Abstract
In vitro generated OVA-specific IL-17–producing CD8 T effector cells (Tc17) from OT-1 mice, adoptively transferred into B16-OVA tumor-bearing mice, controlled tumor growth in early and late stage melanoma. IL-17, TNF, and IFN-γ from the Tc17 effectors all played a role in an enhanced recruitment of T cells, neutrophils, and macrophages to the tumor. In addition, Tc17 cells and recently recruited, activated neutrophils produced further chemokines, including CCL3, CCL4, CCL5, CXCL9, and CXCL10, responsible for the attraction of type 1 lymphocytes (Th1 and Tc1) and additional neutrophils. Neutrophils were rapidly attracted to the tumor site by an IL-17 dependent mechanism, but at later stages the induction of the chemokine CXCL2 by Tc17-derived TNF and IFN-γ contributed to sustain neutrophil recruitment. Approximately 10–50 times as many Tc17 effectors were required compared with Tc1 effectors to exert the same level of control over tumor growth. The recruitment of neutrophils was more prominent when Tc17 rather than Tc1 were used to control tumor and depletion of neutrophils resulted in a diminished capacity to control tumor growth.
Spontaneously arising tumors present Ag to the immune system and are recognized by host T cells. The tumor cells, however, are minor variants of the host tissue and express multiple cell surface molecules designed to prevent and abort immune responses to self. It has long been recognized that these protective mechanisms can be circumnavigated if tumor specific host T cells are activated by tumor Ags in vitro and adoptively transferred back into the host. Under these conditions, activated tumor-specific T cells have the capacity to rapidly eliminate large established tumor burdens. Although this is abundantly true in experimental mouse models, adoptive immunotherapy has met with only modest success in humans because in the case of spontaneously tumors the multiple inhibitory mechanisms have already become established and appear to block or disable most of the aggressive properties of the donor cells.
In the last decade, several clinical studies have demonstrated the ability of adoptively transferred tumor-reactive T cells to mediate regression of established tumors (1–3). Initially the effector cells were obtained from PBLs treated with high doses of IL-2, inducing the generation of lymphokine-activated killer cells, which were capable of lysing tumor cell lines in vitro and controlling established pulmonary metastases in a mouse model (4, 5). However, cancer patients treated with lymphokine-activated killer cells therapy had poor responses and experienced toxic effects in different organs due to the administration of IL-2 (6). CD8 CTLs are thought to play a crucial role in tumor rejection, and many groups have focused their efforts on the identification of Ag HLA class I restricted cytotoxic T cell peptides and tumor-associated Ags recognized by CD8 T cells, to isolate, expand, and transfer a large number of activated antitumor CTLs. In fact, this approach has been shown to induce tumor regression in several animal models and human clinical trials (7, 8). In some models, the ability of CTLs to kill tumor or stromal cells is crucial (8–12), but other groups have shown that IFN-γ produced by CD8+ T cells is key for the recruitment of inflammatory T cells and the expansion of other antitumor T cells clones and that perforin and FasL play no role (13–16).
Although it is generally accepted that cytotoxic CD8 T cells are the most effective cell type in adoptive immunotherapy, it is clear that their efficacy is not solely confined or perhaps even dependent on their cytolytic properties and multiple additional effector mechanisms are used in the elimination of the tumor targets.
We showed, earlier, that CD8 T cells can be differentiated into two subsets based on their differential cytokine secretion. Type I CD8+ cytotoxic T cells (Tc1) (17, 18) secrete IFN-γ and IL-2, whereas type 2 CD8+ cytotoxic cells (Tc2) secrete IL-4, IL-5, and IL-10. In our earlier studies we showed that both Tc1 and Tc2 tumor-specific effector populations could eliminate established melanoma, thymoma, or breast cancer tumors. But it was clear that Tc1 and Tc2 used quite different mechanisms in controlling tumor growth. Tumor-bearing mice treated with Tc1 cells survive longer after T cell transfer and Tc1 had an stronger therapeutic effect than Tc2. Tc1 effectors were dependent on their ability to secrete IFN-γ, whereas Tc2 operated by mechanisms that were dependent on the ability to secrete both IL-4 and IL-5 (19–23).
