Abstract
The lifespan of dendritic cells (DCs) can potentially influence immune responses by affecting the duration of DCs in stimulating lymphocytes. Significant differences in the lifespan have been reported for various DC subsets, however, the molecular mechanisms for regulating such differences between DC subsets remain unclear. In this study, we compared the apoptosis signaling molecules in two major DC subjects, the myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). We observed a lower ratio between anti-apoptotic Bcl-2/Bcl-xL and pro-apoptotic Bax/Bak in shorter-lived myeloid DCs (mDCs) than in longer-lived plasmacytoid DCs (pDCs) or T cells. Transfection with Bcl-2 or Bcl-xL prolonged the survival of mouse primary mDCs in vitro, while deletion of Bcl-2 accelerated DC turnover in vivo. In addition, the ratios between anti-apoptotic Bcl-2/Bcl-xL and pro-apoptotic Bax/Bak could be regulated in DCs. Signaling from toll-like receptors (TLRs) up-regulated Bcl-xL and improved DC survival. Our data suggest that differential expression of apoptosis signaling molecules regulates the lifespan of different DC subsets.
Keywords: dendritic cells, apoptosis
1. Introduction
Cells in the hematopoietic lineage undergo constant self-renewal. Various cell types have distinctly different half-lives in vivo. In addition, developmental and activation statuses could also affect the turnover rates in each cell lineage. For example, naïve T cells are relatively long-lived, while activated T cells have shorter lifespan to ensure the removal of T cells that have been significantly expanded after antigen stimulation (Lenardo et al., 1999; Tough and Sprent, 1994; Tough and Sprent, 1998). A small portion of activated T cells may survive longer as memory T cells (Li et al., 2003; Liu et al., 2004; Sprent and Surh, 2002). The turnover of the cells in the immune system not only plays an important role in regulating lymphocyte homeostasis, but also may be important for the maintenance of immune tolerance (Lenardo et al., 1999; Rathmell and Thompson, 2002). Although the precise molecular mechanisms governing the rates of self-renewal for different cell lineages are unclear, apoptosis appears to play an important role in this process (Adams et al., 1999; Lenardo et al., 1999; Rathmell and Thompson, 2002).
DCs represent the most efficient antigen presenting cells in capturing, processing and presenting antigens for lymphocyte activation (Banchereau and Steinman, 1998; Lanzavecchia and Sallusto, 2001; Liu, 2001; Shortman and Liu, 2002; Steinman et al., 2003). It is conceivable that the lifespan of DCs can influence the duration for DCs in stimulating lymphocytes, thereby affecting the outcome of lymphocyte activation and immune responses. Several reports have suggested rapid turnover of DCs in vivo (Hou and Van Parijs, 2004; Ingulli et al., 1997; Kamath et al., 2002; Nopora and Brocker, 2002). DCs may also undergo accelerated clearance from the lymphoid organs after interacting with antigen-specific T cells (Ingulli et al., 1997). Tracking DC turnover by BrdU labeling has revealed prominent differences in the rates of self-renewal among different DC subsets (Kamath et al., 2002), suggesting that the lifespan of different DC subsets varies significantly in vivo. In particular, mDCs in the mouse spleens were labeled more rapidly than pDCs by BrdU, indicating that mDCs undergo faster turnover and have a shorter lifespan than pDCs in vivo (O’Keeffe et al., 2002). However, the roles of apoptosis signaling in regulating the rates of survival and turnover of different DC subsets remain to be defined.
Several lines of evidence indicate that apoptosis can regulate DC survival and functions (Hou and Van Parijs, 2004; Nopora and Brocker, 2002). It has been shown that overexpression of Bcl-2 in DCs can prolong DC survival and enhance the immunogenicity of DCs in transgenic mice (Nopora and Brocker, 2002). In addition, induction of cell death in DCs has been shown to limit the priming of antigen-specific cytotoxic T cells (Jung et al., 2002), while inhibition of apoptosis in DCs enhances the potency of DC-based vaccine in triggering tumor-specific immune responses (Kim et al., 2005; Peng et al., 2005; Pirtskhalaishvili et al., 2000). DCs may also play an important role in regulating immune tolerance (Banchereau et al., 2004; Steinman et al., 2003; Steinman et al., 2000). Immunization with excessive activated antigen-pulsed DCs has been shown to induce systemic and tissue-specific autoimmune responses (Ludewig et al., 1998; Roskrow et al., 1999). In addition, we have observed that targeted inhibition of apoptosis in DCs with a caspase inhibitor can induce spontaneous T cell activation and the development of systemic autoimmunity in transgenic mice (Chen et al., 2006). Therefore, apoptosis in DCs potentially regulate the scope of immune responses and immune tolerance.
