Abstract
Four cases of pediatric cardiac tumours (PCTs) associated with ventricular arrhythmias are reported. Sudden cardiac death attributable to the tumour occurred in two children. A third child received an implantable cardioverter defibrillator and the fourth had persistent ventricular arrhythmia despite medical therapy. Most PCTs are considered benign; however, the development of malignant arrhythmias may complicate the management of these tumours in some patients. The literature regarding the arrhythmogenic potential of PCTs and the use of implantable cardioverter defibrillators in these patients is reviewed. The series highlights the deficiency of prognostic information for this cohort.
Keywords: Cardiac tumour, Implantable cardioverter defibrillator, Sudden cardiac death, Ventricular tachycardia
Abstract
Les auteurs rendent compte de quatre cas de tumeurs cardiaques en pédiatrie (TCP) associées à une arythmie ventriculaire. Deux enfants sont décédés subitement de problèmes cardiaques attribuables à la tumeur. Un troisième enfant a reçu un défibrillateur interne et le quatrième présentait une arythmie ventriculaire persistante malgré une médicothérapie. La plupart des TCP sont considérées comme bénignes. Cependant, l’apparition d’arythmies malignes peut compliquer la prise en charge de ces tumeurs chez certains patients. Les auteurs analysent les publications portant sur le potentiel arythmogène des TCP et le recours aux défibrillateurs internes chez ces patients. La série souligne les lacunes de l’information pronostique au sein de cette cohorte.
Cardiac tumours have been identified in 0.02% to 0.04% of the pediatric population (1,2). The vast majority of these tumours are benign, with minimal growth potential. Some can become quite large, resulting in compression of cardiac chambers or vital structures such as conduction tissue or coronary vasculature, as well as obstruction of cardiac valves and outflow tracts (1,3). In the absence of hemodynamic impairment, observation is the standard care in most cases because many tumours spontaneously regress with time (1,3,4). However, some tumours display the potential for malignant arrhythmias, with an increased risk of sudden cardiac death (1,5).
We report a series of patients with pediatric cardiac tumours (PCTs) who developed ventricular arrhythmias. We review the literature regarding the arrhythmogenic potential as well as the use of implantable cardioverter defibrillators (ICDs) in children with these lesions. The series highlights the deficiency of prognostic information for this cohort.
CASE PRESENTATIONS
Case 1: Ventricular fibroma and sudden cardiac death
A six-year-old boy with a juvenile nasopharyngeal angiofibroma was found to have biventricular hypertrophy on a routine preoperative electrocardiogram. His cardiac examination was normal. A chest radiograph demonstrated mild cardiomegaly. An echocardiogram showed a 4 cm × 4.5 cm echogenic mass in the lateral left ventricular (LV) wall in the sulcus between the great arteries. There was no intra-cardiac flow disturbance. The mass was most consistent with a ventricular fibroma.
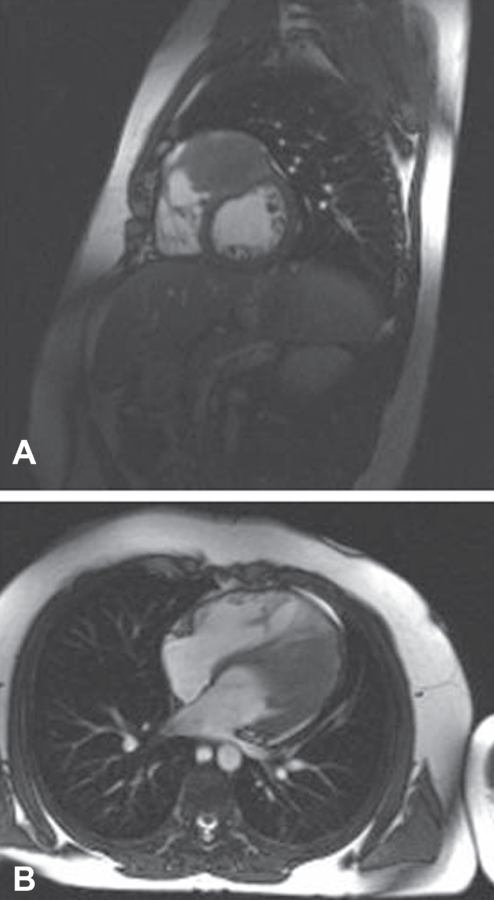
Serial echocardiograms showed no change in tumour size and no compression of the outflow tracts, but did show mild septal dyskinesia at the site of tumour involvement. A cardiac magnetic resonance image (MRI) at 12 years of age showed a 5 cm × 5 cm × 5.5 cm mass contiguous with the superolateral LV wall, minimal enhancement and no compression of the LV or outflow tracts. The study was repeated two years later, showing an increase in tumour size to 7 cm × 6 cm × 4 cm and splaying of the outflow tracts but no outflow tract obstruction (Figure 1). The child had no documented arrhythmias on serial follow-up until an exercise stress test at 15 years of age demonstrated occasional premature ventricular beats.
