Abstract
Research has suggested that lycopene may be metabolized by eccentric cleavage, catalyzed by β-carotene oxygenase 2 (BCO2), resulting in the generation of apo-lycopenals. Apo-6′-lycopenal and apo-8′-lycopenal have been reported previously in raw tomato. We now show that several other apo-lycopenals are also present in raw and processed foods, as well as in human plasma. Apo-lycopenal standards were prepared by in vitro oxidation of lycopene, and a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method using atmospheric pressure chemical ionization in negative mode was developed to separate and detect the apo-6′-, 8′-, 10′-, 12′-, 14′-, and 15′-lycopenal products formed in the reaction.
Hexane/acetone extracts of raw tomato, red grapefruit, watermelon, and processed tomato products were analyzed, as well as plasma of individuals who had consumed tomato juice for eight weeks. Apo-6′-, 8′-, 10′-, 12′-, and 14′-lycopenals were detected and quantified in all food products tested, as well as plasma. The sum of apo-lycopenals was 6.5 µg/100 g ‘Roma’ tomato, 73.4 µg/100 g tomato paste, and 1.9 nmol/L of plasma. We conclude that several apo-lycopenals, in addition to apo-6′- and 8′-lycopenal, are present in lycopene containing foods. In addition, the presence of apo-lycopenals in plasma may derive from the absorption of apo-lycopenals directly from food and/or human metabolism.
Keywords: apo-lycopenal, lycopene, metabolism, tomato
INTRODUCTION
Epidemiological studies have repeatedly correlated increased consumption of tomato and tomato products with reduction in the incidence of a number of cancers and other chronic diseases (1–5). Many researchers have suggested that lycopene, the most abundant carotenoid in red tomatoes, may be responsible for this effect (6–8). It has also been suggested that metabolites of lycopene may be bioactive (9, 10). Khachik et al. (11) first reported lycopene derivatives in human blood plasma, which were later identified as two diastereomeric forms of 2,6-cyclolycopene-1,5-diol (12). These researchers proposed that these two structures were either absorbed from tomato products in the diet, or were metabolic products of 2,6-cyclolycopene-1,5-epoxide, a compound which has been identified in raw tomatoes, tomato juice, and tomato paste (13).
Lycopene may be metabolized in humans by a mechanism analogous to that of β-carotene, another C40 hydrocarbon carotenoid. The most widely characterized pathway of β-carotene metabolism is the formation of two molecules of retinal by the cleavage of β-carotene at the central double bond by β-carotene oxygenase 1 (BCO1) (14). A second enzyme, β-carotene oxygenase 2 (BCO2), has been shown to cleave β-carotene eccentrically (15). Interestingly, a central cleavage product of lycopene has never been identified in a mammalian system. Thus, a focus upon BCO2 cleavage has stimulated efforts to oxidize lycopene to produce aldehydes which could result from BCO2 cleavage. Kim and colleagues (16) produced a series of aldehyde cleavage products of lycopene using a variety of oxidative methods. Likewise, a similar series of aldehyde derivatives were generated by Caris-Veyrat et al. (17) using potassium permanganate as an oxidant.
We have limited knowledge of lycopene metabolites in mammals. Apo-lycopenals have been reported in laboratory animals consuming high lycopene diets. Apo-10′-lycopenol was found in the lung tissue of ferrets consuming a high lycopene diet for 9 weeks (18). In a separate study, apo-8′-lycopenal, and putatively apo-12′-lycopenal were observed in the hepatic tissue of rats consuming a high lycopene diet for 30 days (19). Interestingly, these apo-lycopenal products are not limited to mammalian systems. Apo-6′-lycopenal and apo-8′-lycopenal were previously identified in extracts of tomato paste (20) and raw tomatoes (21).
Given the highly unsaturated structure of lycopene, containing 11 conjugated double bonds available for oxidative cleavage, we hypothesized that an array of apo-lycopenals, in addition to apo-6′- and apo-8′-lycopenal, might be present in tomatoes or tomato products, and possibly as mammalian metabolites. We chose to focus our search on the long chain non-volatile products (> 20 carbons, i.e. acycloretinal or longer) that would result from a double bond cleavage and apply highly sensitive and selective tandem HPLC-MS/MS techniques to detect and identify apo-lycopenals by comparison to synthesized compounds and authentic standards. We also sought to identify and quantify apo-lycopenals in other lycopene-containing foods (red grapefruit, watermelon). Additionally, we identified and quantified apo-lycopenals in the plasma of humans consuming a daily serving of tomato juice.
MATERIALS AND METHODS
Chemicals
Apo-6′-lycopenal, apo-8′-lycopenal, apo-12′-lycopenal, and β-apo-12′-carotenal were purchased from Carotenature (Lupsingen, Switzerland). Pure lycopene was isolated and crystallized from tomato paste following a procedure outlined below. Acetone, acetonitrile, butylated hydroxytoluene (BHT), calcium carbonate, chloroform, dichloromethane, hexane, methanol, methyl tert.-butyl ether, potassium hydroxide, te trahydrofuran, and toluene were purchased from Fisher Scientific (Pittsburgh, PA). Tetrahydrofuran was stabilized before use by passing the solvent through a column of alumina. Formic acid, ethylenediaminetetraacetic acid (EDTA), diatomaceous earth, β-apo-8′-carotenal, and β-carotene were purchased from Sigma Aldrich (St. Louis, MO).
Isolation of lycopene from tomato paste
We found it necessary to isolate pure lycopene from tomato paste due to our observation that analytical grade lycopene standard purchased from two commercial sources contained detectable levels of apo-lycopenals. Sixty grams of tomato paste was blended in an Oster blender with 12 g CaCO3, and 250 mL of MeOH to dehydrate the tissue. The homogenate was filtered through a Whatman #1 filter using a Buchner funnel. The MeOH rinse was discarded, and the filtrant was then mixed with 150 mL of acetone and filtered again. The acetone rinse was also discarded. The filtrant was then mixed with 150 mL of hexane/acetone (1:1) and filtered again. Next, the hexane/acetone filtrate (containing carotenoids and lipids) was placed into an Erlenmeyer flask and 75 mL of 30% KOH in MeOH solution was added in addition to a stir bar. The Erlenmeyer flask was placed into a large beaker containing ice, and the mixture was stirred on a stir plate for 2 h to saponify the components. The mixture was then transferred to a 2 L separatory funnel and washed three times with water. The aqueous phase was drained and discarded, and the hexane was placed in a 500 mL round bottom flask and dried under reduced pressure in a 40 °C water bath.