The Bcl-2 family proteins play a major role in regulating mitochondrion-dependent apoptosis (Adams et al., 1999; Gross et al., 1999). They share one or more Bcl-2 homology (BH) domains and can be divided into three subfamilies: 1) the anti-apoptotic subfamily proteins, such as Bcl-2, Bcl-xL and Mcl-1; 2) the pro-apoptotic Bax-and Bak-like proteins; and 3) the pro-apoptotic BH3-only subfamily. Bax and Bak can oligomerize to form pores on the outer mitochondrial membrane that cause mitochondrial membrane permeabilization. The anti-apoptotic Bcl-2 family proteins can bind to pro-apoptotic Bax or Bak to sequester and inhibit these molecules (Rathmell and Thompson, 2002). BH3-only proteins are the upstream sensors for different apoptosis signaling (Strasser, 2005). BH3-only proteins either inhibit the anti-apoptotic molecules as “de-repressors”, or directly activate pro-apoptotic Bax or Bak, resulting in the aggregation of Bax or Bak to trigger mitochondrial membrane permeabilization, which then leads to the release of cytochrome c from the mitochondrial intermembrane space to the cytosol and activation of caspases that demolish the cell(Gross et al., 1999; Strasser, 2005; Willis and Adams, 2005).
In the present study, we characterized the regulation of DC lifespan by apoptosis in different DC subsets. We examined the expression of Bcl-2 family proteins in DC subsets, including CD11c+CD11b+ myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) that are CD11clow and plasmacytoid dendritic cell antigen-1-positive (PDCA-1+; Miltenyi Biotec, Germany) from the mouse spleens. We found that the relative expression of anti-apoptotic versus pro-apoptotic molecules correlated with the differential turnover rates of mDCs, pDCs and T cells in vivo. Activation of DCs through TLRs up-regulated anti-apoptotic Bcl-xL and improved DC survival. In addition, overexpression of Bcl-2 and Bcl-xL by nucleofection prolonged DC survival. Our results suggest that the balance between anti- and pro-apoptotic molecules controls the lifespan of different DC subsets.
2. Materials and methods
2.1 Mice
Wild type and bcl-2+/- mice on the C57BL/6 background were obtained from the Jackson Laboratory. All mice were housed in a specific pathogen-free animal facility at the Baylor College of Medicine, and experiments were performed according to federal and institutional guidelines and with the approval of the Baylor College of Medicine Institutional Animal Care and Use Committee.
2.2. Purification of DC subsets and T cells
Mouse spleens were treated with liberase (Roche) (0.4 mg/spleen) and incubated at room temperature for 20 min. Single cell suspension was prepared and the red blood cells were lysed with the ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.2). The cells were blocked with 1 μg/ml CD16/CD32 and 10 μg/ml rat IgG on ice for 15 min, and then incubated with 1 μg/ml biotinylated antibodies to CD3, Thy1.2, CD19, IgM, DX5, and TER119 (BD Biosciences) on ice for 30 min, followed by incubation with streptavidin-conjugated Biomag beads (Qiagen) on ice for 30 min. After removal of the cells bound to the Biomag beads with a Cell Separation Magnet (BD Biosciences), live cells were purified by Ficoll-gradient separation and incubated with anti-PDCA-1 MACS beads (Miltenyl Biotec) at 4 °C for 15 min. The cells bound to anti-PDCA-1 beads were purified with a MACS column and were >90% positive for CD11c and PDCA-1 staining (Fig. 2A). The unbound cells were incubated with anti-CD11c MACS beads (Miltenyl Biotec) and the cells were purified with a MACS column. Approximately 95% cells were CD11c+ and more than 90% were CD11c+CD11b+ mDCs (Fig. 2A). DCs were either used for lysis and Western blot, or cultured in RPMI complete medium containing 10 μg/ml anti-CD40 plus 1 μg/ml protein A, 5 μg/ml LPS (Sigma), 50 μg/ml lipoteichoic acid (LTA; Sigma), 0.5 μM phosphorothioate-stabilized CpG oligonucleotide (TCCATGACGTTCCTGATGCT), 100 ng/ml polyinosinic-polycytidylic acid (poly I:C; Sigma) or 100 ng/ml peptidoglycan (PGN; Sigma) and lysed for Western blot analysis. To purify T cells, splenocytes were incubated with biotinylated-anti-Thy1.2 (BD Biosciences). And Thy1.2+ cells were purified with streptavidin-conjugated MACS beads (Miltenyi Biotec).
Figure 2.
