SUMMARY
Endothelium lining the cardiovascular system is highly sensitive to hemodynamic shear stresses that act at the vessel luminal surface in the direction of blood flow. Physiological variations of shear stress regulate acute changes in vascular diameter and when sustained induce slow, adaptive, structural-wall remodeling. Both processes are endothelium-dependent and are systemically and regionally compromised by hyperlipidemia, hypertension, diabetes and inflammatory disorders. Shear stress spans a range of spatiotemporal scales and contributes to regional and focal heterogeneity of endothelial gene expression, which is important in vascular pathology. Regions of flow disturbances near arterial branches, bifurcations and curvatures result in complex spatiotemporal shear stresses and their characteristics can predict atherosclerosis susceptibility. Changes in local artery geometry during atherogenesis further modify shear stress characteristics at the endothelium. Intravascular devices can also influence flow-mediated endothelial responses. Endothelial flow-induced responses include a cell-signaling repertoire, collectively known as mechanotransduction, that ranges from instantaneous ion fluxes and biochemical pathways to gene and protein expression. A spatially decentralized mechanism of endothelial mechanotransduction is dominant, in which deformation at the cell surface induced by shear stress is transmitted as cytoskeletal tension changes to sites that are mechanically coupled to the cytoskeleton. A single shear stress mechanotransducer is unlikely to exist; rather, mechanotransduction occurs at multiple subcellular locations.
Keywords: atherosclerosis, endothelial mechanotransduction, hemodynamics
INTRODUCTION
The interaction between hemodynamics and the endothelium is an important determinant of cardiovascular function in mammalian evolution, development, survival and morbidity. Shear stress is the force per unit area created when a tangential force (blood flow) acts on a surface (endothelium)—wherever flow occurs, shear stress exists. In studies of this dynamic environment, physiology and pathology converge with fluid dynamics, biomechanics, and cell and molecular biology. Shear-induced mechanotransduction (the conversion of mechanical stresses to biochemical responses) is particularly important in arteries, in which blood flow regulates vascular tone and structure. This regulation occurs via mechanically stimulated release of potent, shear-responsive, endothelial-derived factors such as nitrovasodilators, prostaglandins, lipoxygenases, hyperpolarizing factors, growth factors and other related molecules.1–5 In contrast to vessel changes seen during acute vasoregulation, sustained changes of local hemodynamics promote adaptive structural remodeling of the artery wall through endothelium-dependent regulation of gene and protein expression.6,7
The endothelium is critical to mammalian survival; this layer of cells maintains anticoagulant properties, and enables physiological control of vasoregulation and modulation of vascular permeability. It also mediates both the pathological consequences of and protective responses to acute and chronic inflammation, wound healing and major cardiovascular disorders such as atherogenesis.8 Blood flow is an important local regulator of these functions and exerts its effects through endothelial mechanotransduction. Flow also seems to control key aspects of embryonic cardiovascular development,9 particularly the induction of late-onset genes.10 Interactions between shear stress and the endothelium clearly regulate important developmental, homeostatic and adaptive mechanisms in arteries; however, they are also an important influence in cardiovascular pathology, particularly site-specific susceptibility to and progression of atherosclerosis. The aims of this Review are to examine the influence of hemodynamic shear stress on the regulation of endothelial function, consider the localized, spatial characteristics of flow that are of pathological importance for vascular dysfunction, and outline the intracellular spatial organization of endothelial mechanotransduction.
CLINICAL IMPLICATIONS OF SHEAR STRESS
In the arterial circulation, shear stress has a critical role in determining where most vascular pathology originates.11 Furthermore, shear stress is implicated in the development of endothelial phenotypic changes that are associated with increased atherosclerosis susceptibility,12 initiation and development,13 and phenotypic changes in which the metabolic balance is disrupted and becomes protective, pathological, or both.12 The most commonly encountered site-specific clinical states that illustrate shear stress mechanisms in the endothelium are associated with site-specific susceptibility to atherosclerosis and the consequences of interventional therapy including angioplasty, bypass grafts or the deployment of devices such as stents. The local geometry—modified by the presence of a plaque or device—influences the magnitude, directionality and spatiotemporal distribution of shear stress (Figures 1–3). Similar principles of fluid dynamics apply to the geometry of normal vessels, in which endothelial mechanisms mediated by shear stress are probably responsible for an increased susceptibility to pathogenesis at locations of atypical geometry.11,12,14
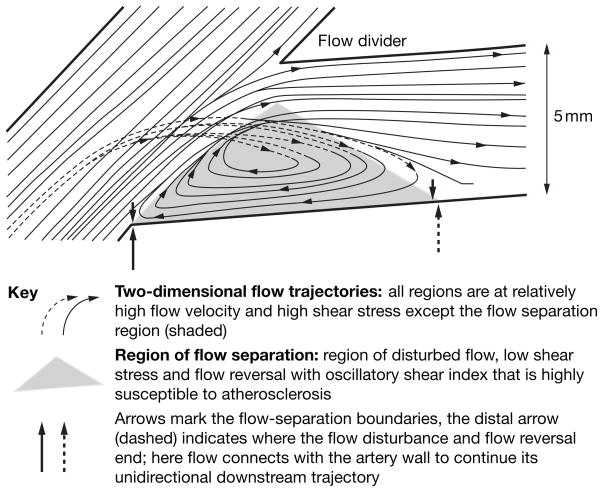
Figure 1.
