Abstract
Herein, we describe the cases of 4 patients who each experienced a myocardial infarction in association with nonbacterial thrombotic endocarditis. We discuss the clinical presentation of this rare condition, distinguish between infective and nonbacterial thrombotic endocarditis via a review of the medical literature, and present treatment options for myocardial infarction that is associated with nonbacterial thrombotic endocarditis.
Key words: Acute disease; cerebral infarction/etiology/pathology; diagnosis, differential; echocardiography, transesophageal; endocarditis/complications/diagnosis/etiology/pathology/therapy; heart valve diseases/complications/diagnosis/pathology; infection/complications; myocardial infarction/complications; neoplasms/complications; stroke/complications; thrombolytic therapy/contraindications
Nonbacterial thrombotic endocarditis (NBTE) is defined as noninfectious cardiac valvular vegetations with negative blood cultures.1 Approximately 80% of patients who have NBTE also have an underlying malignancy.2 Typically, the presenting symptom of NBTE is systemic or pulmonary embolization.3
Here, we describe and discuss the cases of 4 patients with NBTE who presented with myocardial infarction (MI).
Case Reports
Patient 1
A 76-year-old man with stage IIIB adenocarcinoma of the lung presented at the hospital with recurrent episodes of confusion. A computed tomographic scan of the head revealed multiple infarcts that involved the bilateral cerebellar hemispheres and the left frontoparietal, left posterior temporal, and left occipital regions. A transesophageal echocardiogram (TEE) showed a 1.3 × 1.1-cm vegetation on the anterior leaflet of the mitral valve, a normal left ventricular ejection fraction (LVEF), and no other intracardiac mass or thrombus. The patient was started on antibiotic therapy; however, the blood cultures were negative. One week later, he experienced substernal chest pain. Upon examination, his blood pressure was 122/70 mmHg, and a systolic murmur was audible at the left lower sternal border. Laboratory tests showed elevated troponin I with a peak value of 3.82 ng/mL (normal value, <0.4 ng/mL). A 12-lead electrocardiogram (ECG) showed sinus tachycardia with right bundle branch block and nonspecific ST–T wave changes. The diagnosis of non-ST-elevation myocardial infarction (NSTEMI) was made, and the patient was treated with aspirin, β-blockers, nitrates, and angiotensin-converting enzyme (ACE) inhibitors. Heparin was not administered due to the patient's history of recent hematuria and thrombocytopenia. He died within 4 days due to multiorgan failure.
Patient 2
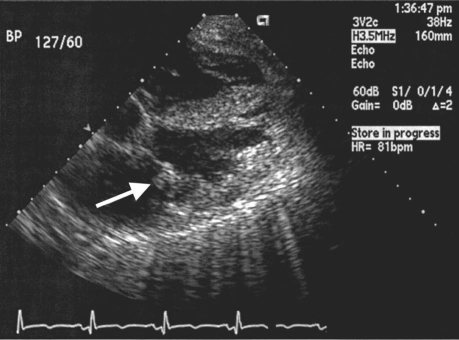
A 53-year-old man with esophageal cancer presented at the hospital with chest pain and dyspnea. He was intubated and was treated for respiratory failure. Upon examination, his blood pressure was 117/50 mmHg, and a systolic murmur was heard at the apex. Laboratory tests revealed elevated serum troponin I with a peak value of 7.82 ng/mL (normal value, <0.4 ng/mL). A 12-lead ECG showed ST-segment elevation in leads II, III, and aVF, which suggested an acute inferior-wall MI (Fig. 1). The diagnosis of ST-elevation myocardial infarction (STEMI) was made. A transthoracic echocardiogram (TTE) disclosed a 1.1 × 0.6-cm vegetation on the anterior leaflet of the mitral valve, mild mitral regurgitation, normal LVEF, and no other intracardiac mass or thrombus (Fig. 2). Blood cultures were negative, and the patient was given no antibiotic therapy. A computed tomographic scan of the brain showed 2 areas of cortical ischemia that involved the right middle cerebral artery (MCA). The patient was treated with aspirin, β-blockers, and unfractionated heparin. He developed renal failure and died within 4 days due to multiorgan failure.