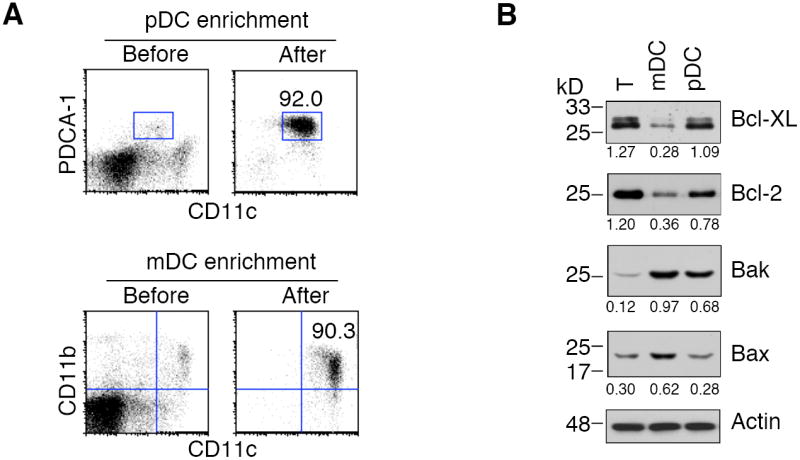
Expression of Bcl-2 family proteins in splenic DC subsets. (A) T cells, mDCs and pDCs were purified from mouse spleens. An example of the purities of enriched mDCs and pDCs after enrichment from mouse spleens was shown. (B) T cells, mDCs and pDCs isolated from mouse spleens were lysed for Western blot analysis of Bcl-2 family proteins. The intensities of protein bands were quantitated using the Kodak Image Station 440CF 1D Image Analysis Software. The ratios of the intensities between the Bcl-2 family proteins and actin were indicated. The data are representative of two independent experiments.
2.3. Antibodies and Western blot
Cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 1x protease inhibitor cocktail from Roche) on ice for 1 h. Cell lysates were quantitated by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA), and used for Western blot by probing with various antibodies, followed by horseradish peroxidase-conjugated secondary antibodies (Southern Biotechnology, Birmingham, AL). The blots were then developed using Supersignal Dura substrate (Pierce). Antibodies for Western blot analyses were: monoclonal mouse antibody to Bax (clone 6A7), Bcl-xL (clone 2H12, BD Biosciences), HA1.1 (clone 16B12, Covance) and α-tubulin (clone B-7, Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal rabbit antibodies to Bcl-2, Bak (Upstate Biotechnologies), and polyclonal goat anti-actin (Santa Cruz Biotechnology). The intensities of protein bands were quantitated using the Kodak Digital Science Image Station 440CF 1D Image Analysis Software (Eastman Kodak Co., Rochester, NY).
2.4. Apoptosis assays for DCs
DCs (0.5×105/well) were incubated with different treatments in 96-well flat-bottom tissue culture plates for 0 h (as control) or indicated time. The cells were then harvested and stained with propidium iodide or FITC-Annexin V (Sigma) plus propidium iodide to quantitate live cells by flow cytometry. Percentages of apoptosis were calculated as described (Hornung et al., 1997).
2.5. Transfection of bone marrow-derived DCs by nucleofection
To transfect primary bone marrow-derived DCs, bone marrow cells were cultured in 10 ng/ml GM-CSF and 10 ng/ml IL-4 (Biosources) for 5 days. The cells were collected at day 5 and resuspended in 100 μl Macrophage Nucleofector Solution (107/ml) containing 1 μg pMAX-GFP plus 3 μg pSH1-bcl-2-HA, pSH1-bcl-xL-HA or control pSH1 plasmid. The cells were electroporated in a Nucleofector II using program Y-001 (Amaxa). Then, 500 μl of pre-warmed RPMI complete medium containing 10 ng/ml GM-CSF and 10 ng/ml IL-4 were added to the transfection cuvette, and the cells were gently transferred to 5 ml pre-warmed RPMI complete medium containing GM-CSF and IL-4 in 6-well plates. We routinely found that approximately 80% of the transfected cells remained viable after overnight culture and between 30 and 55% cells were positive for GFP (Chen et al., unpublished observations). Two days after transfection, live cells were purified by Ficoll-gradient separation and used for Western blot or apoptosis assays.
2.6. Tracking BrdU-labeled DCs and flow cytometry
Mice were injected daily with BrdU intraperitoneally (1 mg/mouse). Spleens were harvested at indicated time, treated with 0.4 mg/ml liberase (Roche) at room temperature for 10 min followed by lysis of red blood cells with ACK lysis buffer. After blocking with 10 μg/ml rat IgG and 1 μg/ml anti-CD16/CD32 (BD Biosciences), 106 cells were incubated with fluorochrome-conjugated antibodies to different DC and T cell markers for 15min on ice. Cells were then used for BrdU staining with the FITC BrdU Flow Kit (BD Biosciences). After fixation with the Cytofix/Cytoperm buffer (BD Biosciences), the cells were permeabilized with Cytoperm Plus Buffer (BD Biosciences) and treated with 300 μg/ml DNase and 25 mM MgCl2 at 37°C for 45 min. The cells were then stained with FITC-anti-BrdU (BD Biosciences) and analyzed by flow cytometry on an EPICX flow cytometer (Beckman Coulter). The data were analyzed by the FlowJo software (TreeStar). The conjugated antibodies to CD11c, CD11b, CD4 and CD8 were obtained from BD Biosciences. PE-conjugated anti-PDCA-1 was from Miltenyi Biotec. CD11c+CD11b+ mDCs, PDCA-1+ pDCs and T cells were gated to analyze BrdU staining.