Flow separations at an arterial branch can predispose or contribute to pathogenesis. Flow separation and the flow disturbance that occurs in the separated region are proatherogenic—complex, transient vortices form and dissipate (but not completely) throughout each heartbeat. The primary characteristics of disturbed flow are low average shear stress, constantly changing gradients of shear stress, oscillatory flow (and shear stress) because of flow reversal, and multifrequency, multidirectional, secondary flows. High shear stress protects against atherosclerosis as long as it remains below levels that detach the endothelium (estimated >40 N/m2 ; rare). In pulsatile flow within arteries, the separation region expands and contracts with the cardiac cycle. Excellent illustrations of computational imaging of human arterial hemodynamics19 can be viewed online.80
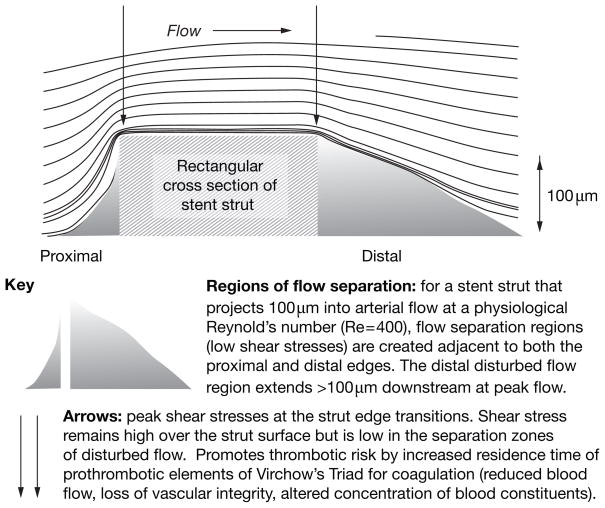
Figure 3.
Flow separations around a stent strut that predispose to or contribute to pathogenesis.
For Poiseuille flow (see Box 1), shear stress (τ) is directly proportional to the velocity of blood flow, and inversely proportional to the cube of the arterial radius (R), where Q is flow rate and μ is fluid viscosity. See Equation 1:
| (1) |
Box 1. Definitions of key flow-related terms.
Poiseuille flow is the name given to the mathematical model of steady, laminar, fully developed flow through a straight, circular tube of constant cross-sectional area. Poiseuille flow rarely exists in large arteries because they are not straight, contain branches that perturb steady flow, and the cross-sectional area varies. Nevertheless, the Navier-stokes equations used to solve for Poiseuille flow are of great value in the estimation of hemodynamic values.
Newtonian fluids are fluids that exhibit a linear relationship between the shearing stress and rate of deformation (shearing strain). Although blood is a non-Newtonian fluid, its flow characteristics observed by various imaging techniques in large arteries approximate Newtonian fluid behavior.
Reynolds number (Re) is a dimensionless ratio between inertial and viscous forces. At low Re, viscous forces predominate while at high Re, inertial forces are most important, which gives rise to turbulent (chaotic) flow.
Oscillatory shear index provides a measure of the shear stress experienced at a point in space by taking into account shear stresses that act in directions other than that of the bulk flow.
Thus, small changes in R greatly influence τ, and vice versa.
Shear stress and systemic endothelial changes
High shear stress is generally beneficial as it promotes adaptive dilatation or structural remodeling of the artery wall through endothelium-mediated mechanisms.15 However, dysfunctional endothelium is an early manifestation of cardiovascular diseases such as hypercholesterolemia, diabetes and hypertension, and systemic inflammatory disorders. In this context, vascular dysfunction is usually defined as impaired, flow-mediated dilatation throughout an artery bed (in contrast to impairment at a discrete lesion site; see below). The dominant mechanism that defines widespread endothelial dysfunction is impaired expression of constitutive endothelial nitric oxide synthase (eNOS).2,3 This enzyme is essential for the synthesis and release of the potent vasodilator, antioxidant and anti-inflammatory mediator, nitric oxide. Shear stress induces eNOS transcription and translation and thus results in greater availability of nitric oxide. Human and animal model studies have demonstrated that regular, moderate exercise can reverse systemic endothelial dysfunction via changes in cardiac output16 and possibly altered pulsatility.17 This beneficial change is important and independent of improvement in all known risk factors.16
Shear stress and site-specific endothelial changes
Anatomical
Of high clinical importance are ‘site-specific’ endothelial functional phenotypes associated with particular characteristics of flow and shear stress that develop at curves (e.g. the aortic arch), branches and bifurcations in arteries.18–21 As the schematic Figure 1 shows, the flow departs from pulsatile, unidirectional shear stress to create flow-separation zones that include flow reversal, oscillatory shear stress and sometimes turbulence (chaotic flow). Such regions are susceptible to the focal development of atherosclerosis, whereas adjacent undisturbed flow regions are not.
Lesion-related
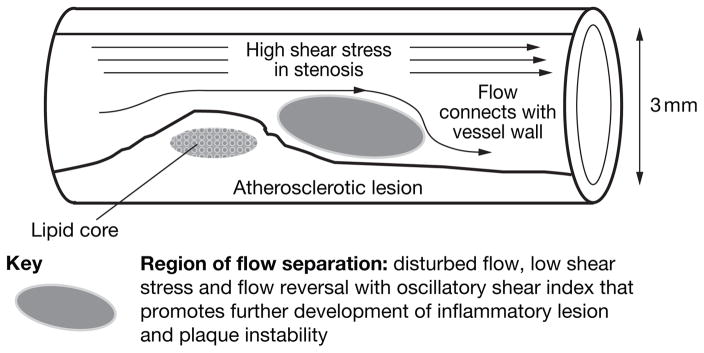
A developing atherosclerotic lesion can itself alter the local shear stress pattern on the endothelium. When a substantial stenosis is present, an increased velocity of flow through the narrowed luminal space can create flow separation (disturbed flow) in the region immediately downstream, analogous to a jetting effect across the stenosis (Figure 2). The disturbed flow created by a lesion has some similar characteristics to those seen in prelesional sites susceptible to shear-induced changes—albeit at a reduced scale. Consequently, lesion-induced disturbed flow may contribute to the growth of the lesion over time. In vitro and in vivo studies of endothelial cells have demonstrated that such an environment promotes proinflammatory gene and protein expression that is conducive to increased atherosclerosis-susceptibility,20,21 plaque growth and instability,22 and increased risk of thrombosis.23
Figure 2.