The dried extract was removed from the round bottom flask, transferred to a vial, and 1:1 chloroform/MeOH solution was added. The vial was placed into a 55 °C water bath, and the mixture was stirred until all of the dried extract dissolved. The water bath containing the dissolved lycopene was removed from the heat so urce and allowed to slowly cool to room temperature to induce lycopene crystallization. The sample was then transferred to the −20 °C freezer for two hours to facilitate further crystallization. Afterwards, the supernatant was filtered off and the crystals were washed four times with ice cold MeOH. The crystals were dried under vacuum and analyzed for apo-lycopenals using the LC-MS/MS method detailed below. Purity was determined by HPLC-photodiode array (PDA).
Synthesis of apo-lycopenals for identification
A series of apo-lycopenals was generated from lycopene using ozone as an oxidant. The lycopene used for this oxidation was isolated as above with modifications. That is, the initial blending of 360 g of tomato paste with MeOH (600 mL) and CaCO3 (70 g) also included 100 g of diatomaceous earth. Further, once the combined acetone/hexane 1:1 filtrate was prepared, it was washed with 3 × 100 mL portions of saturated NaCl solution and the organic layer was evaporated. The resulting crude tomato oleo resin was transferred to a LiChroprep RP-18, 40–63 µM, reverse phase column (EM Separations, Gibbstown, NJ) which was washed with 150 mL of MeOH/acetonitrile/CH2Cl2 45:45:10 (22) and then pure CH2Cl2 to elute 188 mg of lycopene which as used as obtained. For ozonolysis, according to the procedure of Schwartz et al. (23), 4 mg (0.01 mmol) of this lycopene and 4-methylmorpholine-N-oxide (40 mg; 0.34 mmol) were dissolved in 8mL of CH2Cl2 and the solution divided into 4 × 2mL aliquots. Phosphate buffer (2 mL of 25 mM, pH 6) was also added to each aliquot. Ozone was generated by a model T-816 laboratory ozonator (Welsbach, Philadelphia, PA) with oxygen pressure of 8 psi, dielectric voltage of 105 V, and ozone was bubbled into CH2Cl2 for 30 s. Different aliquots (0.5, 1, 1.5 and 2 mL) of this saturated ozone solution were added to the four lycopene containing vials which were immediately shaken to mix with the buffer and quench the reaction. The CH2Cl2 layers were then separated, concentrated, and the residues subjected to HPLC-MS/MS analysis as described below.
HPLC-MS/MS Method for separating apo-lycopenals in the oxidized lycopene mixture
An HPLC method was developed to resolve long-chain apo-lycopenals produced in the mixture of lycopene oxidation products. The mixture was separated by a model 2996 HPLC (Waters) connected to a model 2996 PDA (Waters) using a 150 mm × 4.96 mm i.d., 5 µm, YMC C30 column (Waters Corp., Milford, MA). Composition of solvents was as follows: A= 88:5:5:2 MeOH/water/methyl tert.-butyl ether/0.1% aq. formic acid solution; B = 78:20:2 methyl tert.-butyl ether/MeOH/0.1% aq. formic acid solution. A gradient of A and B was used to separate the synthesized compounds at a flow rate of 1.3 mL/min, column temperature was 30 °C. The gradient was as follows: isocratic at 0% B for 1 min, ramp to 4.5% B over 0.1 min, linear gradient to 90% B over 19.9 min, linear gradient to 100% B over 6 min, and 4 min to return to 0% B. The HPLC was interfaced with a Q-Tof Premier quadrupole time-of-flight hybrid mass spectrometer (Micromass UK Ltd., Manchester, UK) interfaced using an atmospheric pressure chemical ionization (APcI) source operated in negative ion mode. Additional settings were as follows: corona current = 30 µA, cone = 35 V, desolvation temp. = 400 °C, desolvation gas flow = 300 L/h, and gas for collisionally induced dissociation (CID) was argon at 4 ×10−3 bar.
Extraction of foods
Foods used for analysis were purchased from a local supermarket (Columbus, OH). Three different brands of each processed tomato product (catsup, spaghetti sauce, pizza sauce, tomato soup, tomato paste, tomato juice) were tested. Raw red vine ripened tomatoes, cherry tomatoes, ‘Roma’ tomatoes, ruby red grapefruit, and watermelon were also tested. Raw fruits were purchased, diced, and mixed in an Oster blender to produce a puree before extraction.
Foods were extracted following the method developed previously (24). All extractions were performed under red light. Hexane extracts were dried immediately under argon gas and stored at −80 °C for not more than one week before analysis. Samples were reconstituted in 1:1 methyl tert.-butyl ether /MeOH before being injected into the UPLC for analysis. Each extraction of food products was performed in duplicate.
UPLC-MS/MS Method for Food
A method was developed to quantify the apo-lycopenals present in lycopene-containing foods. Separation was performed on a Waters Acquity UPLC (Waters Corp., Milford, MA). The column used was a 50 mm × 2.1 mm i.d., 1.7 µm particle size, BEH C18 (Waters Corp., Milford, MA). Solvent A = 80:20 MeOH/0.1% aqueous formic acid solution. Solvent B = 85:15 MeOH/tetrahydrofuran. Flow rate was 0.7 mL/min, column temperature = 40 °C. Gradient was as follows: 0% B to 90% B over 1 min, to 95% B over 0.7 min, to 100% B over 0.8 min which was held for 0.5 min, then quickly back to 0% B for 2 min. Quantitation of compounds was performed on a Quattro Ultima triple quadrupole mass spectrometer (Micromass UK Ltd., Manchester, UK) interfaced with the UPLC via an APcI source operated in negative ion mode. The mass spectrometer was operated in selected reaction monitoring (SRM) mode transmitting the radical ion form of the parent [M•−] in the first quadrupole, fragmenting it by collision induced dissociation (argon-filled chamber at 3×10−3 mBar) and selecting the corresponding [M-69]− daughter ion with the last quadrupole to deliver to the detector. Mass transitions were as follows: 284>215, 310>241, 350>281, 376>307, 416>347, 442>373, 536>467 for acycloretinal, apo-14′ -12′, -10′, -8′, -6′-lycopenal and lycopene, respectively. Instrumental pa rameters included: corona current = 30 µA, cone =35 V, desolvation temp. = 400 °C, desolvation gas flow = 700 L/h.
