular disease
Safety data from the pharmacotherapy trials showed that adverse events were similar in both the active and placebo arms of the trials. Studies have reported that the safety profile of bupropion in patients with CVD is similar to that observed in the general population (30,31). In 1996, Joseph et al (32) reported that the nicotine patch did not cause a significant increase in cardiovascular events in high-risk outpatients with cardiac disease. The total number of primary end points (death, MI, cardiac arrest and admission to the hospital due to increased severity of angina, arrhythmia or congestive heart failure) in the nicotine group was similar to that in the placebo group (16 versus 23, respectively; P=0.23) (32). There was an insufficient number of RCTs reporting safety data among the pharmacotherapy RCTs to pool these safety data.
DISCUSSION
Our meta-analysis was designed to estimate the efficacy of various behavioural therapies and pharmacotherapies for smoking cessation in cardiac patients. We found that both behavioural and pharmacotherapy treatments are efficacious in cardiac patients. However, the magnitude of the effect was small for such a high-risk group.
Although behavioural therapies as a group are superior to usual care, there are insufficient data to draw conclusions regarding the optimal length, duration and type of behavioural therapy to administer. The intensity of the behavioural intervention applied in each of the studies varied widely. Furthermore, RCTs also tested a broad spectrum of behavioural therapies – some studies tested smoking cessation advice and others tested multiple individual or group counselling sessions. Consequently, further studies are required before we can develop guidelines for smoking cessation in cardiac patients. In particular, large, multicentre, head-to-head RCTs are required to identify which types of behavioural therapies are most efficacious in cardiac patients. Additional RCTs are also required to identify the optimal length and duration of each type of behavioural intervention.
Additional RCTs are also needed to examine the effect of combination therapy involving both behavioural therapy and pharmacotherapy. Combination therapy has been shown to improve abstinence rates in the general population of smokers (33,34) and published guidelines now recommend combining multiple individual or group counselling sessions with NRT (35). Combination therapy may prove to be more efficacious than either behavioural therapy or pharmacotherapy alone in cardiac patients. However, we identified only one pharmacotherapy RCT in cardiac patients that included a combined behavioural component. However, both the active and placebo arms received the same behavioural intervention and thus, the effect of pharmacotherapy alone in comparison with combination therapy could not be examined (36).
We identified only four RCTs examining the efficacy of pharmacotherapy in cardiac patients, and we could not identify any previous meta-analyses that examined the efficacy of smoking cessation pharmacotherapies in cardiac patients. The paucity of research in this area may relate to several factors. First, physicians may be reluctant to enroll cardiac patients in pharmacotherapy RCTs due to safety concerns. However, the two safety trials conducted to date suggest that smoking cessation pharmacotherapy use has a similar safety profile in cardiac patients as that observed in the general population (30,32). Second, researchers may believe that there is no need to replicate studies performed among general populations in cardiac populations. However, cardiac patients may have different safety profiles than the general population and are at a high risk of cardiac events if they continue to smoke. Furthermore, they often have different motivations to quit smoking than the general population. Receiving a cardiac diagnosis is thought to be a ‘teachable moment’, a naturally occurring health event thought to motivate individuals to spontaneously adopt risk-reducing health behaviours (37). Evidence of this phenomenon has been found in a number of studies (10,14,19,23,38). For example, in the Framingham Heart Study (38), men were 1.9 times more likely to quit smoking than the general smoking population following the development of CAD. In another study, Wilkes and Evans (10) found that patients with chronic disease, including heart disease, expressed a greater desire (45% versus 30%) and need for assistance (38% versus 23%) to quit smoking than age-matched controls in the general population. Finally, the magnitude of the effect sought in this population must be greater given that they are at such high immediate risk. Thus, there remains an important need to examine the safety and efficacy of smoking cessation therapies in this patient population.
Limitations
Several potential limitations of our meta-analysis should be noted. First, there were substantial methodological variations in the RCTs included in the present meta-analysis. RCTs varied in their definitions of behavioural therapy, usual care, patient characteristics, and the intensity and duration of therapy. Nevertheless, we considered them to be similar enough to be pooled. Furthermore, we used random effects models rather than fixed effects ones. Thus, our models incorporate both between-study and within-study variability, and account for heterogeneity. Second, we identified only four pharmacotherapy studies in cardiac patients. Thus, the estimates produced in our meta-analysis of pharmacotherapies have wide CIs. Third, the four pharmacotherapy RCTs examined three different medications. Consequently, heterogeneity was present and meta-analysis, even via random effects, may not have been fully appropriate. Nevertheless, it represents the totality of available evidence for pharmacotherapies to date in this specific patient population. Fourth, there were insufficient data available to fully examine the safety of these therapies in cardiac patients. Finally, as is true for all systematic reviews and meta-analyses, the results of the present study are also limited by the possibility of publication bias, particularly among the behavioural therapy RCTs. The two largest behavioural therapy RCTs were not statistically significant, while the results of the smallest RCTs were significant. These findings support the theory that studies with null results are less likely to be published than those with significant results (ie, publication bias) (39). This is particularly true for smaller studies. However, our meta-analysis produced relatively strong ORs for efficacy and publication bias would have to be quite strong to overturn these results. Thus, it is highly unlikely that our results are due to the effects of publication bias.