More recently, we have demonstrated that a new subset of IL-17–producing CD8 T effectors cells, termed Tc17, can be generated in vitro and that they have a quite different phenotype from either the Tc1 or Tc2 subsets. Tc17 effectors secrete the signature cytokines IL-17A and IL-17F and little or no IFN-γ, IL-4, or IL-5. They also secrete abundant quantities of TNF, IL-21, and IL-22 as well as chemokines, including CCL3, CCL4, CCL5, CXCL9, and CXCL10 (this paper). In marked contrast to Tc1 or Tc2, in vitro generated Tc17 express no message for perforin, are negative for granzyme B by intracellular staining, and express no cytolytic activity against Ag-loaded targets (24). The Tc17 effectors express message for RORγt but are negative for T bet and GATA 3 and thus seem not to be contaminated with Tc1 or Tc2 differentiated cells. Similar populations have been described in the human (25).
They express low levels of FasL-mediated activity on reinjection in vivo (H. Hamada, unpublished observations) and many of the IL-17–secreting cells become double positive for IFN-γ and IL-17 by day 8 after influenza infection. In a previous study, we found that adoptive transfer of Tc17 cells to naive mice protected against lethal influenza A infection (24) and we decided to test the ability of this new CD8 T cell subset to bring about the rejection of already established B16-OVA melanoma tumors.
Other investigators have already noted the presence of both IL-17+ CD4−, and CD8 T cells in multiple mouse and human tumors. In addition, an analysis of infiltrating lymphocytes isolated from peripheral prostate aspirate showed a good correlation between the presence of Th17 and a lower pathologic Gleason score, suggesting Th17 cells participate in control of prostate cancer (26, 27). Muranski et al. (28) demonstrated that Th17-polarized cells mediated tumor killing of advanced B16 melanoma in a murine model. However, the participation of IL-17–producing CD8 T cells in the control of tumor growth has not been previously described in experimental models.
IL-17 is a T cell proinflammatory cytokine produced by TcRα/β+ CD4− and CD8− thymocytes, as well as by activated T cells (29), γδ T cells (30, 31), and invariant NKT cells (32). IL-17 induces the production of TNF, IL-6, IL-1β, IL-8, and MCP 1 from various cell types (33, 34). In addition, IL-17 is involved in the induction of cyclooxygenase (COX)2 and inducible NO synthase (iNOS)2 in chondrocytes (35), induction of PG E2-mediated osteoclast differentiation factor expression in osteoblasts (36), promotion of stem cell factor and G-CSF–mediated granulopoiesis (37), and enhancement of allorejection (38). Th17 and Tc17 secrete a number of additional cytokines on restimulation, including TNF, IL-21, and IL-22 (24, 39). The role of IL-17–secreting cells in tumor progression is controversial. On the one hand, some studies showed induction of protective tumor immunity in transplantable tumor models using IL17-expressing tumor cell lines (40, 41), whereas other groups had suggested that it can promote tumor growth through an increase in inflammatory angiogenesis or by blocking apoptosis of the tumor cells (42–44).
We sought here to determine whether the Tc17 effector cells are effective at controlling tumor growth, the mechanism by which control is achieved, and the relative effectiveness of the Tc17 subset to other subsets of CD8 T cells.