Plasma analysis
Seven human plasma samples from a previous dietary intervention study were analyzed for apo-lycopenals. In this study, healthy men and women consumed a diet supplemented with soy-fortified tomato juice for 8 weeks (delivering 21.9 mg of lycopene/day). Blood was sampled after 8 weeks of juice consumption. Samples were collected into 10 mL EDTA tubes, centrifuged immediately to remove red blood cells, and stored at −80 °C until extraction. Written informed consent was obtained from all study participants, and this study was approved by the Institutional Review Board of the Ohio State University, protocol # 2004H009.
Plasma (2 mL) was extracted using HEAT (hexane/ethanol/ acetone/toluene; 10:6:7:7, v/v/v/v) as the extraction solvent (25). Briefly, plasma was mixed with an equal volume of ethanol containing 0.1% butylated hydroxytoluene, 0.5 mL of a saturated NaCl solution, and 10 mL of HEAT. The mixture was vortexed and then centrifuged for 5 min at 300 × g. The upper non-polar layer was removed with a glass pipette and the remaining aqueous plasma mixture was extracted two more times. The non-polar layers were combined and dried under argon. The dried extract was stored at −80 °C until analysis by HPLC-MS/MS.
HPLC-MS/MS method for human plasma
The C30 HPLC method used to separate the lycopene oxidation mixture was also used to separate apo-lycopenals present in human plasma extracts with slight modifications (solvent A for plasma analysis was 80:20 MeOH/0.1% aqueous formic acid solution). Both the C30 and the C18 methods were tested with plasma extracts, and the C30 method provided twice the sensitivity of the C18 method. This superior sensitivity was likely due to matrix components which were diluted or resolved from analytes in the longer C30 method. Since levels of apo-lycopenals are low in plasma, the more sensitive C30 method was chosen for quantitation of these compounds in plasma.
The C30 method was run on a UPLC instrument, which is capable of operating under the same parameters as a traditional HPLC system. A Waters Acquity UPLC (autosampler, pump, and PDA detector) was interfaced with the Quattro Ultima mass spectrometer (operated using the same settings as for food analysis) to quantify apo-lycopenals in the plasma extracts.
Recovery Experiments
Recovery experiments help validate that analytes are not being lost during extraction. Since aldehydes (i.e. apo-lycopenals) have the ability to react with nucleophiles, we hypothesized that the aldehydes might covalently bind to protein in the food or blood plasma matrix and be underestimated. To determine whether such reactions were occurring during our extraction process, we performed the lipophilic extraction of both the plasma and the food in the presence of excess formaldehyde.
Data Analysis
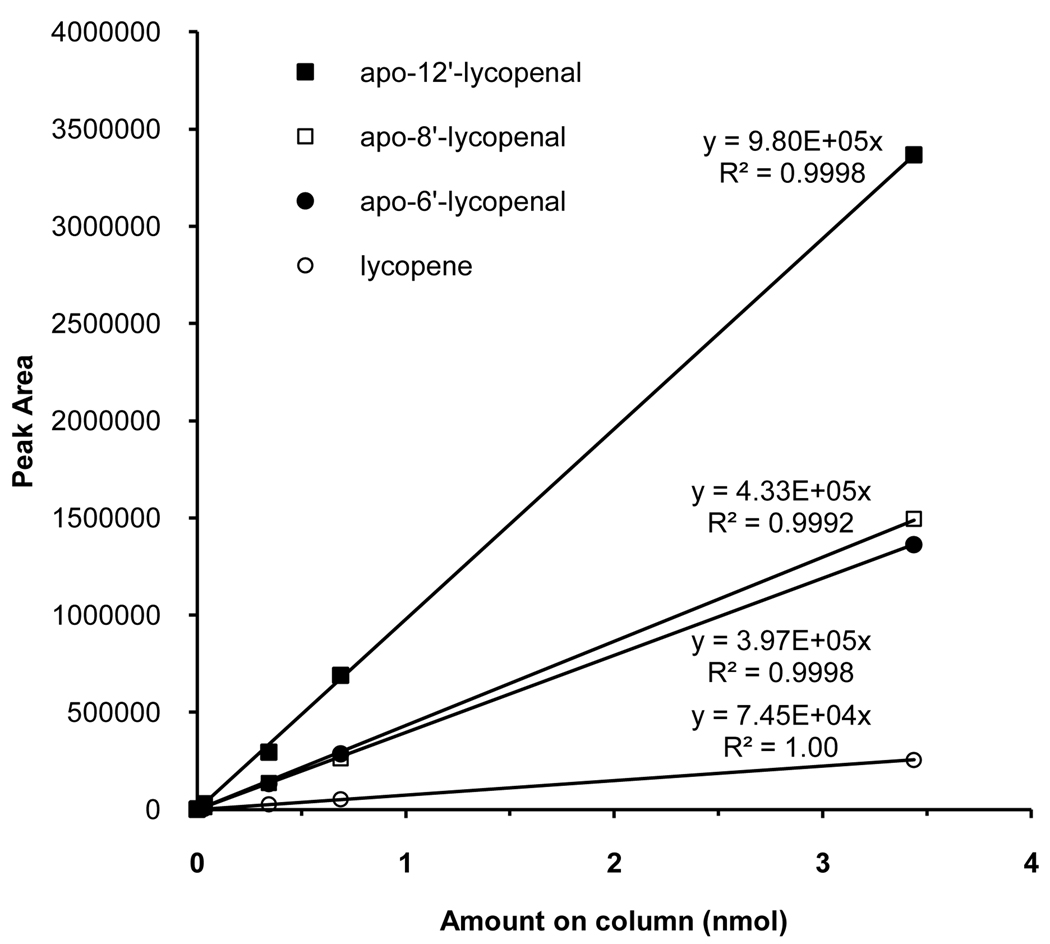
Quantification of compounds in plasma and food samples is based on external calibration curves of apo-6′-, 8′-, 12′-lycopenal from Carotenature, and all-trans-lycopene purified by crystallization. Peak areas of the [M-69]− daughter fragment (see UPLC-MS/MS Method for Food) were used to generate the calibration curves. Concentrations of lycopene and apo-lycopenals in the samples were calculated by comparing their peak areas to the respective calibration curve. In lieu of authentic apo-10′-lycopenal standard, apo-10′-lycopenal levels were estimated using the average response of apo-8′-lycopenal and apo-12′-lycopenal standards. In lieu of authentic apo-14′-lycopenal standard, levels of apo- 14′-lycopenal were estimated using the response of apo-12′-lycopenal standard.