Figure 1).
Cardiac magnetic resonance images demonstrating a large fibroma high in an interventricular septum splaying both outflow tracts but producing no outflow tract obstruction
While playing noncompetitive rugby a few months later, he had a head-to-head collision with another player, ran another short distance and then collapsed backwards on the grass, unresponsive. Immediate cardiopulmonary resuscitation was performed by his coach until paramedics arrived but he could not be resuscitated. The postmortem head and spine MRI showed no cervical fracture or dislocation, no intracranial pathology and no change in the cardiac tumour size. The postmortem study described a 9 cm × 7 cm × 5 cm mass histologically consistent with a fibroma. It protruded into the LV outflow tract but did not encircle the outflow tracts or interfere with the coronary arteries.
Case 2: Atrial myxoma and sudden cardiac death
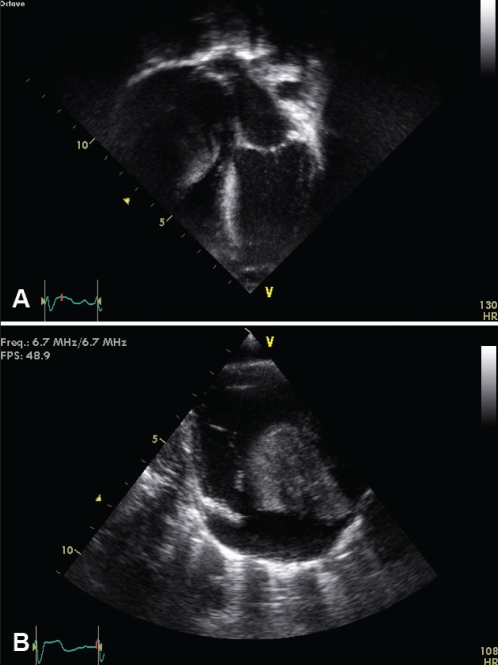
A macrosomic term baby had mild dysmorphic features suggestive of Simpson-Golabi-Behmel syndrome. Cytogenetic testing was normal. The chest radiograph showed cardiomegaly. His cardiac examination and electrocardiogram were normal. The echocardiogram and chest computed tomography demonstrated a 3.5 cm × 3.5 cm homogeneous solitary tumour arising from the anterolateral right atrium encroaching on the right ventricular (RV) cavity, obstructing the tricuspid valve and impairing pulmonary blood flow (Figures 2A and 2B). The tumour was consistent with an atrial myxoma. Because the right coronary artery coursed through the mass, the tumour was not considered resectable. The baby had a left modified Blalock-Taussig shunt on day 4 of life to augment pulmonary blood flow. He developed refractory hemodynamically significant supraventricular tachycardia (SVT) on day 10 of life that was treated with digoxin initially, then propafenone and amiodarone. His SVT resolved beyond the neonatal period. Serial imaging showed no change in tumour size; however, by two months of age, he had adequate pulmonary blood flow through the tricuspid valve, a mildly restrictive Blalock-Taussig shunt and no obstruction in the right coronary artery. His ventricular function was normal.
Figure 2).
