Abstract
BACKGROUND:
Cold temperature is known to commonly precipitate angina pectoris in patients with symptomatic coronary artery disease (CAD). It was previously shown that the ischemic threshold was lower during exercise testing at −8°C than at +20°C in patients with a history of cold-induced angina, whereas it was unchanged in patients without cold-induced angina. Few data exist on the impact of more severe cold exposure on myocardial ischemia.
OBJECTIVE:
To determine the effect of extreme cold exposure (−20°C) on the ischemic threshold among CAD patients.
METHODS:
Thirteen men with CAD and documented exercise-induced ischemia performed two treadmill exercise tests, one at +20°C and one at −20°C, in random order. Electrocardiograms were recorded every 30 s and analyzed separately in random order by two experienced readers blinded to temperature.
RESULTS:
The mean (± SD) time to ischemic electrical threshold was 53±60 s lower at −20°C than at +20°C (P=0.008), corresponding to a relative change of −8.0±10.5%. All other exercise parameters, including total exercise time and rate-pressure product, were unchanged during exposure to extreme cold.
CONCLUSION:
Exposure to extreme cold (−20°C) lowers the ischemic threshold during exercise testing in patients with CAD, even if asymptomatic or without a history of cold-induced angina. Patients with CAD and evidence of exertional angina or myocardial ischemia wishing to perform exercise at extremely low temperatures should discuss this matter with their physicians.
Keywords: Cold, Coronary disease, Exercise test, Myocardial ischemia
Abstract
HISTORIQUE :
On sait que le froid précipite souvent une angine de poitrine chez les patients atteints d’une coronaropathie symptomatique. On a déjà démontré que le seuil ischémique était plus faible pendant une épreuve à l’effort à −8 °C qu’à +20 °C chez des patients ayant des antécédents d’angine induite par le froid, tandis qu’il demeurait inchangé chez les patients dénués de tels antécédents. Il existe peu de données sur les répercussions d’une plus grande exposition au froid sur l’ischémie myocardique.
OBJECTIF :
Déterminer l’effet d’une extrême exposition au froid (−20 °C) sur le seuil d’ischémie chez les patients ayant une coronaropathie.
MÉTHODOLOGIE :
Treize hommes ayant une coronaropathie présentant une ischémie documentée à l’effort ont effectué deux épreuves sur tapis roulant, l’une à +20 °C et l’autre à −20 °C, dans un ordre aléatoire. On a enregistré leurs électrocardiogrammes toutes les 30 s et deux lecteurs expérimentés, qui ne connaissaient pas la température, les ont analysés séparément et de manière aléatoire.
RÉSULTATS :
Il fallait un temps moyen (± ÉT) de 53±60 s de moins à −20 °C qu’à +20 °C (P=0,008) avant d’atteindre le seuil électrique d’ischémie, ce qui correspond à un changement relatif de −8,0±10,5 %. Tous les autres paramètres de l’épreuve, y compris le temps total de l’épreuve et le produit du taux et de la tension, demeuraient inchangés pendant l’exposition à un froid extrême.
CONCLUSION :
L’exposition à un froid extrême (−20 °C) réduit le seuil d’ischémie pendant l’épreuve à l’effort de patients ayant une coronaropathie, même s’ils sont asymptomatiques ou sans antécédents d’angine induite par le froid. Les patients ayant une coronaropathie et des indications d’angine à l’effort ou d’ischémie myocardique qui souhaitent faire de l’exercice par temps très froid devraient en discuter avec leur médecin.
Exposure to cold temperature has been shown to precipitate angina pectoris in approximately 40% of patients with symptomatic coronary artery disease (CAD) (1–3). In addition, emerging data suggest that extreme cold is associated with an increased risk of myocardial infarction and sudden cardiac death (4,5). An increase in peripheral vascular resistance resulting in higher myocardial oxygen demand as well as disturbances of coronary artery vasomotion are the two proposed mechanisms accounting for the reduction in ischemic threshold during exposure to cold temperature. We previously showed that exercise testing performed at a temperature of −8°C resulted in a 30% reduction in time to 1 mm ST depression relative to exercise testing at +20°C among individuals with a history of cold-induced angina, whereas the ischemic threshold was unchanged in cold-tolerant patients (3). Few data exist on the impact of more severe environmental cold exposure on ischemic threshold among CAD patients, including the effect of extreme cold exposure on ischemic threshold in those without cold-induced angina. The main objective of the present study was to determine the effects of extreme cold exposure (−20°C) on exercise parameters among CAD patients with documented exercise-induced ischemia.