Values for apo-lycopenals in raw fruits and vegetables are the average of duplicate extractions. Apo-lycopenal levels in processed products are the average from the measurement of three different brands of product extracted in duplicate.
RESULTS AND DISCUSSION
Synthesis of apo-lycopenals
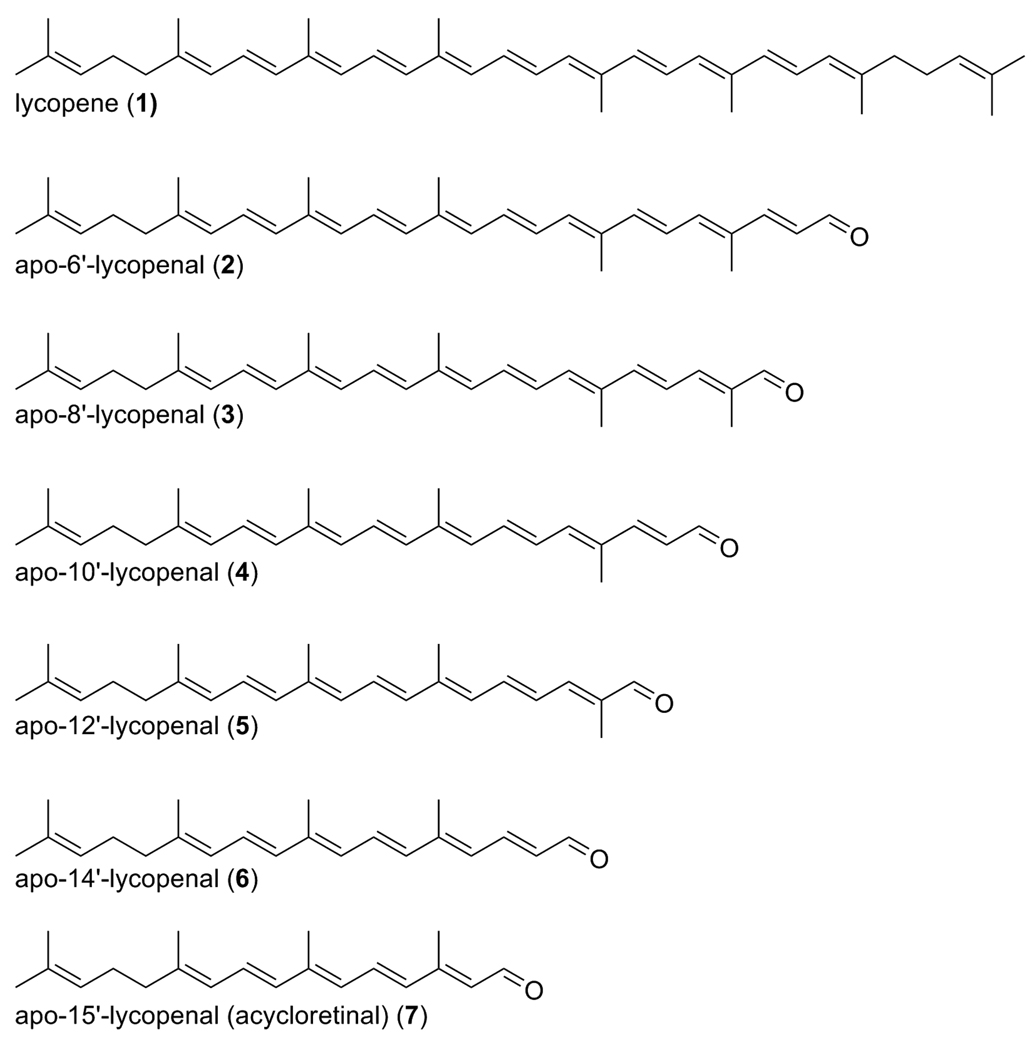
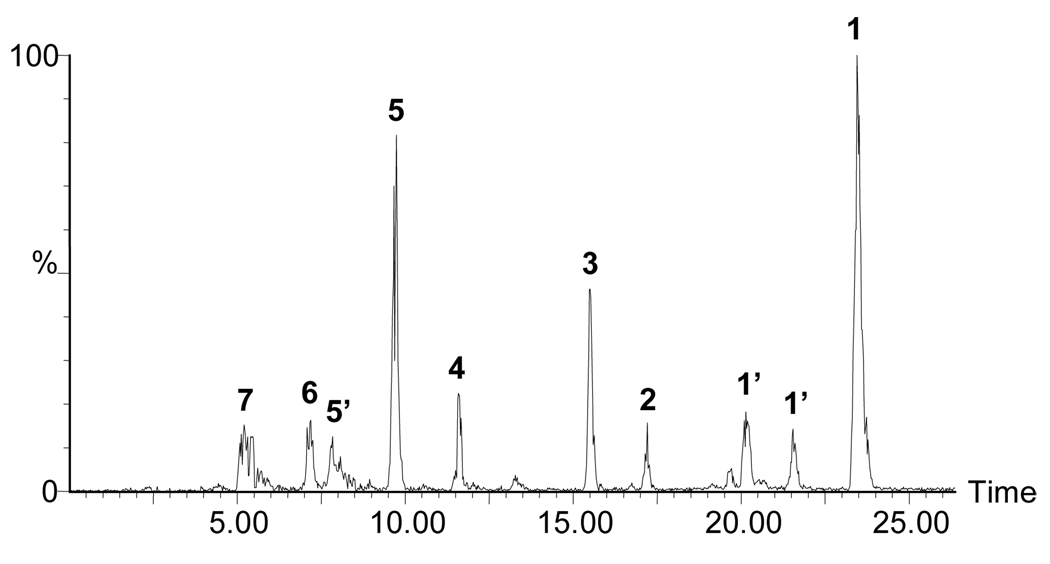
Figure 1 displays the structures of all-trans lycopene and the long chain apo-lycopenals observed after oxidation of lycopene with ozone. Oxidation occurs at each double bond in the chain, leading to the series of products detected. Figure 2 shows an HPLC-MS chromatogram of the separated mixture of apo-lycopenals and lycopene after lycopene ozonolysis. All apo-lycopenals in the series (apo-6′-, 8′-, 10′-, 12′-, 14′-, and 15′-lycopenal) display the characteristic isoprenyl loss upon CID in the mass spectrometer (M-69) (26). The m/z of the synthesized apo-lycopenals were within 2 ppm mass accuracy of the respective theoretical m/z values and elute in order of increasing molecular weight as expected. Identity of apo-6′-, 8′-, and 12′-lycopenal were confirmed by retention time (RT) and ultraviolet (UV) spectra coincident with commercial standards. MS/MS chromatograms for the m/z values consistent with apo-10′-,14′-, and 15′-lycopenal were observed in the oxidation mixture and tentatively identified based on anticipated RT (relative to the RT of the apo-6′-, 8′-, and 12′-lycopenal standards), UV spectra, and characteristic M-69 MS/MS transition.