CONCLUSION
Our meta-analysis highlights the need for more RCTs examining the efficacy of smoking cessation pharmacotherapies in cardiac patients. These RCTs should examine both traditional smoking cessation pharmacotherapies, such as NRTs and bupropion, as well as newer alternatives. Promising new pharmacotherapies include varenicline, which recently received United States Food and Drug Administration approval (40). Varenicline blocks the reinforcing effects of continued nicotine use and relieves the symptoms of nicotine withdrawal (40). Two other new therapies include rimonabant, an antagonist to cannabinoid type 1 receptors (41), and the nicotine vaccine, which neutralizes nicotine in the blood and reduces nicotine uptake into the brain (42). Our meta-analysis highlights the need for head-to-head RCTs to identify which smoking cessation therapy is superior in cardiac patients, as well as RCTs examining the efficacy of combination therapy (behavioural and pharmacotherapy). Cardiac patients are at an increased risk of cardiac events if they continue to smoke and, consequently, improved smoking cessation in this high-risk patient population is likely to result in substantial public health benefits.
Acknowledgments
The authors of this study thank Meriam Abouelouafaa for her help with data abstraction. Dr Eisenberg had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
RELATIONSHIPS TO DISCLOSE/POTENTIAL CONFLICTS OF INTEREST: Dr Eisenberg is a member of Pfizer Canada Inc’s Varenicline Advisory Board and a Senior Physician-Scientist of the Fonds de la recherche en santé du Québec (FRSQ). Mr Filion is supported, in part, by a bursary from the FRSQ. Dr Rinfret is a Junior Physician-Scientist of the FRSQ. Dr Pilote is a Senior Physician-Scientist of the FRSQ and holds the William Dawson Chair at McGill University (Montreal, Quebec). Dr Joseph is a scientist for the Canadian Institutes for Health Research. Dr O’Loughlin holds a Canada Research Chair in the Early Determinants of Adult Chronic Disease.
REFERENCES
- 1.Ockene J, Kristeller JL, Goldberg R, et al. Smoking cessation and severity of disease: The Coronary Artery Smoking Intervention Study. Health Psychol. 1992;11:119–26. doi: 10.1037//0278-6133.11.2.119. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29:1422–31. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 3.Samet JM. The 1990 Report of the Surgeon General: The Health Benefits of Smoking Cessation. Am Rev Respir Dis. 1990;142:993–4. doi: 10.1164/ajrccm/142.5.993. [DOI] [PubMed] [Google Scholar]
- 4.Reducing tobacco use: A report of the Surgeon General – executive summary. Nicotine Tob Res. 2000;2:379–95. [PubMed] [Google Scholar]
- 5.Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 6.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, for the Varenicline Phase Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Hays JT, Hurt RD, Rigotti NA, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation: A randomized, controlled trial. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 9.Hurt RD, Sachs DPL, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 10.Wilkes S, Evans A. A cross-sectional study comparing the motivation for smoking cessation in apparently healthy patients who smoke to those who smoke and have ischaemic heart disease, hypertension or diabetes. Fam Pract. 1999;16:608–10. doi: 10.1093/fampra/16.6.608. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. The Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 12.Bolman C, de Vries H, van Breukelen G. A minimal-contact intervention for cardiac inpatients: Long-term effects on smoking cessation. Prev Med. 2002;35:181–92. doi: 10.1006/pmed.2002.1036. [DOI] [PubMed] [Google Scholar]
- 13.Burt A, Thornley P, Illingworth D, White P, Shaw TR, Turner R. Stopping smoking after myocardial infarction. Lancet. 1974;1:304–6. doi: 10.1016/s0140-6736(74)92607-5. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Budz B, Mackay M, Miller C. Evaluation of a nurse-delivered smoking cessation intervention for hospitalized patients with cardiac disease. Heart Lung. 1999;28:55–64. doi: 10.1016/s0147-9563(99)70043-9. [DOI] [PubMed] [Google Scholar]
- 15.Pozen MW, Stechmiller JA, Harris W, Smith S, Fried DD, Voigt GC. Nurse rehabilitators impact on patients with myocardial-infarction. Med Care. 1977;15:830–7. doi: 10.1097/00005650-197710000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Haskell WL, Alderman EL, Fair JM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89:975–90. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 17.Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients – influence of a smoking-related diagnosis: A pilot study. Heart Lung. 1994;23:151–6. [PubMed] [Google Scholar]