Flow separations at a stenosis that predispose to or contribute to pathogenesis.
Postintervention
Deployment of a stent after angioplasty provides immediate relief of obstructed flow. However, stent strut geometry itself can create small adverse flow disturbances that inhibit re-endothelialization and promote conditions that favor thrombus formation (Figure 3).24 This finding builds on those of preliminary studies that used flow over small steps to model flow separation.25 The endothelium between stent struts is, in turn, influenced by altered hemodynamics,26 which can also influence the transport of therapeutic molecules eluted from drug-coated stents.27 The design of second-generation and third-generation stents with reduced strut heights is beginning to address such stent–artery mechanical issues and has shown some initial success in improved stent performance.28
Bypass of occluded coronary arteries with small arteries obtained from other sites is largely successful because their previous location has preconditioned these vessels to arterial hemodynamics. However, vein grafts transferred to the high-pressure coronary artery circulation frequently develop stenoses, particularly at the artery–vein attachment sites. Here, complex vascular geometry can contribute to flow separation and could be responsible for endothelial dysfunction.29
Of course, one must acknowledge that blood is a non-Newtonian fluid, that Reynolds numbers are high, and that short distances between branches inhibit fully developed Poiseuille flow (see Box 1 for definitions of these terms). Despite these caveats, similar arterial flow perturbations have been recorded across several scales, which all have common features of importance in endothelial pathobiology.
SHEAR STRESS AND ATHEROSCLEROSIS-SUSCEPTIBLE ENDOTHELIAL PHENOTYPES
Transient, unstable flow separation that creates flow disturbance regions that contain oscillating, transient vortices is associated with a predisposition to atherosclerosis at branches, bifurcations and curvatures in the arterial circulation. Here, average flow velocities (and, therefore, shear stresses) are considerably lower than in adjacent undisturbed regions, and are accompanied by steep temporal and spatial gradients of shear stress and multidirectional force vectors. Occasional turbulence (chaotic flow) can add further hemodynamic complexity. Gene-expression patterns of endothelial cells at such locations in normal pigs12 and transgenic mice30 are considerably different to those seen in cells from adjacent, undisturbed, laminar-flow regions. Lesions develop when additional risk factors are present.11,18,31–34
Geometry and flow characteristics in vivo
MRI and ultrasonographic studies of the aortic arch in humans, mice and pigs have identified a complex series of flow-reversal events in the inner curvature of the arch.35 In decelerating systole, the forward motion of blood away from the heart reverses on the inside of the curve to create a separated, oscillatory, flow pattern. These locations are consequently characterized by an absence of preferential endothelial alignment, whereas in regions of unidirectional laminar flow, endothelial cells align in the direction of flow.12,33,34 Beyond the arch in the descending thoracic aorta, the flow reconnects with the wall and becomes unidirectional and less complex—signified by realignment of the endothelium.
Classic studies in pigs have mapped lesion distribution to arterial geometry.36 In 2007, the pig coronary circulation was modeled in fine detail. From the geometry obtained from CT scans and experimentally measured boundary conditions, Huo et al. used finite element modeling to determine a three-dimensional hemodynamic analysis of the pig left anterior descending coronary artery (LAD).37 They reported that low time-averaged wall shear stress and high oscillatory shear index (Box 1), both relative to adjacent sites, coincided with disturbed flows opposite the flow divider and lateral to the junction orifice. Furthermore, they reported that these differences were enhanced when flow rates at the LAD inlet increased. Atherosclerosis-susceptible locations mapped to regions of low shear stress and high oscillatory shear index. Of note, pulsatile unidirectional flow was restored 2.2 cm into the LAD;37 similar spatial relationships have been estimated for human left common, right and circumflex coronary arteries.38 The regions of high oscillatory shear index described above in proximal coronary arteries are predisposed to atherosclerotic disease.