Figure 1.
Series of apo-lycopenals produced after ozone treatment of lycopene.
Figure 2.
An HPLC-MS chromatogram of the products resulting from the oxidation of lycopene with ozone acquired on a quadropole-time-of-flight mass spectrometer (QTof Premier, Micromass, Beverley, MA). The chromatogram displays the sum of ion intensities for the radical anions of lycopene and apo-lycopenals in APcI negative (note: HPLC gradient slightly modified from that used for blood plasma analysis – see methods). 1 = all-trans-lycopene (536.44), 1’ = cis-lycopene isomers (536.44), 2 = apo-6′-lycopenal (442.32), 3 = apo-8′-lycopenal (416.31), 4 = apo-10′-lycopenal (376.28), 5 = apo-12′-lycopenal (350.26), 6 = apo-14′-lycopenal (310.23), and 7 = apo-15′-lycopenal (284.21).
Since many of the foods and blood plasma samples that we analyze contain a significant amount of β-carotene in addition to lycopene, it is necessary to develop HPLC methods that separate apo-lycopenals from potential apo-carotenals which are isobaric, e.g. apo-8′-lycopenal and β -apo-8′-carotenal. In addition, it is desirable to distinguish these species according to their MS/MS behavior. β -carotene was oxidized with ozone and examined using the HPLC-MS/MS method detailed above. The β -apo-carotenals observed were found to have different retention times than their corresponding apo-lycopenals (data not shown). Furthermore, HPLC-MS/MS analysis revealed that the β-apo-carotenals do not produce the M-69 fragment due to cyclization of the terminal isoprenyl group into an ionone ring. Commercial standards of β-apo-8′-carotenal and β -apo-12′-carotenal were further used to verify the RT and UV spectra of the compounds produced in the β-carotene oxidation mixture.
Apo-lycopenals in food extracts
Initial tests showed that the concentrations of apo-lycopenals in food extracts were far below the PDA detection limit necessitating the selectivity and sensitivity of mass spectrometry. Consequently, apo-lycopenals were analyzed using HPLC-MS/MS and identified by coincident behavior with the apo-lycopenal standards according to retention time, accurate mass, and MS/MS fragmentation patterns. Lycopene, apo-6′-, 8′-, 10′-, 12′-, and 14′-lycopenal were observed in all food tested. Interestingly, apo-15′-lycopenal was not detected in any of the food products although it was generated in the lycopene oxidation mixture. Quantities of food apo-lycopenals are listed in Table 1.
Table 1.
Apo-lycopenals in Commonly Consumed Lycopene Containing Foods
| average µg/100g wet weight ± standard deviation | |||||||
|---|---|---|---|---|---|---|---|
| Food | lycopene | apo-6′- lycopenal |
apo-8′- lycopenal |
apo-10′- lycopenala |
apo-12′- lycopenal |
apo-14′- lycopenalb |
|
| ‘Roma’ Tomato | 3,500 | 1.1 | 1.8 | 1.1 | 2.2 | 0.28 | |
| Grape Tomato | 6,200 | 1.6 | 2.2 | 1.2 | 2.3 | 0.38 | |
| Red Vine Ripened Tomato |
3,800 | 3.5 | 4.3 | 2.0 | 2.9 | 0.42 | |
| Ruby Red Grapefruit |
190 | 0.023 | 0.027 | 0.022 | 0.047 | nd | |
| Watermelon | 3,200 | 0.77 | 0.86 | 0.45 | 0.80 | 0.21 | |
| Catsupc | 11,000 ± 1,600 | 4.9 ± 1.3 | 9.3 ± 1.6 | 1.2 ± 0.3 | 5.5 ± 0.2 | 0.27 ± 0.02 | |
| Spaghetti Saucec | 11,000 ± 2,700 | 6.3 ± 1.5 | 10 ± 2 | 1.2 ± 0.1 | 5.3 ± 0.8 | 0.31 ± 0.05 | |
| Pizza Saucec | 15,000 ± 4,100 | 10 ± 4 | 16 ± 7 | 1.7 ± 0.6 | 7.1 ± 2.2 | 0.43 ± 0.11 | |
| Tomato Soupc | 9,600 ± 2,400 | 6.9 ± 3.7 | 11 ± 4 | 1.7 ± 0.8 | 6.1 ± 2.3 | 0.54 ± 0.27 | |
| Tomato Pastec | 34,000 ± 4,100 | 19 ± 5 | 34 ± 3 | 3.7 ± 1.2 | 16 ± 4 | 0.69 ± 0.13 | |
| Tomato Juicec | 8,400 ± 1,800 | 1.8 ± 0.5 | 5.0 ± 1.3 | 0.56 ± 0.13 | 2.8 ± 0.3 | 0.11 ± 0.02 | |
nd = not detected
Level was estimated by averaging apo-8′-lycopenal and apo-12′-lycopenal slopes.
Level was estimated by using apo-12′-lycopenal equivalents.