- 18.Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: Randomised controlled trial. BMJ. 2002;324:87–9. doi: 10.1136/bmj.324.7329.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–9. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Quist-Paulsen P, Gallefoss F. Randomised controlled trial of smoking cessation intervention after admission for coronary heart disease. BMJ. 2003;327:1254–7. doi: 10.1136/bmj.327.7426.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feeney GF, McPherson A, Connor JP, McAlister A, Young MR, Garrahy P. Randomized controlled trial of two cigarette quit programmes in coronary care patients after acute myocardial infarction. Intern Med J. 2001;31:470–5. doi: 10.1046/j.1445-5994.2001.00110.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor CB, Houston-Miller N, Killen JD, Debusk RF. Smoking cessation after acute myocardial infarction: Effects of a nurse-managed intervention. Ann Intern Med. 1990;113:118–23. doi: 10.7326/0003-4819-113-2-118. [DOI] [PubMed] [Google Scholar]
- 23.Rigotti NA, McKool KM, Shiffman S. Predictors of smoking cessation after coronary artery bypass graft surgery: Results of a randomized trial with 5-year follow-up. Ann Intern Med. 1994;120:287–93. doi: 10.7326/0003-4819-120-4-199402150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Miller NH, Smith PM, Debusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients – results of a randomized trial. Arch Intern Med. 1997;157:409–15. [PubMed] [Google Scholar]
- 25.Carlsson R, Lindberg G, Westin L, Israelsson B. Influence of coronary nursing management follow up on lifestyle after acute myocardial infarction. Heart. 1997;77:256–9. doi: 10.1136/hrt.77.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engblom E, Ronnemaa T, Hamalainen H, Kallio V, Vanttinen E, Knuts LR. Coronary heart disease risk factors before and after bypass surgery: Results of a controlled trial on multifactorial rehabilitation. Eur Heart J. 1992;13:232–7. doi: 10.1093/oxfordjournals.eurheartj.a060152. [DOI] [PubMed] [Google Scholar]
- 27.Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Prev Med. 2000;30:261–8. doi: 10.1006/pmed.2000.0644. [DOI] [PubMed] [Google Scholar]
- 28.Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12:239–44. doi: 10.1023/a:1007757530765. [DOI] [PubMed] [Google Scholar]
- 29.Rennard S, Daughton D, Cheney R, et al. Nicotine replacement therapy for patients with coronary-artery disease. Arch Intern Med. 1994;154:989–95. [PubMed] [Google Scholar]
- 30.Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: A multicentre, randomised study. Eur Heart J. 2003;24:946–55. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 31.Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;119:1080–7. doi: 10.1016/j.amjmed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–8. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 33.Hughes JR, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA. 1999;281:72–6. doi: 10.1001/jama.281.1.72. [DOI] [PubMed] [Google Scholar]
- 34.Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: A randomized trial. Am J Med. 2003;114:555–62. doi: 10.1016/s0002-9343(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 35.The Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline. JAMA. 1996;275:1270–80. [PubMed] [Google Scholar]
- 36.Campbell IA, Prescott RJ, Tjeder-Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respir Med. 1991;85:155–7. doi: 10.1016/s0954-6111(06)80295-7. [DOI] [PubMed] [Google Scholar]
- 37.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ Res. 2003;18:156–70. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 38.Freund KM, D’Agostino RB, Belanger AJ, Kannel WB, Stokes J., III Predictors of smoking sessation: The Framingham Study. Am J Epidemiol. 1992;135:957–64. doi: 10.1093/oxfordjournals.aje.a116407. [DOI] [PubMed] [Google Scholar]
- 39.Stern JM, Simes RJ. Publication bias: Evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–5. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorenby DE, Hays JT, Rigotti NA, et al. for the Varenicline Phase Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien CP. Anticraving medications for relapse prevention: A possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 42.Cornuz J, Klingler K, Mueller P, Jungi F, Cerny T.A therapeutic vaccine for nicotine dependence: Results of a phase I and a randomized phase II study J Clin Oncol200;2316 Suppl1008.(Abst) [Google Scholar]