Endothelial phenotypes in vivo at sites of flow disturbance
The coordinated regulation of endothelial gene expression in response to local shear stresses has been proposed to determine regional phenotypes that promote atherosclerosis-susceptibility or atherosclerosis-protection.12,20,21,35 Unidirectional, laminar shear stress correlates with the in vitro induction of transcript profiles considered protective (e.g. antioxidative, anti-inflammatory, antiproliferative) and in situ endothelial expression of candidate genes at locations that are protected from atherosclerosis.39–41 Genetic manipulation of mice also indicates that transcriptional activity of candidate genes is linked to flow disturbances.42,43 However, global and candidate-gene expression studies at discrete endothelial sites in vivo have provided a more comprehensive picture of interacting pathways at sites of flow disturbance.12,39,44,45
Passerini and colleagues used RNA amplification to overcome sampling limitations and provided insights into pathway complexity in endothelial cells in vivo.12 They studied paired replicate analyses of gene expression in freshly isolated endothelium from disturbed flow/atherosclerosis-susceptible and undisturbed flow/atherosclerosis-protected arterial regions. Differential transcriptome analyses of samples from the inner curvature of the aortic arch and descending thoracic aorta of normal adult male pigs revealed that atherosclerosis-susceptible (proinflammatory, procoagulant) and atherosclerosis-protective (antioxidant and anticoagulative) gene expression coexisted in atheroma-susceptible regions. Thus, the concept proposed by Hajra et al. that the endothelium is ‘primed’ for pathological change30 was refined to that of a balanced phenotype in which the presence of disturbed flow tips the balance of gene expression towards atherosclerosis-susceptibility. Disturbed flow characteristics might, therefore, predict the development of atherosclerosis but, in the absence of additional risk factors, pathogenesis is kept in check by equally protective gene expression in the same cells. Work extending the study by Passerini et al. has shown that gene expression differences extend to site-specific differences in protein expression and in post-translational regulation in the endothelium.46 In 2008, Zakkar et al. revealed an interesting mechanism for the containment of proinflammatory activation at atherosclerosis-protected endothelial sites by enhanced expression of mitogen activated protein kinase phosphatase 1 (MKP-1, also known as DUS1).47 In vitro studies showed a reciprocal relationship between MKP-1 and proinflammatory endothelial vascular adhesion protein 1 (VCAM-1); MKP-1 expression was induced by shear, while VCAM-1 was down-regulated. Gene silencing of MKP-1 restored VCAM-1 expression in shear-exposed cells.
The endothelial transcription factor Krüppel-like factor 2 (KLF2) is only induced by flow.39 On the basis of this finding and its in situ expression in atherosclerosis-protected regions, KLF2 has been suggested to underpin the molecular basis of the healthy state of flow-exposed endothelial cells.39,48,49 KLF2 could influence the regulation of up to a third of shear-activated genes.48 The link between KLF2 expression and protection from atherosclerosis in arterial regions suggests that this protein is important for the regulation of hemodynamic-related endothelial phenotype that protects against atherogenesis.
Studies of endothelial phenotypes in the complex flow environment of the arterial plaque surface are limited by technical challenges. However, in 2007, Volger et al. reported differential endothelial expression of chemokines, nuclear factor-κB, p53, transforming growth factor β and other related genes and proteins in advanced plaques, compared with early lesions.13 Although this study did not address the temporal or early spatial phenotypic changes in the endothelium, nor the heterogeneous basis upon which the presumed changes occur, the findings open a promising avenue of investigation.
Important studies in vivo demonstrated eNOS transcript responses when shear stress was experimentally altered by a tapered cast placed around the carotid artery in mice.42 Shear stress increased as the cast tapered and this feature was associated with strong induction of eNOS transcription (protective). Immediately downstream of the cast, in a flow-separation and disturbance region, low expression of eNOS occurred (susceptible). Furthermore, in apolipoprotein–E-knockout mice implanted with the cast, atherosclerosis development was invariably associated with the downstream disturbed flow region.43 These studies demonstrated a causal link between altered hemodynamics and the reduced expression of an important molecule in atherosusceptible regions in vivo. Their finding confirms in vitro predictions and permits extrapolations of other studies.
SHEAR STRESS AND ENDOTHELIAL MECHANOTRANSDUCTION MECHANISMS
Decentralized subcellular mechanisms of endothelial shear stress mechanotransduction
The development of endothelial tissue culture in the 1970s paved the way for controlled studies of the effects of hemodynamic forces upon endothelial cell biology and the mechanisms involved.1,5,33,34,49–52 Wall shear stress depends upon the detailed geometry of the vessel surface. Thus, measurements of fluid shear stresses that treat the endothelial surface as flat and ignore the detailed cell topography are less precise than subcellular surface measurements that reveal the spatial heterogeneity of stress distributions.53
Shear stress mechanotransduction in the endothelium requires several sequential steps:5,54 first, physical deformation of the cell surface; second, intracellular transmission of stress; third, conversion of mechanical force to chemical activity (‘true’ mechanotransduction); and fourth, downstream biochemical signaling with feedback. The temporal relationships are not yet firmly established—the first and second stages, for example, could occur almost simultaneously. However, this model can be used to interpret existing data and can readily accommodate new findings. For example, endothelial membrane-potential changes can be sufficient to stimulate the release of vasoactive molecules in the first, third and fourth mechanotransduction response steps via activation of flow-sensitive ion channels at the luminal membrane and bypass of intracellular stress transmission.55,56 In most cases, however, shear-mediated signaling to subcellular sites distant from the luminal surface emphasizes the central role of the cytoskeletal transmission of forces (the second step), which has led to the development of a ‘decentralized’ model of mechanotransduction.5
Live-cell imaging of endothelial focal adhesions revealed the dynamic nature of cell–substratum interactions. Directional shear stress applied to the luminal endothelial cell surface resulted in reorganization of adhesion sites at the abluminal (attached) surface and caused endothelial cells to align in the direction of flow.57 Furthermore, biochemical measurements of phosphorylation of focal adhesion site proteins in response to shear stress have provided strong evidence for mechanically-initiated signal transduction at these locations.58 Other studies, centered on the shear-induced phosphorylation of junctional molecules, have identified rapid shear signaling responses at intercellular junctions, particularly those that involve platelet endothelial cell adhesion molecule 1 (PECAM-1 also known as CD31 antigen; a protein that localizes to the interendothelial cell adhesion site in confluent endothelium).59,60 The evidence for stress transmission by cytoskeletal elements that link the luminal surface to junctions and focal adhesions is compelling, and was demonstrated by live-cell imaging of filament displacement that used fluorescent reporter molecules.61,62 The nuclear membrane is also subject to cytoskeleton-mediated transmission of stress.63 This feature could influence gene expression, possibly by regulating access of transcription factors through nuclear pores or changing the tensional stresses within DNA. Dalby et al. proposed that the nuclear lamins and cytoskeleton form a continuous system, connected via adhesion sites in the nuclear envelope—and that this system constitutes a mechanism for mechanical regulation of gene expression.64
The model of decentralized mechanotransduction (Figure 4) proposes that although shear stress acts initially at the luminal (apical) cell surface with some responses located there, surface deformation is also transmitted throughout the body of the cell such that multiple elements located away from the luminal surface can, independently or in concert, transduce the mechanical signals into chemical activities at distant subcellular sites. The model accommodates many separate experimental findings in relation to endothelial shear stress responses associated with disparate subcellular locations. In this model, the entire cell can be regarded as an assembly of mechanotransduction sites connected principally by the cytoskeleton, although the dynamic nature of the structural elements themselves is also acknowledged. The degree to which mechanotransduction that originates at one subcellular location can interact with that at another is as yet unclear.
