Abstract
The changes in wall structure of two penicillinase-negative strains of Bacillus licheniformis on addition of penicillin were studied. After addition of penicillin to give a concentration of 1 unit/ml, exponentially growing cells of strain 749 c/72 doubled once and then stopped. Strain 749c/72/IIIg was more resistant and continued growing, but synthesis appeared to become uncontrolled over the surface, producing localized wall thickening at the expense of elongation, and leading to distorted cells and growth in twisted and coiled chains, with an accompanying drop in growth rate. The continued growth can be explained by the existence of a less sensitive transpeptidase, but there is no obvious explanation for the uncontrolled synthesis. The effect of penicillin could be reversed by addition of penicillinase in both strains, although there appeared to be a persistent effect of penicillin which also produced distorted cells for a few generations and inhibited cell separation. The changes in wall structure produced by penicillin and penicillinase appeared all over the cell surface, suggesting that wall synthesis occurred all over the cell. Also a separate process for cross-wall synthesis is suggested since this appeared less sensitive than wall synthesis.
Full text
PDF
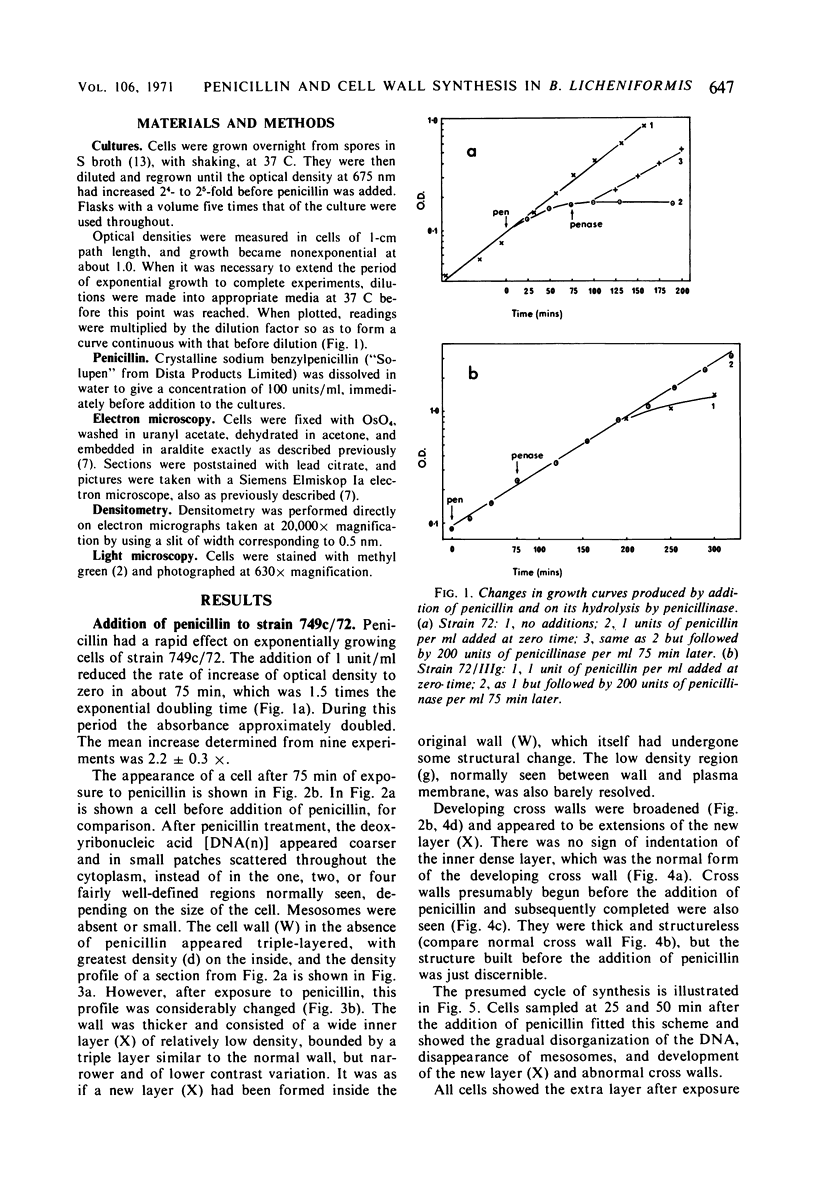











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISSET K. A., HALE C. M. Complex cellular structure in bacteria. Exp Cell Res. 1953 Dec;5(2):449–454. doi: 10.1016/0014-4827(53)90231-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. The cell wall of Escherichia coli: early effects of penicillin treatment and deprivation of diaminopimelic acid. J Gen Microbiol. 1967 Feb;46(2):237–246. doi: 10.1099/00221287-46-2-237. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. A., Pollock M. R. The genetics of Bacillus licheniformis penicillinase: a preliminary analysis from studies on mutation and inter-strain and intra-strain transformations. J Gen Microbiol. 1965 Oct;41(1):7–21. doi: 10.1099/00221287-41-1-7. [DOI] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton P. J. An electron microscopic study of the structure of mesosomal membranes in Bacillus licheniformis. J Ultrastruct Res. 1970 May;31(3):247–259. doi: 10.1016/s0022-5320(70)90129-2. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Pavlik J. G., Rogers H. J., Tanner P. J. Organization of polymers in the cell walls of some bacilli. Nature. 1968 Aug 10;219(5154):642–644. doi: 10.1038/219642a0. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., FRANCOMBE W. H., MAYALL B. H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959 Dec;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. Chemical structure and biosynthesis of bacterial cell walls. Bacteriol Rev. 1963 Mar;27:18–55. doi: 10.1128/br.27.1.18-55.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase adaptation in Bacillus cereus; an analysis of three phases in the response of logarithmically growing cultures to induction of penicillinase formation by penicillin. Br J Exp Pathol. 1952 Dec;33(6):587–600. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970 May;61(2):155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XII. Inhibition of cross-linking by penicillins and cephalosporins: studies in Staphylococcus aureus in vivo. J Biol Chem. 1968 Jun 10;243(11):3169–3179. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]