3. Results
3.1. Faster turnover rates in mDCs than in T cells and pDCs
Because terminally differentiated DCs do not undergo significant cell division, it is possible to track the turnover rates of DCs in lymphoid organs by the rates of their replacement by newly differentiated DCs labeled with BrdU (Kamath et al., 2002). We examined the lifespan of CD11chighCD11b+ mDCs in vivo by BrdU labeling. We observed that almost 40% of CD11chighCD11b+ mDC was labeled with BrdU after two days of BrdU pulsing (Fig. 1). By day 4, approximately 70% mDC were BrdU+ (Fig. 1). Both CD4+ and CD8+ mDCs were rapidly labeled with BrdU, although CD8+ DCs were labeled slightly faster. This is consistent with previous studies that DCs undergo rapid turnover in vivo (Hou and Van Parijs, 2004; Nopora and Brocker, 2002; O’Keeffe et al., 2002).
Figure 1.
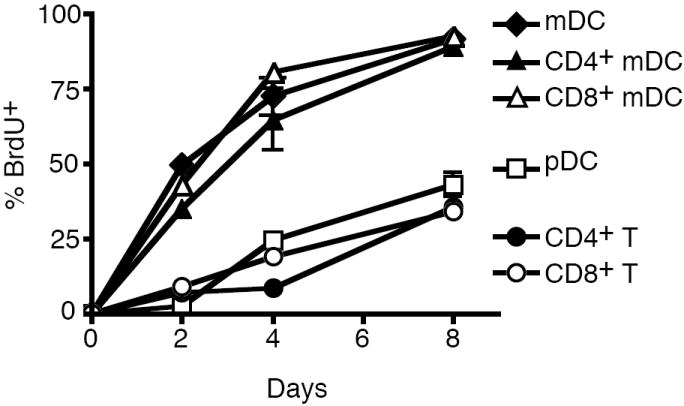
Spontaneous turnover of DC subsets. Two-month-old mice were labeled with BrdU for different days. Spleens were collected at different time and stained for markers of mDCs, pDCs or T cells. After fixing and permeabilization, the cells were stained with FITC-anti-BrdU and analyzed by flow cytometry. The percentages of BrdU+ cells were presented as mean ± SD of 3 mice at each time point. Data shown are representative of four independent experiments.
Differential turnover rates for different DC subsets have been observed (Kamath et al., 2002). In particular, the plasmacytoid DCs (pDCs) have been shown to have a relatively longer lifespan by slower BrdU labeling in vivo (O’Keeffe et al., 2002). We therefore also examined BrdU labeling of pDCs. We observed that less than 10% of CD11clowPDCA-1+ pDCs were positive for BrdU at day 2, and 25% were labeled by day 4. Similar to pDCs, CD4+ or CD8+ naïve T cells also underwent slower rates of BrdU labeling than mDCs (Fig. 1). This suggests that pDCs and T cells have a relatively longer lifespan than mDCs.
3.2. Correlation of the turnover rates in DC subsets with the molecular ratios between anti-apoptotic Bcl2/Bcl-xL and pro-apoptotic Bax/Bak
To determine the molecular mechanisms that account for the differences in the lifespan between mDCs and pDCs in vivo, we enriched CD11clowPDCA-1+ pDCs and CD11c+CD11b+ mDCs from the spleens of C57BL/6 mice (Fig. 2A). Using positive selection with anti-PDCA-1- and anti-CD11c-microbeads (Miltenyi Biotec) in combination with depletion of non-DCs in the spleens (described in details in Materials and Methods), we were able to significantly enrich CD11clowPDCA-1+ pDCs and CD11c+CD11b+ mDCs from mouse spleens (Fig 2A).
We next lysed freshly purified splenic DCs and T cells to examine the expression of apoptosis signaling molecules by Western blot. We detected less anti-apoptotic Bcl-2 and Bcl-xL in mDC than in pDCs or T cells (Fig. 2B). When normalized with actin, T cells expressed >4-fold of Bcl-xL compared with mDCs (Fig. 2B). Similar to T cells, pDCs also expressed >3-fold as much Bcl-xL as in mDCs (Fig. 2B). In addition, T cells contained >3-fold Bcl-2 (Fig. 2B), while pDCs also had >2-fold of Bcl-2 compared with mDCs (Fig. 2B). This suggests that T cells and pDCs expressed more anti-apoptotic Bcl-2 and Bcl-xL than mDCs.
By contrast, mDCs expressed significantly more pro-apoptotic Bax than pDCs or T cells (Fig. 2B). Bak in mDCs was higher (>6-fold) than in T cells, and also slightly higher (30%) in pDCs than in mDCs (Fig. 2B). In addition, mDCs also contained >2-fold Bax compared to T cells or pDCs (Fig. 2B). Therefore, the shorter-lived mDCs had more pro-apoptotic Bax and Bak than longer-lived pDCs or T cells. A lower molecular ratio between anti-apoptotic Bcl-2/Bcl-xL and pro-apoptotic Bax/Bak (Fig. 2B) is correlated with the shorter lifespan of mDCs in vivo (Fig. 1).