METHODS
Study participants
Fifteen patients with stable CAD at the cardiovascular prevention and rehabilitation centre of the Montreal Heart Institute in Montreal, Quebec, were recruited for the study. All patients had documented exercise-induced myocardial ischemia, defined as 1 mm or greater horizontal or downsloping ST segment depression, on the past two treadmill exercise tests performed within a year before study entry, in association with at least one of the following criteria: 70% or greater diameter stenosis of at least one major coronary artery; documented previous transmural myocardial infarction; or reversible perfusion defect on sestamibi exercise testing. Patients with myocardial infarction, unstable angina or coronary artery bypass within three months, coronary angioplasty within six months, overt heart failure, uncontrolled hypertension, change of medication within two weeks of enrollment, a history of serious exercise-induced arrhythmias, or baseline electrocardiogram (ECG) abnormalities potentially interfering with interpretation of the ST segment during exercise, were excluded. All medications were continued during the study.
Study design
In random order, each participant completed two maximal treadmill exercise tests, one at +20°C and one at −20°C, within three weeks or each other. The tests were separated by at least 48 h and performed at the same hour each day, at least 2 h after a light meal. All tests were conducted by an experienced exercise physiologist and a nurse, and were supervised by a cardiologist. The study protocol was approved by the local research ethics board and all patients gave written informed consent.
Exercise testing
Maximal exercise testing was performed on a treadmill (Marquette Electronics Inc, USA) using a ramp protocol. All ramp protocols were individualized for each patient to elicit a maximal effort with an exercise time of approximately 10 min according to a method published previously (6). Heart rate, brachial blood pressure and rating of perceived exertion were recorded before the test and at 1 min intervals during exercise and recovery. A three-channel ECG was continuously monitored and a 12-lead ECG was recorded every 30 s. End points for terminating exercise were severe angina, dyspnea or extreme fatigue. A cold chamber was used to perform treadmill tests at −20°C. This specially designed room (Foster Co, Canada) measured 3.66 m × 3.05 m × 2.59 m and maintained a constant temperature during treadmill tests. A ventilation system recycled the air and a large window allowed constant supervision by staff operating the electronic equipment, which was kept at room temperature outside the cold chamber. Only the patient, the nurse who recorded blood pressure and the treadmill were placed inside the cold chamber; regular calibration showed that all equipment was unaffected by exposure to cold. Verbal communication with the patient and the nurse inside the chamber was possible through an intercom system. All patients wore the same standardized clothing (T-shirt and light pants) for the tests at −20°C and +20°C. Following tests at −20°C, patients were allowed to completely recover at normal room temperature. The treadmill tests at +20°C were performed using the same equipment.
Study end points
Total exercise time, energy expenditure in metabolic equivalent units, heart rate, rate-pressure product and heart rate recovery at 1 min, were measured at peak exercise and at the ischemic threshold. The ST segment was measured 0.08 s after the J point in three consecutive QRS complexes, with a flat baseline and R waves of equal amplitude. The average of the three measurements was compared with the baseline tracing, as previously described (7). Electrical ischemic threshold was defined as the onset of 1 mm or greater horizontal or downsloping ST segment depression compared with baseline. Measurements were performed on separate ECG sheets by two experienced readers who were blinded to temperature condition and recording time; values recorded by the two observers were averaged.
Statistical analysis
Because all exercise parameters met the criteria of normal symmetrical distribution according to the Shapiro-Wilk test and the normal probability plot, parametric tests were used to compare intra-individual changes between normal and cold temperatures. Results are presented as mean ± SD. Differences were considered statistically significant at P<0.05. Analyses were performed using SPSS 16 (SPSS Inc, USA) for Windows (Microsoft Corporation, USA).
RESULTS
Baseline characteristics
Two of 15 patients did not perform the second of two treadmill tests and were excluded from the analysis. Baseline data for the remaining 13 men are presented in Table 1. Briefly, the mean age was 67.4±6.6 years. Five (38%) patients had silent myocardial ischemia, while the remaining eight (62%) patients had a history of angina, one of whom had had cold-induced angina.
TABLE 1.