A Apical four-chamber two-dimensional echocardiographic image demonstrating a large myxoma arising from the anterolateral atrial wall obstructing right ventricular inflow. B Modified parasternal long-axis two-dimensional echocardiographic view demonstrating the myxoma obstructing the tricuspid valve and right ventricular inflow
The child remained clinically well with no cardiac symptoms until almost three years of age. He was climbing a ladder in the playground and fell backward onto pea gravel. The fall was witnessed by his mother, who immediately came and picked him up. He screamed, gasped and stiffened, then became limp and unresponsive. The presenting rhythm was asystole. Return of spontaneous circulation was obtained after prolonged resuscitative measures. Head imaging showed no evidence of skull fracture or intracranial hemorrhage. The echocardiagram showed no change in right atrial mass and no inflow/outflow tract obstruction. Neuroimaging 72 h following the event documented significant and widespread central nervous system dysfunction and clinical findings of brain death.
Case 3: Ventricular rhabdomyoma and malignant ventricular arrhythmia
A term newborn developed respiratory distress and cyanosis at 12 h of age. A physical examination was normal. A chest radiograph showed a large globular heart. An electrocardiogram demonstrated left-axis deviation and LV hypertrophy with strain pattern. The echocardiogram showed a 3 cm × 5 cm homogeneous mass within the anterolateral LV wall. The systolic function was decreased and there was mild mitral regurgitation. A cardiac MRI in the first week of life showed an extensive solitary tumour within the LV free wall extending into the interventricular septum. The tumour compressed the RV cavity and produced dynamic narrowing of the RV outflow tract, distortion of the superior vena cava, and elevation of the main and branch pulmonary arteries. No obstruction to the LV outflow tract was observed. A head MRI showed multiple cortical areas of increased signal intensity consistent with tuberous sclerosis. Cytogenetic testing was normal.
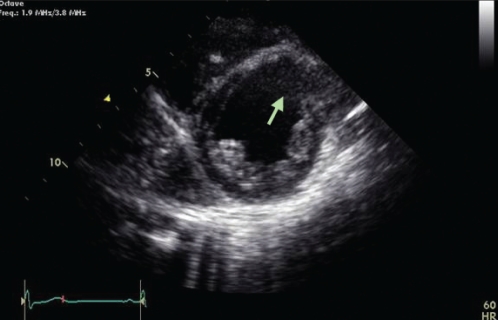
The baby thrived clinically. Serial echocardiograms over the next three years showed gradual reduction in the size of the tumour consistent with the natural history of cardiac rhabdomyomas, but the LV dilation and systolic dysfunction persisted. By four years of age, the tumour had completely regressed, leaving a dyskinetic thinned irregular area of scarring (Figure 3). Serial Holter studies starting at the age of three years documented frequent monomorphic ventricular ectopy, but at eight years of age, he was noted to have nonsustained ventricular tachycardia (VT). He underwent cardiac catheterization with electrophysiological study, which showed dyskinesis of the LV anterior wall and upper septum at the site where the tumour had regressed. Sustained polymorphic VT was easily induced. A single-lead ICD was placed shortly after the study. The patient has had no discharges from the ICD; however, he continues to have frequent ventricular ectopy.
Figure 3).
Parasternal short-axis image at the level of the left ventricular papillary muscles demonstrating the paradoxical rightward movement of the thinned anteroseptal wall in systole (arrow). This portion of the septum was the location of the rhabdomyoma that had regressed spontaneously
Case 4: Ventricular fibroma and malignant ventricular arrhythmia
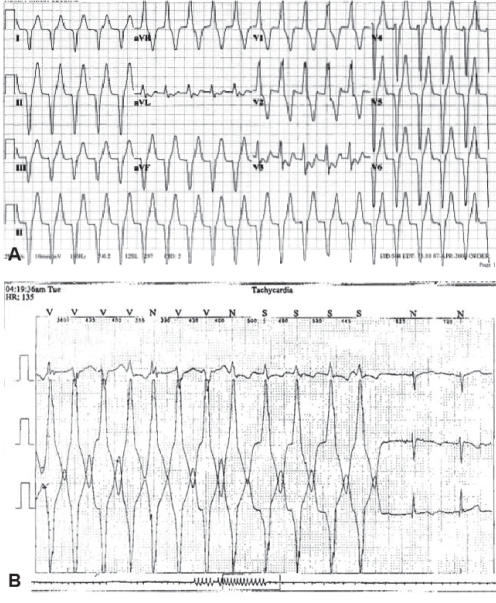
A nine-year-old boy with a solitary large 3.5 cm × 2 cm intramural mass – most likely a fibroma – in the LV free wall, was followed clinically. He had documented ventricular arrhythmia, including prolonged episodes of monomorphic VT at a ventricular rate of 130 beats/min to 175 beats/min as well as a slower wide complex rhythm, all of which were asymptomatic (Figures 4A and 4B). Beta blockade was initiated with some improvement in the frequency and duration of the arrhythmias. However, persistent arrhythmia prompted a conversion to sotalol. He continued to have asymptomatic episodes of VT, albeit less frequently. Serial echocardiograms demonstrated no change in the size of the tumour or hemodynamic impact from the mass. He has remained on sotalol with no cardiac symptoms.