Fig. 1 Patient 2. A 12-lead electrocardiogram shows ST-segment elevation in leads II, III, and aVF.
Fig. 2 Patient 2. Transthoracic echocardiogram shows a vegetation (arrow) on the anterior leaflet of the mitral valve.
Patient 3
A 55-year-old man with stage IV adenocarcinoma of the lung presented at the hospital with dyspnea, for which he was intubated. His blood pressure was 120/70 mmHg, and auscultation revealed crepitations and bilateral wheezing. A chest radiograph showed bilateral pulmonary consolidation that spared the apices, and small bilateral effusions. A 12-lead ECG showed ST-segment elevation in leads V1 through V3, which suggested an acute MI. Laboratory tests revealed elevated troponin I with a peak value of 1.30 ng/mL (normal value, <0.4 ng/mL). The diagnosis of STEMI was made. The patient was started on antibiotic therapy. Blood cultures were negative. A TTE disclosed a 1.2 × 0.6-cm vegetation on the posterior leaflet of the mitral valve, mild mitral regurgitation with a normal LVEF, and no other intracardiac mass or thrombus. The patient was treated with aspirin, ACE inhibitors, diuretics, and intravenous nitroglycerin. He developed disseminated intravascular coagulation with renal failure and died within 3 days due to multiorgan failure.
Patient 4
A 44-year-old woman with stage IIIB non-small-cell lung cancer presented at the hospital with slurred speech and abnormal gait, dragging her left foot. A computed tomographic scan of the brain showed a large right-MCA infarct. A TTE revealed a vegetation on the aortic valve and mild-to-moderate aortic insufficiency. A TEE disclosed a 0.8 × 0.4-cm vegetation on the right coronary cusp, a normal LVEF, and no other intracardiac mass or thrombus. The patient was empirically started on antibiotic therapy. Blood cultures were negative. Two days later, she developed substernal chest pain. Upon examination, her blood pressure was 130/80 mmHg, and auscultation revealed a diastolic murmur that was consistent with aortic regurgitation. Laboratory tests showed elevated troponin I with a peak value of 1.79 ng/mL (normal value, <0.4 ng/mL). A 12-lead ECG showed sinus rhythm with no ischemic changes. The diagnosis of NSTEMI was made, and the patient was treated with aspirin, β-blockers, and clopidogrel. She died 2 months later due to progression of the cancer.
Discussion
Nonbacterial thrombotic endocarditis is characterized by the deposition of small masses of fibrin and other blood components on the leaflets of the cardiac valves. In contrast with vegetations of infective endocarditis (IE), the valvular lesions of NBTE are sterile and only loosely attached to the underlying valve. They usually occur along the line of closure of the leaflets or cusps. Histologically, the lesions are composed of bland thrombus without accompanying inflammatory reaction or valvular damage,4 although grossly valvular thickening has been found in some patients.5 The vegetations in NBTE vary in size from 0.1 to 2 cm at their greatest diameter.5 Approximately 82% of the involved valves in NBTE are normal and undamaged.6 Nonbacterial thrombotic endocarditis is associated with malignancy and other conditions that promote hypercoagulability, such as burns, sepsis, and the presence of indwelling catheters.7 The incidence and frequency of a particular type of malignancy seem to vary geographically. In an autopsy sample of patients in the United States, the prevalence of endocarditis was only 0.96%; lung tumor was the most frequently observed malignancy in association with it.8 In another study from the United States, mucin-producing adenocarcinoma of the lung was the most typically associated malignancy, followed by adenocarcinoma of the ovary.9 In contrast, in an autopsy study in Japan, the prevalence of NTBE was 2.4%, and malignant neoplasms of the lower gastrointestinal tract, thyroid, and female genitourinary system were more prevalent than was lung neoplasm.5 Of our 4 patients with NBTE, 3 had lung cancer and 1 had esophageal cancer.