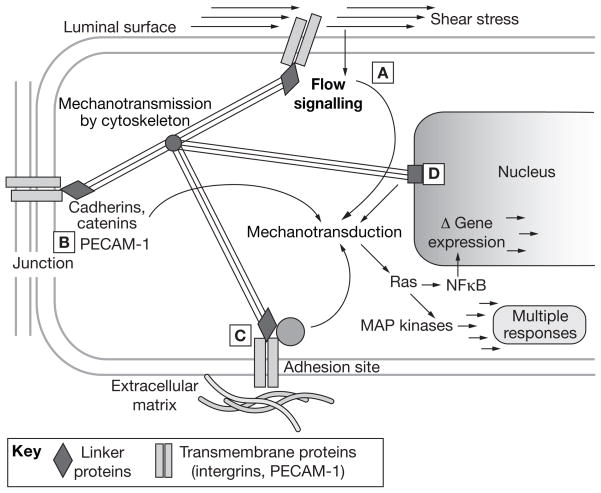
Figure 4.
The decentralized model of endothelial mechanotransduction by shear stress. The cytoskeleton has a central role in the transmission of tension changes throughout the cell. (A) Direct signaling can occur through deformation of the luminal surface, possibly via the glycocalyx. Examples include localized activation of potassium, sodium and calcium ion channels, phospholipase activity leading to calcium signaling, G-protein activation and caveolar signaling. (B) Mechanotransduction is also mediated via junctional signaling: that is, the transmission of forces to intercellular junction protein complexes via the cortical and/or filamentous cytoskeleton. VEGFR2 located at the luminal surface (see A) or near the junction (see B) can associate with VE-cadherin, β-catenin, and phosphatidylinositol 3 kinase to phosphorylate Akt and the primary transmembrane protein at this location, PECAM-1.59 (C) Cytoskeletal forces are also transmitted to adhesion sites. Transmembrane integrins bound to the extracellular matrix serve as a focus for deformation. This deformation results in autophosphorylation of FAK, which binds the SH2 domain of c-Src, a kinase family that phosphorylates paxillin and p130cas and leads to integrin-dependent activation of MAP kinases via Ras GTPase. A second parallel integrin-mediated pathway involves the activation of Shc, which binds Src family kinases through SH2 domains. Shc phosphorylation leads to Ras-MAP kinase activity.81 Ras releases the trans-acting NFκB from its cytosolic inhibitor, and thus enables its translocation to the nucleus where it binds to the promoters of multiple target genes. A third integrin-mediated pathway involves rhoA activation, which profoundly influences actin assembly and, therefore, transmission of mechanical stimuli. The multiple roles of small GTPases in mechanotransduction are reviewed in depth by Tzima.82 (D) Nuclear deformation is also likely to result in mechanically induced signaling, possibly via lamins in the nuclear membrane. Other possible direct effects include macromolecular conformation changes that are relevant to gene regulation. Of note, the locations are based on direct or indirect experimental evidence, are not mutually exclusive, and are probably interconnected. Permission obtained from Cambridge University Press © Davies PF and Helmke BP (2008) Endothelial Mechanotransduction. In Cellular Mechanotransduction: Diverse Perspectives from Molecules to Tissue (Eds Mofrad RK and Kamm RD)54. Abbreviations: Akt, protein kinase B; c-Src, tyrosine-protein kinase; FAK, focal adhesion kinase; MAP, mitogen-activated protein; NFκB, nuclear factor κB; p130Cas, Crk-associated substrate; PECAM-1, platelet endothelial cell adhesion molecule 1; Ras, small GTPase; Rho, small GTPase related to Ras; SH2, Src homology 2 domain; Shc, SH2-combining adaptor protein; VE-cadherin, vascular endothelial cadherin; VEGFR2, vascular endothelial growth factor receptor 2.
Physical deformation
Displacement of one or more cellular elements is required to initiate mechanically induced signaling responses. As shear stress acts at the luminal cell surface, local membrane structures can participate in mechanotransduction. Examples include activation of ion channels and G proteins, and changes in phospholipid metabolism and membrane fluidity.54 The distribution of forces that act on the luminal surface of the endothelial monolayer is determined by the microgeometry of the surface, secondary to the bulk characteristics of the blood flow. Detailed mapping of the monolayer surface by atomic force microscopy followed by computational modeling of flow has identified considerable heterogeneity in the cell–cell and subcellular distribution of stress concentrations.53 As expected, regions of the cell that extend furthest into the flow are subjected to the highest shear stress forces. The ‘tallest’ structure was considered to be the area of the cell surface that extends over the nuclear region, which has a typical peak height of 5–7 μm. However, specialized structures have been identified that, if expressed, can extend considerably further than that distance into the luminal blood flow. Two of these extended cellular structures have elicited great interest: the glycocalyx, a highly charged, glycoprotein-rich extension of the cell surface, and primary cilia, which connect to the cytoskeleton at their base, extend through the luminal cell surface and project into the flow region.