Baseline characteristics of participants (n=13)
| Clinical variables | |
|---|---|
| Age, years | 67.4±6.6 |
| Body mass index, kg/m2 | 28.1±3.2 |
| Waist circumference, cm | 99.4±9.3 |
| Canadian Cardiovascular Society functional class, n (%) | |
| 0 | 5 (38) |
| I | 4 (31) |
| II | 4 (31) |
| III or IV | 0 (0) |
| Risk factors, n (%) | |
| Diabetes mellitus | 2 (15) |
| Hypertension | 5 (38) |
| Dyslipidemia | 12 (92) |
| Smoking | 1 (8) |
| Positive family history | 8 (61) |
| Medical history, n (%) | |
| Previous myocardial infarction | 7 (54) |
| PCI | 3 (23) |
| CABG | 3 (23) |
| Medications, n (%) | |
| Acetylsalicylic acid | 12 (92) |
| Beta-blockers | 9 (69) |
| Calcium channel blockers | 5 (38) |
| ACE inhibitors | 2 (15) |
| Statins | 12 (92) |
| Nitrates | 3 (23) |
Data presented as mean ± SD unless otherwise indicated. ACE Angiotensin-converting enzyme; CABG Coronary artery bypass grafting; PCI Percutaneous coronary intervention
Effects of cold on the study end points
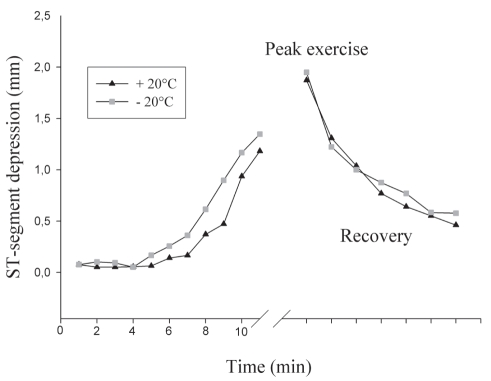
The mean time to electrical ischemic threshold was 53±60 s lower at −20°C than at +20°C (P=0.008), corresponding to a relative change of −8.0±10.5% (Table 2 and Figure 1). All but two patients had a shorter time to onset of ischemia at −20°C. Symptoms of angina were reproduced in two patients only, both at −20°C and +20°C, and without a significant difference of time to symptom onset. Maximal ST depression and exercise capacity were similar at both temperatures, as were rate-pressure products at all time points during the exercise tests (Table 2). No significant ventricular or supraventricular arrhythmias occurred at either temperature.
TABLE 2.
Main exercise test parameters at +20°C and −20°C
| Parameters | +20°C | −20°C | P* |
|---|---|---|---|
| At ischemic threshold† | |||
| Exercise time, s | 591±109 | 538±85 | 0.008 |
| Work, METs | 6.1±1.5 | 5.7±1.1 | 0.185 |
| Heart rate, beats/min | 119±22.5 | 114±17 | 0.175 |
| Rate-pressure product, beats/min•mmHg•102 | 193±47 | 195±45 | 0.831 |
| At peak exercise | |||
| Exercise time, s | 740±93 | 740±75 | 0.974 |
| Work, METs | 8.0±2.0 | 8.0±1.8 | 0.697 |
| Heart rate, beats/min | 133±23 | 135±22 | 0.239 |
| Rate-pressure product, beats/min•mmHg•102 | 232±62 | 242±65 | 0.231 |
| ST segment depression, mm | 2.0±0.5 | 2.0±0.6 | 0.759 |
| At 1 min postexercise | |||
| Heart rate recovery, beats/min | 18.1±3.9 | 17.4±6.1 | 0.536 |
Data presented as mean ± SD.
Paired Student’s t test;
1 mm ST segment depression. METs Metabolic equivalent units
Figure 1).
ST segment depression during treadmill exercise tests performed at +20°C and −20°C
DISCUSSION
The principal finding of the present study was that exposure to extreme cold (−20°C) lowered the ischemic threshold during exercise testing in patients with CAD, even in the absence of anginal symptoms or without a history of cold-induced angina. To our knowledge, the present study was the first to evaluate the effects of extreme cold (using a cold chamber) among CAD patients, and the first to demonstrate a significant effect of cold temperature on myocardial ischemia in patients without a history of cold intolerance. Despite a lowering of the ischemic threshold, exposure to extreme cold was not associated with a decrease in total exercise time, nor was it associated with any deleterious effects on cardiovascular function including serious ventricular arrhythmias.
Previous studies have generally reported a lowering of the ischemic threshold on exposure to cold solely in patients suffering from cold-induced angina, suggesting that individual susceptibilities to cold exist (3,8–11). Most of these studies, however, used temperatures ranging from +6°C to −10°C. In contrast, we found that exposure to extreme cold (−20°C) in a cold chamber (mimicking weather conditions typically found in northern or temperate climates), resulted in an approximate 10% reduction in time to onset of ischemia in individuals who, in large part, were not cold intolerant. Our data, along with previous reports, suggest that the effect of cold on myocardial ischemia may be dose dependent; the ischemic threshold may be lowered even in subjects without cold-induced angina on exposure to extreme cold.