3.3. Anti-apoptotic Bcl-2 and Bcl-xL prolong the survival of DCs
The relative expression of pro-apoptotic and anti-apoptotic molecules is correlated with the rates of spontaneous turnover of mDCs and pDCs in vivo (Fig. 1 and Fig. 2B). Changing the balance between pro-apoptotic and anti-apoptotic molecules could potentially alter the lifespan of DCs. It has been shown that overexpression of Bcl-2 in DCs slows down DC replacement in the spleens of CD11c-bcl-2 transgenic mice by BrdU labeling (Nopora and Brocker, 2002). This indicates that Bcl-2 indeed contributes to prolonging the lifespan of DCs in vivo. We also examined the rates of self-renewal of DC subsets in bcl-2-/- mice. Because most bcl-2-/- mice do not survive beyond 4 weeks, we performed BrdU labeling in 3-week-old bcl-2-/- mice and wild type littermates and examined BrdU labeling of DC subsets in the spleens two days later. As expected, we observed that more mDCs and pDCs in the spleens of bcl-2-/- mice were labeled with BrdU than in wild type controls (Fig. 3A). The increased rates of self-renewal of both mDCs and pDCs in bcl-2-/- mice suggest that Bcl-2 helps to prolong the lifespan of both mDCs and pDCs in vivo.
Figure 3.
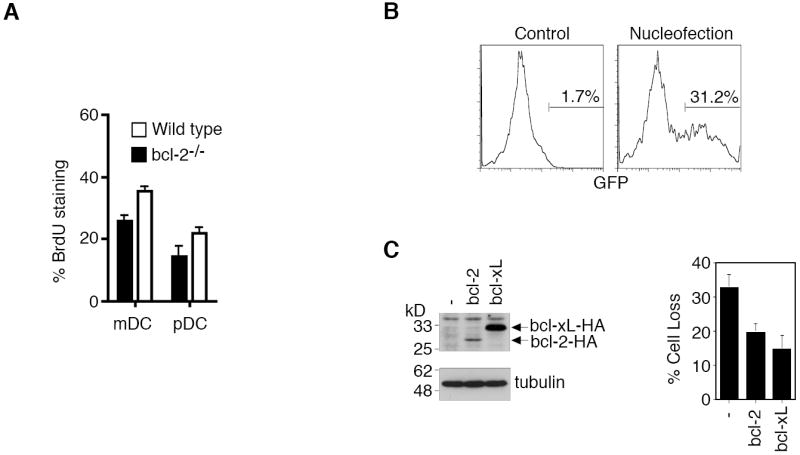
Bcl-2 and Bcl-xL in regulating DC survival. (A) Three week-old wild type and bcl-2-/- mice were labeled with BrdU and the percentages of BrdU+ cells in splenic DCs were determined 48 h later. (B) Bone marrow-derived CD11c+ DCs were transfected with pMAX-GFP by nucleofection. Two days later, the cells were collected to analyze GFP expression by flow cytometry. An example of GFP expression in DCs after a typical nucleofection is shown. (C) Two days after transfection with GFP plus Bcl-2-HA or Bcl-xL-HA, DCs were lysed for Western blot analyses by probing with anti-HA1.1 or anti-α-tubulin (left panels). The cells were also cultured for 24 h, and cell loss of GFP+ DCs was quantitated by flow cytometry (right panel). Data shown are representative of three experiments and are presented as mean ± SD.
To ensure that Bcl-2 and Bcl-xL can prolong the survival of DCs by inhibiting apoptosis, we transfected Bcl-2 and Bcl-xL into mouse bone marrow-derived DCs by the nucleofection method (Amaxa). This nucleofection protocol yielded 30-50% transfection efficiency for primary mouse CD11c+ bone marrow-derived DCs as indicated by the expression of co-transfected GFP (Fig. 3B), while approximately 80% cell viability was routinely achieved after nucleofection (data not shown). Western blot analysis indicates that transfection led to the expression of Bcl-2 or Bcl-xL in DCs (Fig. 3C, left panels). Despite similar transfection efficiencies and the use of an identical expression vector, we consistently detected better expression of Bcl-xL than Bcl-2 in DCs after nucleofection (Fig. 3C, left panels). We observed that spontaneous cell death of bone marrow-derived DCs was suppressed by nucleofection with Bcl-2 or Bcl-xL (Fig. 3C, right panel), supporting the roles of these anti-apoptotic molecules in inhibiting cell death in DCs. Anti-apoptotic Bcl-2 and Bcl-xL therefore likely promote the lifespan of DCs by inhibiting apoptosis.
3.4. Increased expression of anti-apoptotic Bcl-xL in DC subsets after TLR-stimulation
We next examined whether the molecular ratios between anti-apoptotic Bcl-2/Bc-xL and pro-apoptotic Bax/Bak could be regulated by different stimulation signals. We purified splenic mDCs and pDCs and treated them with different stimuli. We observed that the activation of DCs by CD40 crosslinking or various TLR stimuli significantly increased the expression of Bcl-xL in mDCs (Fig. 4A). Bcl-2 was also up-regulated to some extents in mDCs, but the induction was not as significant as Bcl-xL (Fig. 4A). CpG, but not other stimuli, up-regulated Bcl-xL in pDCs (Fig. 4A). In comparison, pro-apoptotic Bax and Bak were not significantly induced in either mDCs or pDCs after various treatments (Fig. 4A). This suggests that stimulation of DCs with TLR stimuli up-regulated the anti-apoptotic Bcl-xL, but not pro-apoptotic Bax/Bak.