The glycocalyx
The glycocalyx can project up to 0.5 μm from the endothelial plasma membrane.65,66 In the high-flow environment of the arterial endothelium, it is the outermost interface between the cell and shear flow and thus its distribution and thickness may be spatially important for mechanical signaling. Its deformation could contribute to force transmission, and its thickness could modulate bidirectional transport of molecules between the cell monolayer and blood, and adhesion of circulating cells. Selective cleavage of glycocalyx components, particularly the glycosaminoglycan heparan sulfate, was found to abolish both flow-mediated endothelial nitric oxide production67 and monolayer realignment.68 Huang et al. used subdiffraction-limited single fluorophore protein imaging at high resolution to measure the glycocalyx thickness in cultured, live arterial-endothelial cells and found it to average 350 μm ± 170 μm.69 Variation in the glycocalyx spatial distribution and composition could prove important in mechanotransduction (for example, under shear stress the glycocalyx redistributes to perijunctional regions68). Theoretical calculations that assume the endothelial glycocalyx is evenly distributed over the cell surface suggest that very little shear stress reaches the plasma membrane;70 however, such a conclusion is inconsistent with the immediate deformation of the cytoskeleton and nucleus recorded at the onset of shear stress.71,72 These inconsistencies will resolve as more information is obtained about the mechanical properties and detailed cellular distribution of the endothelial glycocalyx in arteries.
Primary cilia
Primary cilia can extend into the lumen up to several micrometers from the endothelial luminal surface. Endothelial expression of these structures was noted in 1987 overlying atherosclerotic lesions.73 Interest now centers on their role in mechanotransduction. Poelmann and colleagues reported that approximately 25% of arterial endothelial cells express a single primary cilium and that ciliated cells are more prevalent in regions of low and disturbed blood flow.74 In vitro, steady shear stress causes cilia disassembly within 2 h.75 That cilia might have a role in mechanically induced signaling is an attractive idea because they are linked directly to the cytoskeleton; however, cells without cilia are clearly also flow-responsive, and the relative contribution of cilia to mechanotransduction remains to be assessed.
Force transmission (mechanotransmission)
The deformation of a connected system under tension enables transmission of forces throughout the connected elements. In the endothelium this system comprises the cytoskeletal filaments distributed throughout the cell body and the submembranous, spectrin-like, peripheral cortical cytoskeleton. These elements themselves are interconnected and are also linked to membrane proteins throughout the cell. They provide elastic stiffness and maintain the shape and structure of the cell. Interference with cytoskeletal assembly and dynamics has been observed to inhibit flow responses in various experimental systems.76 Demonstrations of intermediate filament displacement,61 actin filament deformation,62 and microtubule-directed motion of mitochondria77 (all initiated by flow) support the view that forces at the luminal cell surface are transmitted to ‘remote’ cellular sites via cytoskeletal deformations and displacements that could be a function of the prestressed, pretensioned cytoskeleton.77 Furthermore, deformation effects could even extend to adjacent cells, communicated through cell junctional structures and possibly the extracellular matrix.
Mechanical force conversion to chemical activity (‘true’ mechanotransduction)
This critical event—the coupling of force to chemical activity—can occur simultaneously at multiple locations. A single mechanism seems unlikely when the multiplicity of subcellular sites at which responses have been measured is considered. Proposed mechanisms include mechanical induction of changes in the conformation or mobility of membrane proteins, direct force effects on ion channels, separation of assembled junctional proteins, deformation of caveolar structures, and physical changes in integrin dynamics.5 Shear stress-induced foci of strain might also cause displacement of local soluble or bound cofactors that are engaged in homeostatic regulation, analogous to pressure-induced conformational changes in membrane proteins.78 Of note, adhesion sites and junctions are particularly rich in the enzymes, adaptor proteins and cofactors necessary to elicit biochemical mechanotransduction responses.78
Immediate and downstream signaling responses
At multiple subcellular sites, fast responses include activation of ion channels located in the luminal membrane, intracellular calcium ion release, cleavage of membrane phospholipids, changes in membrane fluidity, and the phosphorylation of various proteins, all of which activate secondary signaling pathways. Their spatial organization and coordination is poorly understood but many involve local phosphorylation events and diffusible signaling molecules. Ingber has proposed that stresses applied through the cytoskeleton can directly alter the conformation of bound proteins to change their biochemical activities.79 Multiple responses with different time scales are important as they enable the cells to discriminate efficiently between acute and sustained flow changes.
CONCLUSIONS
The evolution of flow-related responses in the endothelium is one of many specializations essential to the operation of an efficient vascular transport system. The diversity of endothelial functions is reflected in the variety of mechanotransduction mechanisms, and suggests that a single mechanism for coordinated mechanotransduction in endothelial cells is unlikely. A useful approach could be to determine how mechanotransduction occurs as an intracellular ‘systems’ response for each physiological or pathological context of interest, with appreciation of the potential redundancy of elements within the system. To some extent this approach is gaining ground through studies that reflect the focus of different investigators (e.g. investigators with clinical objectives).
New, therapeutic, pharmacological approaches directed at prominent, intracellular regulators of shear stress responses could be beneficial; however, caution is advised as these regulators are also central to other fundamental cellular processes. An alternative approach is the renormalization of undesirable flow characteristics, both systemically and at sites that require intervention; in this respect, control of blood pressure and increased exercise are therapeutic. Exercise reverses flow-mediated vasodilation in a number of arterial beds and can induce beneficial localized changes in hemodynamics when cardiac output is elevated (e.g. by periodically shifting the location of flow separation regions for extended periods to provide relief from a propathological environment). Direct interventions include modifications to the designs of stents and other devices that aim to optimize local flow characteristics.