The exact mechanism by which exposure to cold temperature lowers the ischemic threshold remains a question of debate. Early studies attributed cold-induced angina to sympathetic activation, resulting in increased peripheral vascular resistance and blood pressure, and leading to a greater cardiac workload (increased rate-pressure product) for any given exercise intensity (1,10). In the present study, we did not find any significant difference in heart rate or rate-pressure product at any time point between the exercise tests performed at +20°C and at −20°C. This suggests that an increase in peripheral vascular resistance was not the only factor influencing exercise-induced ischemia. Previous data from our group also reported a lower ischemic threshold and a lower rate-pressure product at the ischemic threshold among CAD patients with cold-induced angina, suggesting potentially deleterious effects of cold on coronary vasomotion (3). This finding is in agreement with a study (12) using scintigraphic imaging that demonstrated increased exercise-induced myocardial perfusion abnormalities during exposure to cold temperature. The potentially conflicting results regarding hemodynamic responses to cold may be explained by several factors. First, various cold stimuli have been used, including environmental cold (1,3,10,11,13,14), cold air inhalation (9,12,15), regional cutaneous cold exposure (cold pressure test) (2,8,16,17) or a combination of the above (16,17). It is now understood that the cold pressure test cannot be used to simulate hemodynamic responses during exercise in cold weather because exercise abolishes the abnormal increase in coronary resistance induced by the cold pressure test in CAD patients (18). Cold air inhalation in a warm environment also does not result in the same cardiovascular physiological responses as generalized exposure to cold air; with skin cooling, stimulation of cold receptors leading to sympathetic activation and peripheral vasconstriction may predominate. Second, varying cold temperatures were used. Third, different CAD samples were studied with different sensitivities to cold. In conclusion, it seems reasonable to believe that in most cases, both increased cardiac workload and alterations in coronary vasomotion contribute to lowering the ischemic threshold on exposure to cold temperature.
The difference in susceptibility to cold temperature among patients remains ill defined. It was first postulated that patients with poor exercise tolerance or low coronary reserve were most affected because of a relatively greater decrease in work performance in a cold environment, thereby being classified as cold intolerant (8). More recent data have shown that cold-induced sympathetic activation may cause either tachycardia as a result of beta-1 receptor stimulation or bradycardia if the baroreceptor response to alpha-2 receptor-mediated vasocontriction is normal. Marchant et al (11) demonstrated that cold-intolerant patients have impaired baroreceptor function that is associated with a steeper rise in heart rate and rate-pressure product during exercise in the cold compared with cold-tolerant patients. This leads to an increased myocardial oxygen demand and thus, to a lower ischemic threshold. This compensating baroreceptor-mediated decrease of heart rate may be overcome at extremely low temperatures and could explain our findings of a decreased ischemic threshold even in cold-tolerant patients. Another hypothesis is that the central effects of cold on coronary vasomotion might predominate over peripheral mechanisms at extremely low temperatures.
Limitations
The current work has several limitations. First, the small sample size may have limited our ability to detect small differences in exercise parameters such as total exercise time. Second, we could not fully reproduce potential environmental conditions that occur in real life. Factors increasing cold exposure, such as wind and snow, were not included in our protocol and participants did not wear thick winter clothing that could protect against cutaneous cold exposure. Third, there is no definitive proof that significant ST segment depression truly represented ischemia, notably in asymptomatic patients. We cannot exclude that other physiological mechanisms, such as hyperventilation, may have elicited the ST segment changes, although hyperventilation has never been shown to be associated with extreme cold exposure. Given the cohort under study, there is a high probability that the observed ST segment depression was indeed the result of myocardial ischemia. Furthermore, previous studies, including the Asymptomatic Cardiac Ischemic Pilot (ACIP) trial (19), used similar criteria for defining myocardial ischemia on treadmill testing as those used in the present study. Finally, the present study was not designed to precisely elucidate the underlying mechanisms responsible for the reduction in ischemic threshold on exposure to cold temperature, since rate-pressure product is an inaccurate and indirect method of assessing myocardial oxygen consumption.
CONCLUSION
Exposure to extreme cold (−20°C) lowers the ischemic threshold during exercise testing in patients with CAD irrespective of the presence or absence of anginal symptoms or a history of cold-induced angina. Although we failed to observe any detrimental effects, performing exercise in extreme cold under real-world conditions may be very different from performing exercise in conditions controlled in the laboratory. Patients with CAD and evidence of exertional angina or myocardial ischemia wishing to perform exercise at extremely low temperatures should discuss this matter with their physicians.