At our institution, 2,463 autopsy records from 1972 through 1976 showed that 1.3% of the patients had NBTE and that the annual incidence increased from 0.8% in 1972 to 2.3% in 1976.9 The prevalence of vegetations caused by NBTE varies widely in different populations (range, 0.3%–9.3%).7 Nonbacterial thrombotic endocarditis typically involves the left side of the heart and most frequently affects the mitral valve (70%) and the aortic valve (33%).10 In our series, 3 patients (75%) had vegetations on the mitral valve and 1 (25%) on the aortic valve.
Clinical diagnosis of NBTE may be difficult to make, because murmurs are noted in only one third of affected patients.8 The clinical suspicion of NBTE should be high when patients present with neurologic symptoms and thromboembolic complications—and TEE should be performed promptly, because TTE may fail to show the vegetation.11
The typical clinical presentation of NBTE is systemic embolization; stroke is the usual manifestation.3,10 The incidence of stroke in patients who have IE is 19%,12 whereas it is more than 33% in patients who have NBTE.10 The most commonly involved territory is the MCA, usually affecting both hemispheres.13 In 1 study, 41% of patients who have NBTE had embolic events that affected multiple territories, including the cerebral, coronary, renal, splenic, pulmonary, and gastrointestinal circulation.6
Two of our patients initially presented with stroke and 2 with embolic MI. Three had emboli in the brain, and all had MCA involvement. Two had emboli in the kidneys, and 1 of those patients developed disseminated intravascular coagulation.
In the absence of angiographic evaluation and pathologic specimens, the diagnosis of coronary embolization due to NTBE in our patients was primarily clinical. However, in all 4, the blood cultures were negative, and the presence of valvular vegetations with multiple embolic events supported a diagnosis of NBTE. Due to the advanced stages of the cancers and to other comorbid conditions in our patients, we concluded that they would not have benefited from more aggressive treatment or invasive approaches to the MI.
The incidence of MI in patients who have IE is 2.9%,14 versus 7.5% in patients who have NBTE.5 Chest pain is the chief symptom of MI in patients who have endocarditis.15 Elevated levels of cardiac enzymes are found in 81% of such patients.15 In our series, 3 patients experienced chest pain, and all 4 had elevated troponin I. The clinical presentation of MI in patients who have endocarditis is similar to that of the general population; however, due to the absence of collateral vessels, the effects of coronary occlusion may be more severe in patients who experience embolic MI.16 The mechanisms of myocardial ischemia in patients who have IE are coronary compression secondary to periannular aortic valve complications, coronary embolism, obstruction of the coronary ostium due to large vegetations, the presence of arteriosclerotic coronary lesions, and severe aortic regurgitation.14
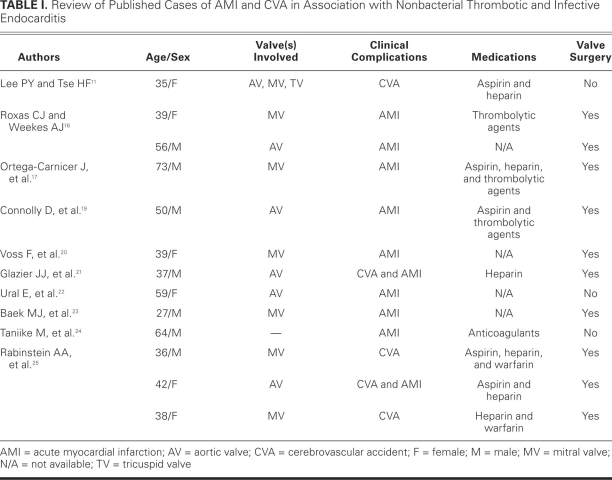
The initial treatment of patients who experience MI due to endocarditis is similar to that for atherosclerotic MI: aspirin, β-blockers, and ACE inhibitors. However, the administration of thrombolytic agents is controversial, because the use of thrombolytic agents in patients who sustained a MI due to endocarditis has led to fatal intracerebral17,18 and gastrointestinal19 bleeding. Other treatments have included percutaneous coronary intervention,20–22 coronary artery bypass grafting,16 direct surgical embolectomy,23 and catheter embolectomy techniques.24 Table I shows a summary of other cases in the medical literature.11,16,17,19–25
TABLE I. Review of Published Cases of AMI and CVA in Association with Nonbacterial Thrombotic and Infective Endocarditis