Figure 4).
A A 12-lead electrocardiogram with rhythm strip illustrating monomorphic ventricular tachycardia at a ventricular rate of 130 beats/min with right bundle branch block morphology and a superior axis suggesting an origin in the left ventricular apical region. B Ambulatory Holter monitor recording from the same patient displaying a 5 s salvo of monomorphic ventricular tachycardia converting to normal sinus rhythm in the last two recorded beats
DISCUSSION
PCTs and arrhythmias
Cardiac tumours are rare; more than one-half of PCTs are diagnosed within the first year of life, with some detected on a fetal echocardiogram (1). The majority of PCTs are benign. Rhabdomyomas are the most common cardiac tumour in children (45%), followed by fibromas (25% to 30%) (2,4). Less common PCTs include myxoma, lipoma, teratoma, hemangioma, mesothelioma and Purkinje cell tumour (1,3).
A rhabdomyoma is a hamartoma consisting of glycogen-rich myocytes (1). They may be single or multiple. They are not encapsulated but are well delineated, measuring up to 10 cm in largest diameter. Calcification or hemorrhage within the tumour is uncommon. They are predominantly found within the ventricles but can occur in the atria or caval veins. Lesions may obstruct cardiac valves or inflow/outflow tracts. They may also produce atrial or ventricular arrhythmias, sinus node dysfunction, heart block and pre-excitation. Many cases are sporadic but an association with tuberous sclerosis is reported in 30% to 50% of patients (1,4). Spontaneous regression of the tumours occurs in more than one-half of cases. Regression may leave a scarred thin chamber wall. Surgical resection may be considered if the tumour produces hemodynamic compromise or malignant arrhythmias.
Fibromas are usually solitary, nonencapsulated tumours consisting of fibroblasts and dense connective tissue (1). They can be quite large. They frequently occupy an intramural position within the LV septum or free wall, but they can also be found in the atria or RV (1,2,4). Focal calcification and necrosis may be seen. More than one-third of cases are diagnosed in infancy. Lesions may obstruct valves and inflow/outflow tracts. They produce conduction abnormalities, and atrial or ventricular arrhythmias in 13% of patients. These tumours have been reported as the cause of sudden cardiac death in 10% to 30% of cases. (2,5,6). Mechanisms of arrhythmia include compression of the His bundle resulting in conduction block, possible creation of re-entry pathways producing VT, and regions of asynchronous refractoriness within the tumour mass producing electrical instability, bradyarrhythmias and ventricular fibrillation (2,6). The tumours typically exhibit slow growth; spontaneous regression is very rare. They infiltrate the myocardium and may grow around coronary arteries and conduction tissue, making them unresectable (1).
Myxomas are the most common cardiac tumour in the adult population. They are believed to be derived from mesenchymal cell precursors (7). They form intracavitary masses predominantly in the left atrium attached to the fossa ovalis, but may be seen more commonly in the right atrium in children (1,7). They occur less frequently in the ventricles attached to valve tissue or chordae tendineae. They are often pedunculated but may have a broad attachment to the chamber. Calcification and cystic changes have been described. Complications of myxomas include thrombosis and emboli of tumour fragments, atrial or ventricular arrhythmias and atrioventricular valve obstruction (1,7).