Standard deviation reflects variation between three different commercial manufacturers of the same food product
The mass spectrometric method was very sensitive to, and selective for, apo-lycopenals. However, there were mass spectrometer performance limitations in terms of day-to-day and run-to-run variability. To mitigate this instability, standards were run daily. In addition, we investigated a number of possible internal standards (etretinate, astaxanthin, 13C40 β-carotene) yet none of these candidates improved run-to-run relative standard deviation (RSD). The best solution is to use stable isotopes of the apo-lycopenals as int ernal standards, and efforts are being made to synthesize these for future studies.
Lycopene was present at levels roughly ~1000 times higher than each apo-lycopenal observed. Apo-8′-lycopenal was the predominant apo-lycopenal found in all foods, followed by apo-6′-lycopenal and apo-12′-lycopenal. In contrast, apo-10′- and 14′-lycopenal were present in smaller quantities. The levels of apo-lyocopenals varied across three different commercial manufacturers of a given product, as reflected in higher standard deviations in certain products.
Apo-lycopenals in human plasma
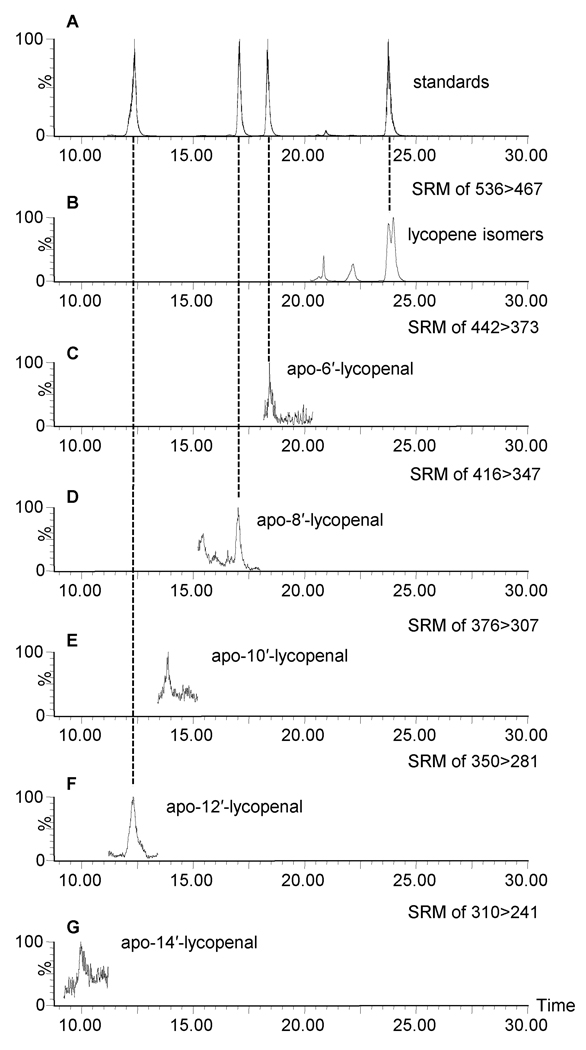
Apo-6′-,8′-,10-′,12′-, and 14′-lycopenal were observed in the plasma of all seven individuals tested who consumed tomato juice daily for eight weeks. Figure 4 shows a chromatogram of lycopene and apo-lycopenals observed in one subject, representative of all plasma samples analyzed. Table 2 provides the average concentration and the standard deviation of apo-lycopenals observed over seven individuals of apo-lycopenals observed over seven individuals. Apo-15′-lycopenal was not detected in the plasma of any of the seven subjects. As shown in Table 2, apo-8′- and 12′-lycopenal were the predominant apo-lycopenals observed in blood. Relative to lycopene, apo-lycopenals were present at levels roughly 1,000 times lower, similar to their relative abundance in food.
Figure 4.
HPLC-MS/MS analysis of lycopene and apo-lycopenals in the blood plasma extract of one representative individual. (A) MS chromatogram of the collection of SRM channels for all-trans lycopene (536>467), apo-6′- (442>373), -8′- (416>347), and -12′-lycopenal (350>281) standards and (B-G) lycopene and apo-lycopenals in blood plasma. Parent radical anion m/z values are shown in the upper right of each trace, with the respective transition to M-69 daughter ions that were monitored.
Table 2.
Apo-lycopenals in Blood Plasma of Seven Subjects Consuming a High Tomato Juice Diet for 8 Weeks.
| average nmol/L ± standard deviation | ||||||
|---|---|---|---|---|---|---|
| lycopene | apo-6′- lycopenal |
apo-8′- lycopenal |
apo-10′- lycopenala |
apo-12′- lycopenal |
apo-14′- lycopenalb |
|
| blood plasma | 1,089 ± 380 | 0.12 ± 0.07 | 0.63 ± 0.32 | 0.28 ± 0.10 | 0.73 ± 0.38 | 0.12 ± 0.04 |
Level was estimated by averaging apo-8′-lycopenal and apo-12′-lycopenal slopes.
Level was estimated by using apo-12′-lycopenal equivalents.
Lycopene preservation during extraction
Lycopene is extremely sensitive to degradation by heat, light, and oxygen; therefore, the presence of apo-lycopenals in such small quantities stimulated our concern that they may form as degradation products during sample processing and analysis. During extraction, we maintained standard precautions to assure chemical stability, including minimizing handling time, extracting under red light, drying samples under nitrogen, and storing immediately at −80 °C until ana lysis. We also performed several experiments to determine if specific technical procedures impacted apo-lycopenal levels. First, we extracted raw tomato with 2% BHT in the extraction solvent as BHT is commonly used as an antioxidant in the extraction of compounds sensitive to oxidation. The presence of BHT did not change the amount of apo-lycopenals observed in the extract. We also performed the extraction of raw tomato with EDTA at various concentrations to determine if the apo-lycopenal formation was catalyzed by free metals or metal-dependent enzymes (e.g. oxidases) during extraction. There was no change in the amount of apo-lyocopenals observed between the no EDTA condition and as a function of EDTA concentration. In fact, neither BHT nor EDTA appear to protect against such generation. In summary, apo-lycopenals were likely not generated artifactually during extraction through free radical or metal-catalyzed reactions. However, we were able to increase the level of apo-lycopenals if we kept a closed vial containing lycopene dissolved in tetrahydrofuran or CH2Cl2 at room temperature overnight.