Preventing the recurrence of embolic events is a challenge in patients who have NBTE. In NBTE, the most effective anticoagulant to prevent recurrent embolic events is unfractionated heparin.3,26,27 Current guidelines recommend the use of either full-dose intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin.27 In cancer-related coagulopathy, anticoagulant treatment should be lifelong, because discontinuation of anticoagulants has led to catastrophic thromboembolic events in some patients.28 Warfarin may not provide optimal anticoagulation in NBTE, because it may not prevent recurrent thromboembolic events in patients who have malignancy-associated coagulopathy.26,28 The reason for this is unknown, but it may be due to non-vitamin-K–dependent agents that are involved in the process of thrombotic coagulopathy in malignancy.28 No large studies have specifically investigated the use of warfarin in patients who have NBTE. Anticoagulants are contraindicated in IE because they increase the rate of cerebral hemorrhage.29,30 In patients who have IE, aspirin does not reduce the risk of embolic events and is associated with an increased risk of bleeding.31
Systemic embolization is usually an indication for valve replacement in patients who have IE.32 Conversely, in patients who have NBTE, surgery is undertaken only when there is severe valvular dysfunction, anticoagulation is contraindicated, or anticoagulant therapy has failed to prevent recurrent emboli.25 Therapy should also be directed toward the primary tumor, because resolution of NBTE has been reported after removal of the tumor.33 The overall in-hospital mortality rate for patients who experience MI due to IE is 64%.14 There are no large-scale studies of MI in patients who have endocarditis, and there is no consensus regarding the optimal management of these patients. The treatment of MI due to NBTE is a therapeutic challenge, and each case should be approached in the context of its clinical situation.
Footnotes
Address for reprints: Syed Wamique Yusuf, MD, Department of Cardiology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1451, Houston, TX 77030
E-mail: syusuf@mdanderson.org
References
- 1.Durie NM, Eisenstein LE, Cunha BA, Plummer MM. Quadrivalvular marantic endocarditis (ME) mimicking acute bacterial endocarditis (ABE). Heart Lung 2007;36(2):154–8. [DOI] [PubMed]
- 2.Ahmed A, Hargrave K, Fisher JR, Kothari MJ. Neurological sequelae of infectious endocarditis [serial on the Internet]. emedicine. 2009 Feb 17 [cited 2010 Jan 14]. Available from: http://emedicine.medscape.com/article/1165712-overview
- 3.Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83(4):746–56. [DOI] [PubMed]
- 4.Kim HS, Suzuki M, Lie JT, Titus JL. Nonbacterial thrombotic endocarditis (NBTE) and disseminated intravascular coagulation (DIC): autopsy study of 36 patients. Arch Pathol Lab Med 1977;101(2):65–8. [PubMed]
- 5.Chino F, Kodama A, Otake M, Dock DS. Nonbacterial thrombotic endocarditis in a Japanese autopsy sample. A review of eighty cases. Am Heart J 1975;90(2):190–8. [DOI] [PubMed]
- 6.Steiner I. Nonbacterial thrombotic endocarditis–a study of 171 case reports [in Czech]. Cesk Patol 1993;29(2):58–60. [PubMed]
- 7.Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113(3): 773–84. [DOI] [PubMed]
- 8.Rosen P, Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am J Med 1973;54(1):23–9. [DOI] [PubMed]
- 9.Bedikian A, Valdivieso M, Luna M, Bodey GP. Nonbacterial thrombotic endocarditis in cancer patients: comparison of characteristics of patients with and without concomitant disseminated intravascular coagulation. Med Pediatr Oncol 1978;4(2):149–57. [DOI] [PubMed]