Figure 4.
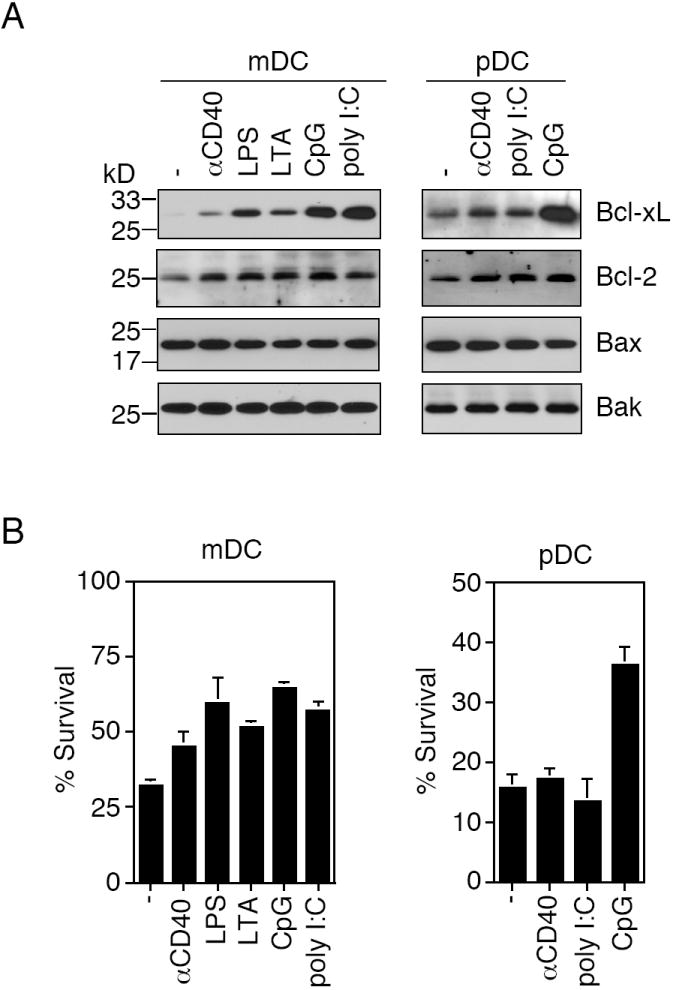
Regulation of expression of Bcl-2 family proteins in splenic DC subsets and splenic DC survival by stimulation. (A) mDCs and pDCs purified from the spleens of C57BL/6 mice were treated with anti-CD40 or different TLR stimuli for 24 h. Cells were lysed for Western blot analyses of Bcl-2, Bcl-xL, Bax and Bak. (B) Splenic mDCs purified from mouse spleens were cultured at 37 °C for 24 h with anti-CD40, LPS, LTA, CpG or poly I:C. pDCs purified from mouse spleens were cultured at 37 °C for 24 h with anti-CD40, poly I:C or CpG, followed by analysis of cell survival. Data shown are representative of three independent experiments and are presented as mean ± SD.
3.5. TLR signaling protects DCs from spontaneous cell death
We also measured spontaneous cell death in purified splenic DCs. We observed that >70% of splenic mDCs underwent cell death, with only approximately 30% viability remaining after 24 h culture in vitro. This rate of cell death is much faster than that would be predicted from in vivo BrdU labeling of DCs in the spleen (Fig. 1), suggesting that taking DCs out of the spleen microenvironment represents a stress that induced accelerated cell death in DCs. Correlated with the increase in Bcl-xL, CD40 crosslinking and treatments with various TLR stimuli improved the viabilities of mDCs derived from the spleens after 24 h of in vitro culture (Fig. 4B).
Although pDCs exhibited slower turnover rates than mDCs in vivo (Fig. 1), pDCs underwent more accelerated cell death than mDCs in vitro. Purified splenic pDCs underwent 84% cell death, with 16% cell viability remaining after 24 h of in vitro culture (Fig. 4B). This suggests that the removal from the splenic microenvironment represents an even more severe stress for pDCs than for mDCs. Consistent with CpG-induced up-regulation of Bcl-xL, CpG also improved the survival of pDCs to 37% (Fig. 4B). This suggests that CpG inhibited cell death of pDCs in vitro.
Consistent with the findings in splenic DCs, spontaneous apoptosis in bone marrow-derived DCs was also inhibited by treatments with different TLR stimuli after 24 h or 48 h in vitro culture (Fig. 5A), while CD40 crosslinking only showed marginal effects in suppressing spontaneous apoptosis in DCs (Fig. 5A). Nucleofection of Bcl-2 or Bcl-xL inhibited spontaneous apoptosis in DCs, while treatments with LPS or CpG further suppressed apoptosis in DCs transfected with Bcl-2 or Bcl-xL (Fig. 5B). These data further support the possibility that TLR stimuli inhibit spontaneous cell death in DCs.