Tumours of the atrioventricular node are extremely rare. They are believed to be hamartomas derived from endodermal tissue. Compression of the atrioventricular node tissue or its artery may produce mechanical or ischemic injury, respectively, resulting in conduction block (8).
Purkinje cell tumours have been described rarely in infants with arrhythmias or sudden cardiac death. These tumours are hamartomatous nests of myocardial cells with abundant mitochondria (3,4). Surgical excision and catheter ablation have been useful in alleviating arrhythmias in some cases (4).
Cardiac tumours and ICDs
Atrial or ventricular arrhythmias refractory to antiarrhythmic therapy are an indication for surgical resection of the tumour (4). However, proximity to coronary arteries or conduction tissue may make resection difficult (1). Case reports have described the use of ICDs for persistent arrhythmias due to nonresectable tumours (5).
Park and Pollock (9) described a seven-month-old infant with an LV fibroma and recurrent ventricular fibrillation despite multiple attempts to fully resect the tumour. He received an epicardial ICD, which delivered appropriate discharges for persistent ventricular arrhythmias. He received a heart transplant at 18 months of age. Thøgersen et al (10) described a four-month-old infant with multiple LV rhabdomyomas and persistent VT despite antiarrhythmic agents, who received an ICD. Sharma et al (5) described a 13-year-old girl with a large LV fibroma and refractory ventricular arrhythmias appropriately treated by an ICD; she later received a heart transplant.
The American College of Cardiology/American Heart Association/Heart Rhythm Society 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities (11) stated that symptomatic sustained VT in a patient with congenital heart disease not amenable to surgical therapy or catheter ablation is a class I indication for ICD implantation. The guidelines offer no specific recommendations for patients with cardiac tumours as a source of persistent arrhythmia. The role of programmed ventricular stimulation (VSTIM) to assess risk of mortality in this group of patients is also unclear. Alexander et al (12) used VSTIM in selected patients with palliated or repaired congenital heart disease, including tetralogy of Fallot, aortic stenosis and dextro-transposition of the great arteries. The investigators found that patients with low-grade ectopy (isolated beats or couplets) were less likely to have inducible VT than patients with high-grade ectopy (sustained or nonsustained VT). Furthermore, patients with inducible VT had a higher risk of sudden cardiac death, although there were many false negatives (12). Khairy et al (13) reported a multicentre study that examined the prognostic role of VSTIM in repaired tetralogy of Fallot patients in which inducible sustained VT predicted an increased risk of clinical VT or sudden cardiac death. The arrhythmogenic substrate was believed to be the myotomy scar or surgical patch.
PCTs and sudden cardiac death
In case 1, sudden death was believed to be due to possible dynamic LV outflow tract obstruction or ventricular arrhythmia. Because there was no chest impact in the collision, commotio cordis was unlikely. In case 2, sudden death was attributed to a cardiac arrhythmia secondary to the cardiac tumour. In both cases, the tumour may have had some hemodynamic effect; however, both patients had a history of documented arrhythmia, consisting of only isolated premature ventricular beats in the first case, and refractory SVT in the second case. As has been described with hypertrophic cardiomyopathy, acute obstruction of blood flow and resultant ischemia may have precipitated dysrhythmia. In case 3, the patient had a large tumour within the free wall of the LV, resulting in dilation of the chamber, which did not appear to regress with the tumour, creating a thinned dyskinetic scar. The patient was deemed to be at increased risk for sudden cardiac death, with findings of nonsustained VT on ambulatory monitoring and inducible sustained VT. It is difficult to determine the contribution of the tumour or the resultant scar to the finding of inducible sustained VT. An ICD was placed but the patient has not had an appropriate discharge despite frequent ectopy. In case 4, asymptomatic sustained VT responded satisfactorily to antiarrhythmic therapy. These cases illustrate the difficulty in ascertaining the outcomes of arrhythmias in the setting of PCTs.
There is a substantial body of case reports of sudden cardiac death or sustained VT in patients with various tumour types. These reports include patients spanning all age groups, and tumours in various locations within the heart. The tumours can be quite large but some are exceedingly subtle on an echocardiogram and have only been identified because of investigations of persistent arrhythmia. It is difficult to determine risk factors of sudden cardiac death in patients with PCTs and arrhythmias. Further studies are required to risk stratify this cohort.