REFERENCES
- 1.Epstein SE, Stampfer M, Beiser GD, Goldstein RE, Braunwald E. Effects of a reduction in environmental temperature on the circulatory response to exercise in man. Implications concerning angina pectoris. N Engl J Med. 1969;280:7–11. doi: 10.1056/NEJM196901022800102. [DOI] [PubMed] [Google Scholar]
- 2.Shea MJ, Deanfield JE, deLandsheere CM, Wilson RA, Kensett M, Selwyn AP. Asymptomatic myocardial ischemia following cold provocation. Am Heart J. 1987;114:469–76. doi: 10.1016/0002-8703(87)90740-x. [DOI] [PubMed] [Google Scholar]
- 3.Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation. 1989;79:1015–20. doi: 10.1161/01.cir.79.5.1015. [DOI] [PubMed] [Google Scholar]
- 4.Medina-Ramon M, Schwartz J. Temperature, temperature extremes, and mortality: A study of acclimatisation and effect modification in 50 US cities. Occup Environ Med. 2007;64:827–33. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman DE, Platt R, Dery V, et al. Seasonal congestive heart failure mortality and hospitalisation trends, Quebec 1990–1998. J Epidemiol Community Health. 2004;58:129–30. doi: 10.1136/jech.58.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers J, Buchanan N, Smith D, et al. Individualized ramp treadmill. Observations on a new protocol. Chest. 1992;101:236S–41S. [PubMed] [Google Scholar]
- 7.Chaitman BR, Waters DD, Bourassa MG, Tubau JF, Wagniart P, Ferguson RJ. The importance of clinical subsets in interpreting maximal treadmill exercise test results: The role of multiple-lead ECG systems. Circulation. 1979;59:560–70. doi: 10.1161/01.cir.59.3.560. [DOI] [PubMed] [Google Scholar]
- 8.Neill WA, Duncan DA, Kloster F, Mahler DJ. Response of coronary circulation to cutaneous cold. Am J Med. 1974;56:471–6. doi: 10.1016/0002-9343(74)90478-1. [DOI] [PubMed] [Google Scholar]
- 9.Hattenhaur M, Neill WA. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation. 1975;51:1053–8. doi: 10.1161/01.cir.51.6.1053. [DOI] [PubMed] [Google Scholar]
- 10.Lassvik CT, Areskog NH. Angina in cold environment. Reactions to exercise. Br Heart J. 1979;42:396–401. doi: 10.1136/hrt.42.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchant B, Donaldson G, Mridha K, Scarborough M, Timmis AD. Mechanisms of cold intolerance in patients with angina. J Am Coll Cardiol. 1994;23:630–6. doi: 10.1016/0735-1097(94)90747-1. [DOI] [PubMed] [Google Scholar]
- 12.Petersen CL, Hansen A, Frandsen E, et al. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am Heart J. 1994;128:511–6. doi: 10.1016/0002-8703(94)90624-6. [DOI] [PubMed] [Google Scholar]
- 13.Rosengren A, Wennerblom B, Bjuro T, Wilhelmsen L, Bake B. Effects of cold on ST amplitudes and blood pressure during exercise in angina pectoris. Eur Heart J. 1988;9:1074–80. doi: 10.1093/oxfordjournals.eurheartj.a062402. [DOI] [PubMed] [Google Scholar]
- 14.Peart I, Bullock RE, Albers C, Hall RJ. Cold intolerance in patients with angina pectoris: Effect of nifedipine and propranolol. Br Heart J. 1989;61:521–8. doi: 10.1136/hrt.61.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodds PA, Bellamy CM, Muirhead RA, Perry RA. Vasoconstrictor peptides and cold intolerance in patients with stable angina pectoris. Br Heart J. 1995;73:25–31. doi: 10.1136/hrt.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassvik C, Areskog NH. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J. 1980;43:661–7. doi: 10.1136/hrt.43.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown CF, Oldridge NB. Exercise-induced angina in the cold. Med Sci Sports Exerc. 1985;17:607–12. [PubMed] [Google Scholar]
- 18.de Servi S, Mussini A, Angoli L, et al. Effects of cold stimulation on coronary haemodynamics during exercise in patients with coronary artery disease. Eur Heart J. 1985;6:239–46. doi: 10.1093/oxfordjournals.eurheartj.a061847. [DOI] [PubMed] [Google Scholar]
- 19.Pepine CJ, Geller NL, Knatterud GL, et al. The Asymptomatic Cardiac Ischemia Pilot (ACIP) study: Design of a randomized clinical trial, baseline data and implications for a long-term outcome trial. J Am Coll Cardiol. 1994;24:1–10. doi: 10.1016/0735-1097(94)90534-7. [DOI] [PubMed] [Google Scholar]