KEY POINTS
Hemodynamic forces, and in particular shear stresses, are regulators of many physiologic and pathologic aspects of endothelial function in the cardiovascular system
In vivo and in vitro global endothelial analyses reveal that endothelial phenotypes are heterogeneous over regional and focal length scales, which links flow characteristics to cardiovascular disease protection, susceptibility and development
Endothelial responses are sensitive to variations in the characteristics of flow that generate shear stresses; regions with oscillating shear stress and flow reversal correspond with pathologic changes in the artery wall and are a risk factor for atherosclerosis-susceptibility
When shear stresses deform the endothelium, a mechanical perturbation is communicated via the cytoskeleton to multiple sites of mechanotransduction, which include cell–matrix adhesion sites, intercellular junctions and the nuclear membrane
Endothelial responses that are specific to shear stress offer potential therapeutic pharmacological targets, although a single mechanosensor is unlikely to exist
Beneficial systemic effects include maintenance of arterial hemodynamics within normal limits through antihypertensive therapies, regular exercise to promote continuous adaptive remodeling and inhibition of endothelial dysfunction, and (when intervention is required) better design of intravascular devices to optimize flow characteristics
REVIEW CRITERIA
This Review distils the substantial literature published since 1970 relevant to blood flow, atherogenesis and the endothelium. English-language, full-text papers and reviews were selected. Principal search terms used in PubMed were “endothelium and ….” where the second term included “hemodynamics”, “shear stress”, “blood flow”, “biomechanics”, and “mechanotransduction”.
Acknowledgments
The author’s research is supported by grants from the National Heart Lung and Blood Institute of the National Institutes of Health.
Footnotes
Competing interests
The author declared no competing interests.
References
- 1.Pohl U, et al. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci. 2006;361:735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corson MA, et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 4.Griffith TM. Endothelial control of vascular tone by nitric oxide and gap junctions: a haemodynamic perspective. Biorheology. 2002;39:307–318. [PubMed] [Google Scholar]
- 5.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, et al. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circ Res. 2008;102:1275–1285. doi: 10.1161/CIRCRESAHA.108.171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. [Google Scholar]
- 9.Lucitti JL, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichert AM, et al. Endothelial nitric oxide synthase gene expression during murine embryogenesis: commencement of expression in the embryo occurs with the establishment of a unidirectional circulatory system. Circ Res. 2008;103:24–33. doi: 10.1161/CIRCRESAHA.107.168567. [DOI] [PubMed] [Google Scholar]
- 11.Glagov S, et al. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 12.Passerini AG, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volger OL, et al. Distinctive expression of chemokines and transforming growth factor-beta signaling in human arterial endothelium during atherosclerosis. Am J Pathol. 2007;171:326–337. doi: 10.2353/ajpath.2007.061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–499. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson EJ, et al. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–2249. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- 16.Green DJ, et al. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JA. Therapeutic approaches to altering hemodynamic forces. In: Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. pp. 1690–1697. [Google Scholar]
- 18.Suo J, et al. Blood flow patterns in the proximal human coronary arteries: relationship to atherosclerotic plaque occurrence. Mol Cell Biomech. 2008;5:9–18. [PubMed] [Google Scholar]
- 19.Steinman DA, Taylor CA. Flow imaging and computing: large artery hemodynamics. Ann Biomed Eng. 2005;33:1704–1709. doi: 10.1007/s10439-005-8772-2. [DOI] [PubMed] [Google Scholar]
- 20.Davies PF, et al. A spatial approach to transcriptional profiling: mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999;17:347–351. doi: 10.1016/s0167-7799(99)01348-7. [DOI] [PubMed] [Google Scholar]
- 21.García-Cardeña G, et al. Mechanosensitive endothelial gene expression profiles: scripts for the role of hemodynamics in atherogenesis? Ann NY Acad Sci. 2001;947:1–6. [PubMed] [Google Scholar]
- 22.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am J Cardiol. 2000;86:3J–8J. doi: 10.1016/s0002-9149(00)01339-4. [DOI] [PubMed] [Google Scholar]
- 23.Folie BJ, McIntire LV. Mathematical analysis of mural thrombogenesis. Concentration profiles of platelet-activating agents and effects of viscous shear flow. Biophys J. 1989;56:1121–1141. doi: 10.1016/S0006-3495(89)82760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duraiswamy N, et al. Spatial distribution of platelet deposition in stented arterial models under physiologic flow. Ann Biomed Eng. 2005;33:1767–1777. doi: 10.1007/s10439-005-7598-2. [DOI] [PubMed] [Google Scholar]
- 25.DePaola N, et al. Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 26.Moore JE, Berry JL. Fluid and solid mechanical implications of vascular stenting. Ann Biomed Eng. 2002;30:498–508. doi: 10.1114/1.1458594. [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan B, et al. Strut position, blood flow, and drug deposition: implications for single and overlapping drug–eluting stents. Circulation. 2005;111:2958–2965. doi: 10.1161/CIRCULATIONAHA.104.512475. [DOI] [PubMed] [Google Scholar]
- 28.Kastrati A, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 29.Loth F, et al. Transitional flow at the venous anastomosis of an arteriovenous graft: potential activation of the ERK1/2 mechanotransduction pathway. J Biomech Eng. 2003;125:49–61. doi: 10.1115/1.1537737. [DOI] [PubMed] [Google Scholar]
- 30.Hajra L, et al. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caro CG, et al. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–1161. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 32.Fry DL. Atherogenesis: initiating factors. CIBA Found Symposium. 1973;12:96–118. [Google Scholar]