The unknown risk of malignant arrhythmias raises the issue of screening. Serial assessment of pediatric patients with cardiac tumours should include ambulatory Holter monitoring to identify intermittent atrial or ventricular dysrhythmias, some of which may be asymptomatic. Further management of identified arrhythmias would be guided by the tumour type and location, as well as the presence of cardiac symptoms. Antiarrhythmic therapy or surgical resection may be useful in controlling recalcitrant rhythm abnormalities.
CONCLUSIONS
The association of cardiac tumours with atrial and ventricular arrhythmias has been well documented. Although complete resection of the tumour is often curative, some tumour types may grow to incorporate coronary vasculature and conduction tissue, making complete resection impossible. The risk of sudden cardiac death with arrhythmias secondary to the tumour is uncertain. No prognostic indicators have been identified to risk stratify these patients. A registry of these patients may be helpful in determining risk factors.
REFERENCES
- 1.Freedom RM, Lee KJ, MacDonald C, Taylor G. Selected aspects of cardiac tumours in infancy and childhood. Pediatr Cardiol. 2000;21:299–316. doi: 10.1007/s002460010070. [DOI] [PubMed] [Google Scholar]
- 2.Stratemann S, Dzurik T, Fish F, Parra D. Left ventricular cardiac fibroma in a child presenting with ventricular tachycardia. Pediatr Cardiol. 2008;29:223–6. doi: 10.1007/s00246-007-9083-1. [DOI] [PubMed] [Google Scholar]
- 3.Becker AE. Primary heart tumours in the pediatric age group: A review of salient pathologic features relevant for clinicians. Pediatr Cardiol. 2000;21:317–23. doi: 10.1007/s002460010071. [DOI] [PubMed] [Google Scholar]
- 4.Burke A, Virmani R. Pediatric heart tumours. Cardiovasc Pathol. 2008;17:193–8. doi: 10.1016/j.carpath.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Sharma K, Rohlicek C, Cecere R, Tchervenkov CI. Malignant arrhythmias secondary to a cardiac fibroma requiring transplantation in a teenager. J Heart Lung Transplant. 2007;26:639–41. doi: 10.1016/j.healun.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Ottaviani G, Rossi L, Ramos SG, Matturri L. Pathology of the heart and conduction system in a case of sudden death due to a cardiac fibroma in a 6-month-old child. Cardiovasc Pathol. 1999;8:109–12. doi: 10.1016/s1054-8807(98)00030-1. [DOI] [PubMed] [Google Scholar]
- 7.Akyıldız EU, Tolgay E, Öz B, Yılmaz R, Koç S. Cardiac myxoma: An unusual cause of sudden death in childhood. Turk J Pedia. 2006;48:1724. [PubMed] [Google Scholar]
- 8.Cohle SD, Suarez-Mier MP, Aguilera B. Sudden death resulting from lesions of the cardiac conduction system. Am J Forensic Med Pathol. 2002;23:83–9. doi: 10.1097/00000433-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Park JK, Pollock ME. Use of an implantable cardioverter defibrillator in an eight-month-old infant with ventricular fibrillation arising from a myocardial fibroma. PACE. 1999;22:138–9. doi: 10.1111/j.1540-8159.1999.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 10.Thøgersen AM, Helvind M, Jensen T, Andersen H, Jacobsen JR, Chen X. Implantable cardioverter defibrillator in a 4-month-old infant with cardiac arrest associated with a vascular heart tumour. PACE. 2001;24:1699–700. doi: 10.1046/j.1460-9592.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelinese for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 12.Alexander ME, Walsh EP, Saul JP, Epstein MR, Triedman JK. Value of programmed ventricular stimulation in patients with congenital heart disease. J Cardiovasc Electrophysiol. 1999;10:1033–44. doi: 10.1111/j.1540-8167.1999.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 13.Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: A multicenter study. Circulation. 2004;109:1994–2000. doi: 10.1161/01.CIR.0000126495.11040.BD. [DOI] [PubMed] [Google Scholar]