Another possible route to the formation of apo-lycopenals is by metal independent enzymes native to the native food sample matrices which may have the capacity to generate apo-lycopenals during extraction. To test whether samples had this capacity, we added exogenous lycopene (prepared in-house) and performed the extraction. We observed slight changes in apo-lycopenal levels. We also performed the same experiment using blood plasma samples by spiking with a 200-fold excess of lycopene over unspiked plasma samples. We observed no change in the amount of apo-lycopenals as compared to unspiked plasma samples.
Recovery Experiments
No significant improvement in recovery was observed (data not shown). Thus, it appears we are not losing apo-lycopenals due to aldehyde reactivity with the sample matrices during extraction.
Lycopene metabolism in mammals is poorly understood, and the role of metabolites as biomarkers of metabolic degredation pathways or potentially bioactive metabolites remains speculative. Researchers have suggested that lycopene may be metabolized into aldehyde derivatives, and previous reports have identified a few apo-lycopenal products in tomatoes (13), tomato paste (12), and in tissues of rodents fed lycopene (10, 24). We now document that several apo-lycopenals are present in a variety of raw and processed foods containing lycopene. In addition, we report for the first time the presence of a series of apo-lycopenals in human plasma.
The hypothesis that oxidative metabolites of lycopene have biological activity is supported by a few in vitro studies. The products of lycopene oxidized with hydrogen peroxide were shown to increase gap junction communication in rat liver epithelial cells (27). In a separate study, the lycopene derivative 2,6-cyclolycopene-1,5-diol was shown to increase connexin 43 levels in vitro in murine and human cells to a greater extent than the parent lycopene molecule (28). Apo-10′-lycopenoic acid was also shown to inhibit growth of human bronchial cells in vitro and to reduce the number of lung tumors in mice injected with the cancer inducing chemical 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (29). As pure compounds become available, additional studies of bioactivity in experimental models will be a high priority.
The apo-lycopenals we observed in raw and processed fruits could be the result of enzyme-catalyzed metabolism of lycopene. In fact, nine carotenoid cleavage dioxygenase enzymes (CCD) have been identified in the genome of Arabidopsis (30). Of these nine, five enzymes are reportedly involved in the synthesis of abscisic acid, an important hormone regulating multiple plant functions (31). The other four CCD enzymes are being more fully characterized. CCD1 from maize has been shown to cleave lycopene at the 5′-6′ position and 9′- 10′ position (32). While only the short, volatile products of these cleavages were determined by GC-MS (pseudo-ionone and 6-methyl-5-heptene-2-one), the complementary long-chain products of these enzymatic cleavages were likely apo-6′-lycopenal and apo-10′-lycopenal, respectively. CCD7 appears to cleave the 9′-10′ double bond of lycopene, but the resulting product has only tentatively been identified with UV spectroscopic data and retention time (30). It is not clear if CCD8 cleaves lycopene, and there is no literature discussing the ability of CCD4 to act on lycopene. It is also possible that the apo-lycopenals observed in these foods might be formed by enzymes involved in the abscisic acid pathway. In contrast to specific biosynthesis, these aldehydes could be the result of a non-specific lipoxygenase cleavage (33, 34), or produced from photo-oxidation in the fruit (35).
The presence of apo-lycopenals in plasma could be due to enzymatic cleavage of lycopene. It has been demonstrated that BCO1 cleaves β-carotene at the 15-15′ double bond to produce two molecules of retinal in vertebrates (14). In addition, a second carotenoid oxygenase (BCO2), has been shown to eccentrically cleave β-carotene to produce β-apo-10′-carotenal (15). In vitro, BCO2 from ferret has also been shown to cleave both β-carotene and lycopene at the 9′-10′ double bond (18). However, a single cleavage site would not explain the array of aldehydes we observed in plasma.
The feeding of lycopene (as Lycovit 10%) to ferrets resulted in the detection of apo-10′-lycopenol in lung tissue (18). This alcohol was presumed to be a metabolite of the aldehyde (apo-10′-lycopenal) formed enzymatically in vivo. In addition, the liver of rats consuming a lycopene containing diet for 30 days produced apo-8′-lycopenal and putative apo-12′-lycopenal in this tissue (19, 36). These products were also hypothesized to be the result of enzymatic cleavage in the animal.
In summary, we have observed a full series of apo-lycopenals in foods, processed food products, and analytical grade lycopene standard. In addition, we have documented the presence of multiple apo-lycopenals in the plasma of humans consuming tomato juice. This evidence suggests that these products may in fact be absorbed from the food and not solely a product of metabolism in vivo. The presence of apo-lycopenals in the plasma, either from the diet or as metabolic products, supports a hypothesis that these compounds may have bioactivity and potentially mediate some of the health promoting beneficial effects proposed for tomato products. In addition, the development of analytical methods for the measurement of apo-lycopenals in mammalian systems provides a tool for the investigation of lycopene metabolism. The continued improvement of analytical approaches provides investigators with new approaches that will help to determine the importance of apo-lycopenals from the diet or as a product of enzymatic cleavage in vivo.
Figure 3.
Standard calibration curves for the quantification of lycopene (○), apo-6′-lycopenal (●), apo-8′-lycopenal (□), and apo-12′-lycopenal (■)
Acknowledgments
Supported by: National Institutes of Health, R01-HL049879 to EHH; National Institutes of Health, National Cancer Institute, RO1-CA112632 to SKC; United States Department of Agriculture IFAFS Grant No. 2001-52102-11333 to SKC and SJS, and Center for Advanced Functional Foods Research and Entrepreneurship (CAFFRE)/OSU Food Innovation Center.