- 33.Dewey CF, et al. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–188. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 34.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 35.Suo J, et al. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 36.Wolf S, Werthessen NT. Dynamics of arterial flow. Adv Exp Med Biol. 1979;115:1–472. [PubMed] [Google Scholar]
- 37.Huo Y, et al. Flow patterns in three-dimensional porcine epicardial coronary arterial tree. Am J Physiol Heart Circ Physiol. 2007;293:H2959–H2970. doi: 10.1152/ajpheart.00586.2007. [DOI] [PubMed] [Google Scholar]
- 38.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 39.Dekker RJ, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 40.Dai G, et al. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler T, et al. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 42.Cheng C, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 44.Iiyama K, et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 45.De Nigris F, et al. Beneficial effects of antioxidants and l-arginine on oxidation-sensitive gene expression and endothelial NO synthase activity at sites of disturbed shear stress. Proc Natl Acad Sci USA. 2003;100:1420–1425. doi: 10.1073/pnas.0237367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKC ζ in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zakkar M, et al. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res. 2008;103:726–732. doi: 10.1161/CIRCRESAHA.108.183913. [DOI] [PubMed] [Google Scholar]
- 48.Parmar KM, et al. Integration of flow-dependent endothelial phenotypes by Krüppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Cardeña G, Gimbrone MA. Biomechanical modulation of endothelial phenotype: implications for health and disease. Handb Exp Pharmacol. 2006;176:79–95. doi: 10.1007/3-540-36028-x_3. [DOI] [PubMed] [Google Scholar]
- 50.Davies PF, et al. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984;73:1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies PF, et al. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shyy YJ, et al. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbee KA, et al. Subcellular distribution of shear stress at the surface of flow aligned and non-aligned endothelial monolayers. Am J Physiol. 1995;268:H1765–H1772. doi: 10.1152/ajpheart.1995.268.4.H1765. [DOI] [PubMed] [Google Scholar]
- 54.Davies PF, Helmke BP. Endothelial mechanotransduction. In: Mofrad RK, Kamm RD, editors. Cellular Mechanotransduction: Diverse Perspectives from Molecules to Tissue. New York: Cambridge University Press; 2008. [In press] [Google Scholar]
- 55.Olesen S-P, et al. Hemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 56.Gautam M, et al. Flow-activated chloride channels in vascular endothelium. Shear stress sensitivity, desensitization dynamics, and physiological implications. J Biol Chem. 2006;281:36492–36500. doi: 10.1074/jbc.M605866200. [DOI] [PubMed] [Google Scholar]
- 57.Davies PF, et al. Quantitative studies of endothelial cell adhesion: directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 59.Fujiwara K, et al. Is PECAM-1 a mechanoresponsive molecule? Cell Struct Funct. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 60.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 61.Helmke BP, et al. Spatial concentration of intracellular strain induced by hemodynamic shear stress. Biophys J. 2003;84:2691–2699. [Google Scholar]
- 62.Mott RE, Helmke BP. Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1616–C1626. doi: 10.1152/ajpcell.00457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maniotis AJ, et al. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalby MJ, et al. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J Cell Biochem. 2007;102:1234–1244. doi: 10.1002/jcb.21354. [DOI] [PubMed] [Google Scholar]
- 65.Weinbaum S, et al. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 66.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 67.Florian JA, et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 68.Yao Y, et al. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H1023–H1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- 69.Huang H, et al. Three-dimensional sub-diffraction-limited single fluorophore imaging of proteins near the cell membrane: application to the endothelial glycocalyx. Proc Natl Acad Sci USA. in press. [Google Scholar]
- 70.Secomb TW, et al. Effect of the endothelial surface layer on transmission of fluid shear stress to endothelial cells. Biorheology. 2001;38:143–150. [PubMed] [Google Scholar]
- 71.Helmke BP, Davies PF. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30:284–329. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- 72.Stamatas GN, McIntire LV. Rapid flow-induced responses in endothelial cells. Biotechnol Prog. 2001;17:383–402. doi: 10.1021/bp0100272. [DOI] [PubMed] [Google Scholar]
- 73.Haust MD. Endothelial cilia in human aortic atherosclerotic lesions. Virchows Arch A Pathol Anat Histopathol. 1987;410:317–326. doi: 10.1007/BF00711288. [DOI] [PubMed] [Google Scholar]
- 74.Van der Heiden K, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 75.Iomini C, et al. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 77.Hu S, Wang N. Control of stress propagation in the cytoplasm by prestress and loading frequency. Mol Cell Biomech. 2006;3:49–60. [PubMed] [Google Scholar]
- 78.Desai G, et al. Pressure-jump studies of the folding/unfolding of trp repressor. J Mol Biol. 1999;288:461–475. doi: 10.1006/jmbi.1999.2692. [DOI] [PubMed] [Google Scholar]
- 79.Ingber D. Vascular control through tensegrity-based integration of mechanics and chemistry. In: Aird WC, editor. Endothelial biomedicine. Cambridge: Cambridge University Press; 2007. pp. 1786–1792. [Google Scholar]
- 80.Computational imaging of pulsatile flow in a normal human carotid bifurcation. [accessed 20 October 2008]; [ http://www.mie.utoronto.ca/labs/bsl/gallery/carotid.mpg]
- 81.Liu Y, et al. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J Cell Biol. 2008;182:185–196. doi: 10.1083/jcb.200709176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]