Figure 5.
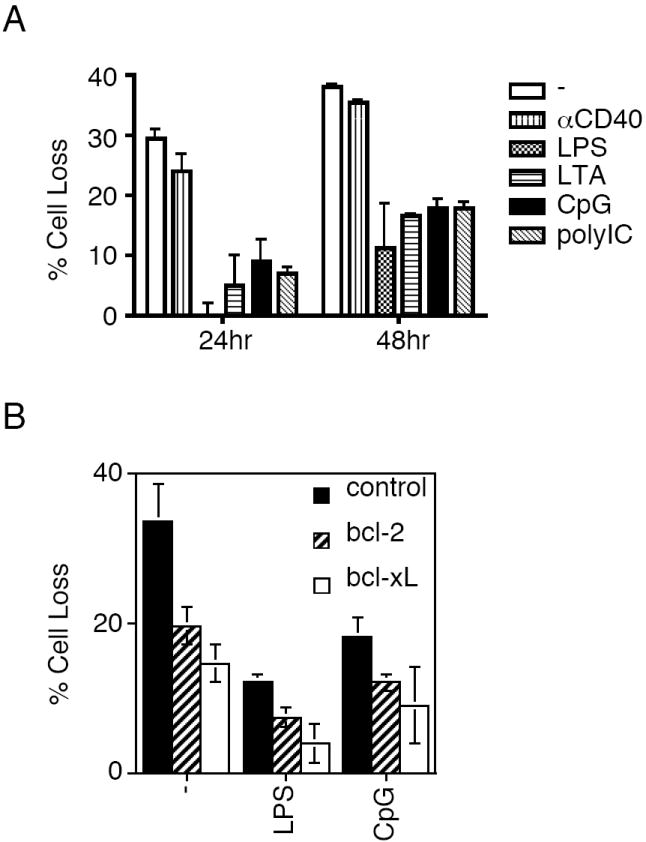
Spontaneous apoptosis in activated DCs. (A) Bone marrow-derived DCs were treated with anti-CD40 or different TLR stimuli. Spontaneous apoptosis were quantitated 24 and 48 h later. Data (mean ± SD) are representative of four experiments. (B) Bone marrow-derived DCs were transfected with pMAX-GFP alone, or pMAX-GFP with bcl-2 or bcl-xL plasmids by nucleofection. Two days later, live cells were purified and cultured in 96-well plates for 24 h with medium alone, or in the presence of LPS or CpG for 24 h. The cells were collected and the loss of GFP+ DCs was quantitated by flow cytometry. Data (mean ± SD) are representative of two experiments. Statistical significance between bcl-2 or bcl-xL transfected group and control group was analyzed by Student’s t test using GraphPad Prism version 4 for Macintosh with P <0.05 considered statistically significant.
4. Discussion
In this study, we investigated the molecular mechanisms that account for the differences in the in vivo lifespan of DC subsets. Our data suggest that the ratios between anti-apoptotic Bcl-2/Bcl-xL and pro-apoptotic Bax/Bak help to determine the lifespan of different DC subsets. BrdU labeling experiments showed that mDCs had a relatively shorter lifespan and underwent faster self-renewal, while pDCs and T cells survived longer and were replaced more slowly. Compared to the longer-lived naïve T cells, the shorter-lived mDCs expressed lower levels of anti-apoptotic Bcl-2/Bcl-xL but higher pro-apoptotic molecules Bax/Bak. By contrast, pDCs that showed longer lifespan had a higher anti-apoptotic to pro-apoptotic molecular ratio similar to naïve T cells. In addition, deletion of Bcl-2 accelerated DC turnover, while transfection of Bcl-2 or Bcl-xL inhibited cell death in DCs. Therefore, the ratios between anti-apoptotic and pro-apoptotic molecules potentially regulate the lifespan of individual DC subsets in vivo.
The molecular ratios of anti-apoptotic Bcl-2/Bcl-xL to pro-apoptotic Bax/Bak could be regulated in DCs by stimulation. We observed that activation of DCs by different TLR ligands up-regulated Bcl-xL in mDCs (Fig. 4). These signals also improved the expression of Bcl-2 in DCs to lesser extents. However, pDCs were only responsive to treatment with CpG. It has been reported that pDCs express TLR9, a receptor for CpG, but lack most other TLRs (Liu, 2001). This may explain why only CpG induced up-regulation of Bcl-xL in pDCs.