ABBREVIATIONS
- BCO1
beta-carotene oxygenase 1
- BCO2
beta-carotene oxygenase 2
- CID
collisionally induced dissociation
References
- 1.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J. Natl. Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 3.Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, Albanes D, Virtamo J, Taylor PR. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol. Biomarkers Prev. 2005;14:2087–2092. doi: 10.1158/1055-9965.EPI-05-0038. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003;133:2336–2341. doi: 10.1093/jn/133.7.2336. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Shikany JM, Liu S, Shagufta Y, Rohan TE. Selected antioxidants and risk of hormone receptor-defined invasive breast cancers among postmenopausal women in the women's health initiative observational study. Am. J. Clin. Nutr. 2008;87:1009–1018. doi: 10.1093/ajcn/87.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heber D, Lu QY. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002;227:920–923. doi: 10.1177/153537020222701013. [DOI] [PubMed] [Google Scholar]
- 7.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 8.Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch. Biochem. Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 9.Carail M, Caris-Veyrat C. Carotenoid oxidation products: from villain to saviour? Pure Appl. Chem. 2006;78:1493–1503. [Google Scholar]
- 10.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: are lycopene metabolites bioactive? Arch. Biochem. Biophys. 2007;458:136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Khachik F, Beecher GR, Goli MB, Lusby WR, Smith JCJ. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal. Chem. 1992;64:2111–2122. doi: 10.1021/ac00042a016. [DOI] [PubMed] [Google Scholar]
- 12.Khachik F, Steck A, Niggli UA, Pfander H. Partial synthesis and structural elucidation of the oxidative metabolites of lycopene identified in tomato paste, tomato juice, and human serum. J. Agric. Food Chem. 1998;46:4874–4884. [Google Scholar]
- 13.Khachik F, Goli MB, Beecher GR, Holden J, Lusby WR, Tenorio MD, Barrera MR. Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J. Agric. Food Chem. 1992;40:390–398. [Google Scholar]
- 14.von Lintig J, Vogt K. Filling the gap in vitamin A research: molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 16.Kim S-J, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36:191–200. doi: 10.1007/s11745-001-0706-8. [DOI] [PubMed] [Google Scholar]
- 17.Caris-Veyrat C, Schmid A, Carail M, Bohm V. Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J. Agric. Food Chem. 2003;51:7318–7325. doi: 10.1021/jf034735+. [DOI] [PubMed] [Google Scholar]
- 18.Hu K-Q, Liu C, Ernst H, Krinsky NI, Russell RM, Wang X-D. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 2006;281:19327–19432. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8'-lycopenal and apo-12'-lycopenal are metabolic products of lycopene in rat liver. J. Nutr. 2006;136:1552–1557. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 20.Winterstein A, Studer A, Ruegg R. Neuere ergebnisse der carotinoidforschung. Chem. Ber. 1960;93:2951–2165. [Google Scholar]
- 21.Ben-Aziz A, Britton G, Goodwin TW. Carotene epoxides of Lycopersicon esculentum. Phytochemistry. 1973;12:2759–2764. [Google Scholar]
- 22.Hakala SH, Heinonen IM. Chromatographic purification of natural lycopene. J. Agric. Food Chem. 1994;42:1314–1316. [Google Scholar]
- 23.Schwartz C, Raible J, Mott K, Dussault PH. 'Reductive ozonolysis' via a new fragmentation of carbonyl oxides. Tetrahedron. 2006;62:10747–10752. doi: 10.1021/ol061001k. [DOI] [PubMed] [Google Scholar]
- 24.Ferruzzi MG, Sander LC, Rock CL, Schwartz SJ. Carotenoid determination in biological microsamples using liquid chromatography with a coulometric electrochemical array detector. Anal. Biochem. 1998;256:74–81. doi: 10.1006/abio.1997.2484. [DOI] [PubMed] [Google Scholar]
- 25.Craft NE, Wise SA, Soares JH. Individual carotenoid content of SRM 1548 total diet and influence of storage temperature, lyophilization, and irradiation on dietary carotenoids. J. Agric. Food Chem. 1993;41:208–213. [Google Scholar]
- 26.van Breemen RB, Schmitz HH, Schwartz SJ. Fast atom bombardment tandem mass spectrometry of carotenoids. J. Agric. Food Chem. 1995;43:384–389. [Google Scholar]
- 27.Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food Chem. Toxicol. 2003;41:1399–1407. doi: 10.1016/s0278-6915(03)00148-0. [DOI] [PubMed] [Google Scholar]
- 28.Bertram JS, King T, Fukishima L, Khachik F. Enhanced activity of an oxidation product of lycopene found in tomato products and human serum relevant to cancer prevention. In: Sen CK, Sies H, Baeuerle PA, editors. Antioxidant and Redox Regulation of Genes. edition no. 1. London, England: Academic Press; 2000. pp. 409–424. [Google Scholar]
- 29.Lian F, Smith DE, Ernst H, Russell RM, Wang X-D. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz SH, Qin X, Loewen MC. The biochemical characterization of two carotenoid cleavage enzymes from arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J. Biol. Chem. 2004;279:46940–46945. doi: 10.1074/jbc.M409004200. [DOI] [PubMed] [Google Scholar]
- 31.Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C. An update on abscisic acid signaling in plants and more. Mol. Plant. 2008;1 doi: 10.1093/mp/ssm022. 198-217-217. [DOI] [PubMed] [Google Scholar]
- 32.Vogel JT, Tan B, Mccarty DR, Klee HJ. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 2008;283:11364–11373. doi: 10.1074/jbc.M710106200. [DOI] [PubMed] [Google Scholar]
- 33.dos Anjos Ferreira AL, Yeum K-J, Russell RM, Krinsky NI, Tang G. Enzymatic and oxidative metabolites of lycopene. J. Nutr. Biochem. 2004;15:493–502. doi: 10.1016/j.jnutbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Robinson DS, Hughes RK, Casey R, Hardy D, West SI. Co-oxidation of beta-carotene catalyzed by soybean and recombinant pea lipoxygenases. J. Agric. Food Chem. 1999;47:4899–4906. doi: 10.1021/jf9901690. [DOI] [PubMed] [Google Scholar]
- 35.Ukai N, Lu Y, Etoh H, Ina K, Oshima S, Ojima FSH, Ishiguro Y. Photosensitized oxygenation of lycopene. Biosci. Biotech. Biochem. 1994;58:1718–1719. [Google Scholar]
- 36.Zaripheh S, Boileau TWM, Lila MA, Erdman JW., Jr (14C)-lycopene and (14C)-labeled polar products are differentially distributed in tissues of F344 rats prefed lycopene. J. Nutr. 2003;133:4189–4195. doi: 10.1093/jn/133.12.4189. [DOI] [PubMed] [Google Scholar]