- 10.Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985–2000. Mayo Clin Proc 2001;76(12): 1204–12. [DOI] [PubMed]
- 11.Lee PY, Tse HF. Transient ischaemic attack in a young woman. Lancet 2000;355(9204):622. [DOI] [PubMed]
- 12.Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke 1990;21(5):695–700. [DOI] [PubMed]
- 13.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke 2002;33(5):1267–73. [DOI] [PubMed]
- 14.Manzano MC, Vilacosta I, San Roman JA, Aragoncillo P, Sarria C, Lopez D, et al. Acute coronary syndrome in infective endocarditis [in Spanish]. Rev Esp Cardiol 2007;60(1):24–31. [PubMed]
- 15.Demin AA, Drobysheva VP. Myocardial infarction in patients with infectious endocarditis [in Russian]. Kardiologiia 2004;44(1):4–9. [PubMed]
- 16.Roxas CJ, Weekes AJ. Acute myocardial infarction caused by coronary embolism from infective endocarditis. J Emerg Med 2008 [Epub ahead of print]. [DOI] [PubMed]
- 17.Ortega-Carnicer J, Ruiz-Lorenzo F, Benedicto A. Thrombolytic therapy for acute myocardial infarction in unsuspected infective endocarditis. Int J Cardiol 2005;103(1):108–10. [DOI] [PubMed]
- 18.Hunter AJ, Girard DE. Thrombolytics in infectious endocarditis associated myocardial infarction. J Emerg Med 2001;21 (4):401–6. [DOI] [PubMed]
- 19.Connolly DL, Dardas PS, Crowley JJ, Kenny A, Petch MC. Acute coronary embolism complicating aortic valve endocarditis treated with streptokinase and aspirin. A case report. J Heart Valve Dis 1994;3(3):245–6. [PubMed]
- 20.Voss F, Bludau HB, Haller C. Mitral valve endocarditis: an uncommon cause of myocardial infarction. Z Kardiol 2003; 92(8):686–8. [DOI] [PubMed]
- 21.Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997;20(10): 885–8. [DOI] [PMC free article] [PubMed]
- 22.Ural E, Bildirici U, Kahraman G, Komsuoglu B. Coronary embolism complicating aortic valve endocarditis: treatment with successful coronary angioplasty. Int J Cardiol 2007;119 (3):377–9. [DOI] [PubMed]
- 23.Baek MJ, Kim HK, Yu CW, Na CY. Mitral valve surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008;33(1):116–8. [DOI] [PubMed]
- 24.Taniike M, Nishino M, Egami Y, Kondo I, Shutta R, Tanaka K, et al. Acute myocardial infarction caused by a septic coronary embolism diagnosed and treated with a thrombectomy catheter. Heart 2005;91(5):e34. [DOI] [PMC free article] [PubMed]
- 25.Rabinstein AA, Giovanelli C, Romano JG, Koch S, Forteza AM, Ricci M. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol 2005;252(3): 352–5. [DOI] [PubMed]
- 26.Sack GH Jr, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56(1):1–37. [PubMed]
- 27.Salem DN, O'Gara PT, Madias C, Pauker SG. Valvular and structural heart disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):593S–629S. [DOI] [PubMed]
- 28.Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau's syndrome. Devastating coagulopathy in the absence of heparin. Am J Med 1985;79(4):423–30. [DOI] [PubMed]
- 29.Delahaye JP, Poncet P, Malquarti V, Beaune J, Gare JP, Mann JM. Cerebrovascular accidents in infective endocarditis: role of anticoagulation. Eur Heart J 1990;11(12):1074–8. [DOI] [PubMed]
- 30.Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med 1999;159(5):473–5. [DOI] [PubMed]
- 31.Chan KL, Dumesnil JG, Cujec B, Sanfilippo AJ, Jue J, Turek MA, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003;42(5):775–80. [DOI] [PubMed]
- 32.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America [published errata appear in Circulation 2005;112(15):2373; Circulation 2007;115(15):e408; Circulation 2007;116(21):e547; and Circulation 2008;118(12):e497]. Circulation 2005;111 (23):e394–434. [DOI] [PubMed]
- 33.Cockburn M, Swafford J, Mazur W, Walsh GL, Vauthey JN. Resolution of nonbacterial endocarditis after surgical resection of a malignant liver tumor. Circulation 2000;102(21):2671–2. [DOI] [PubMed]