Apoptosis plays an important role in regulating lymphocyte homeostasis in the immune system (Lenardo et al., 1999; Rathmell and Thompson, 2002). It has been observed that lpr mice deficient in Fas have accumulated DCs (Fields et al., 2001). DC-specific expression of baculoviral p35 that targets caspase-8 in the Fas signaling pathway induces the accumulation of DCs (Chen et al., 2006; Xu et al., 2001). Therefore, apoptosis also plays an important role in regulating homeostasis of DCs. Previously, we have observed that DCs express abundant signaling molecules in the Fas signaling pathway (Chen et al., 2006). Activated T cells kill DCs through Fas- and perforin-dependent pathways (Chen et al., 2006). Targeting the Fas signaling pathway with p35 transgene inhibited T cell-dependent killing of DCs but did not affect spontaneous DC turnover in the spleens (Chen et al., 2006). However, expression of a bcl-2 transgene slowed the rates of DC turnover (Nopora and Brocker, 2002). Therefore, Fas may be important for regulating T cell-mediated DC apoptosis during interactions with activated T cells, while Bcl-2 family proteins may be more important in regulating spontaneous cell death in DCs, as well as TLR-induced DC survival.
The functional significance for different turnover rates in DC subsets is currently unclear. It has been shown that mDCs are efficient in presenting antigens on MHC class I and MHC class II molecules in humans, however, pDCs are more limited to class II presentation (Schnurr et al., 2005). Rapid turnover of mDCs may favor their engulfment by other DCs and promote antigen processing and presentation (Schnurr et al., 2005; Zimmermann et al., 2004). Faster turnover could also contribute to abilities for mDCs in cross-presentation.
Stimulation of DCs with TLR stimuli is critical for the activation and maturation of DCs (Banchereau and Steinman, 1998; Lanzavecchia and Sallusto, 2001). Maturation of DCs is characterized by increased expression of co-stimulatory molecules and improved abilities in activating antigen-specific T cells (Banchereau and Steinman, 1998; Blander and Medzhitov, 2006). It has been shown that anti-apoptotic Bcl-2 and Bcl-xL enhance the immunogenicity of DCs in inducing antigen-specific immune responses (Hou and Van Parijs, 2004; Nopora and Brocker, 2002). We observed that stimulation with TLR stimuli increased the expression of Bcl-xL and promoted the survival of mDCs and pDCs. Therefore, prolonged survival of DCs may contribute to better stimulation of lymphocytes by mature DCs in addition to increased expression of co-stimulatory molecules.
Acknowledgments
This work was supported by an American Society of Hematology Junior Faculty Scholar Award (to M.C.) and a grant from the NIH (to J.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Huang DC, Puthalakath H, Bouillet P, Vairo G, Moriishi K, Hausmann G, O’Reilly L, Newton K, Ogilvy S, Bath ML, Print CG, Harris AW, Strasser A, Cory S. Control of apoptosis in hematopoietic cells by the Bcl-2 family of proteins. Cold Spring Harb Symp Quant Biol. 1999;64:351–8. doi: 10.1101/sqb.1999.64.351. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–50. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–4. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- Fields ML, Sokol CL, Eaton-Bassiri A, Seo S, Madaio MP, Erikson J. Fas/Fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J Immunol. 2001;167:2370–8. doi: 10.4049/jimmunol.167.4.2370. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hornung F, Zheng L, Lenardo MJ. Maintenance of clonotype specificity in CD95/Apo-1/Fas-mediated apoptosis of mature T lymphocytes. J Immunol. 1997;159:3816–22. [PubMed] [Google Scholar]
- Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol. 2004;5:583–9. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–41. [PubMed] [Google Scholar]
- Kim TW, Lee JH, He L, Boyd DA, Hardwick JM, Hung CF, Wu TC. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–16. [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, Ashton-Rickardt PG. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5:919–26. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopora A, Brocker T. Bcl-2 controls dendritic cell longevity in vivo. J Immunol. 2002;169:3006–14. doi: 10.4049/jimmunol.169.6.3006. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, Hertzog P, Tatarczuch L, Shortman K. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–19. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Kim TW, Lee JH, Yang M, He L, Hung CF, Wu TC. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–93. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtskhalaishvili G, Shurin GV, Gambotto A, Esche C, Wahl M, Yurkovetsky ZR, Robbins PD, Shurin MR. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165:1956–64. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- Roskrow MA, Dilloo D, Suzuki N, Zhong W, Rooney CM, Brenner MK. Autoimmune disease induced by dendritic cell immunization against leukemia. Leuk Res. 1999;23:549–57. doi: 10.1016/s0145-2126(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Schnurr M, Chen Q, Shin A, Chen W, Toy T, Jenderek C, Green S, Miloradovic L, Drane D, Davis ID, Villadangos J, Shortman K, Maraskovsky E, Cebon J. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–72. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–65. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–25. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H. Covalent inhibition revealed by the crystal structure of the caspase-8/p35 complex. Nature. 2001;410:494–7. doi: 10.1038/35068604. [DOI] [PubMed] [Google Scholar]
- Zimmermann VS, Bondanza A, Monno A, Rovere-Querini P, Corti A, Manfredi AA. TNF-alpha coupled to membrane of apoptotic cells favors the cross-priming to melanoma antigens. J Immunol. 2004;172:2643–50. doi: 10.4049/jimmunol.172.4.2643. [DOI] [PubMed] [Google Scholar]