We show in this study that OVA-specific Tc17 effector cells adoptively transferred into tumor-bearing mice control tumor growth in early and late stage melanoma. The Tc17 effectors are a source for various chemokines and cytokines that elicit further chemokines involved in the attraction of inflammatory cells to the tumor site, including CXCR3+ effector cells and neutrophils that accelerate tumor regression. Our studies establish that IL-17, TNF, and IFN-γ are critical for the recruitment of these cells to the tumor. In addition, Tc17 cells and neutrophils that have been recruited to the tumor, produce further chemokines that are important for the attraction of type 1 lymphocytes (Th1 and Tc1) and for the recruitment of additional neutrophils. Soon after Tc17 cell transfer, neutrophils are attracted to the tumor site by an IL-17–dependent mechanism, but at later stages the induction of the chemokine CXCL2 by Tc17-derived TNF and IFN-γ sustained continuous neutrophil recruitment. The rapid recruitment of CXCR3+ type 1 effector cells to the tumor is crucial for the early control of tumor growth, but is dispensable at later stages in our model, suggesting that neutrophils or CCR5+ type 1 effector cells can compensate for the absence of CXCR3+ Th1 or Tc1 cells.
We compared the effectiveness of Tc17 with that of Tc1 effectors and found that ~10–50 times as many Tc17 effectors are required compared with Tc1 to exert the same level of control over tumor growth. The recruitment of neutrophils was more prominent when Tc17 rather than Tc1 were used to control tumor growth but it was not possible be certain whether this represented a different mechanism as the number of Tc17 cells injected was so much greater than for Tc1. We conclude that Tc17, injected alone, were thus less effective than Tc1 for adoptive immunotherapy and hypothesized that the activity that we saw was the consequence of a less effective mechanism of action. Nevertheless, this study establishes the therapeutic potential of Tc17 cells for the treatment of murine experimental melanoma.
Materials and Methods
Mice and cell lines
Specific pathogen-free, C57BL/6 (CD45.2, Thy-1.2), CD45.1, and Thy-1.1 congenic mice were obtained from the Animal Breeding Facility at Trudeau Institute. OT-1 mice on a C57BL/6 (H-2b) background were originally obtained from Dr. M. Bevan (University of Washington, Seattle, WA). These mice express a transgenic TCR Vα2 and Vβ5 specific for the SIINFEKL peptide of OVA in the context of MHC class I, H2-Kb. Homozygous perforin−/−, TNF−/− (OT-1.TNF), and IFN-γ−/− (OT-1.IFN-γ) deficient C57BL/6 mice were generated by backcrossing OT-1 mice onto specified syngeneic deficient mice (H-2b) and the OT-1, cytokine-deficient, mice were selected from the F2 generation. P14 (TCR-transgenic mice specific for the gp33 peptide of lymphocytic choriomeningitis virus [LCMV]), CCR5, CXCR3, IL-17RA, TNFR, and IFN-γR deficient mice were bred at the Trudeau Institute. Mice were housed in the animal facilities at the Trudeau Institute. Animal experiments were conducted in compliance with institutional animal use guidelines.
The OVA-transfected B16 tumor cell line (B16-OVA) was provided by Dr. E. Lord and J. Frelinger (University of Rochester, Rochester, NY). Cells were maintained in RPMI 1640, supplemented with 10% heat-inactivated FBS, 2 mmol/l L-glutamine, 100 μg/ml kanamycin, 50 μmol/l 2-ME, 0.024 mmol/l sodium bicarbonate, and 600 μg/ml G418 (Invitrogen. Carlsbad, CA).
Preparation and generation of OVA-specific CD8 T cells subsets
Spleens and lymph nodes were collected from OT-1 mice (7–8 wks of age) and single-cell suspensions were prepared by mechanical disruption. Cells were washed and resuspended in RPMI 1640 medium supplemented with 10% FBS, 1 mmol/l MEM sodium pyruvate, 0.1 mmol/l MEM nonessential amino acids, 2 mM L-glutamine, 100 IU penicillin, 100 mg/ml streptomycin (Invitrogen), 4.5 × 10−5 M 2-ME (Sigma-Aldrich, St. Louis, MO).
Live cells were obtained by density gradient centrifugation with Lympholyte M (Cedarlane Laboratories, Hornby, Ontario, Canada). Fc receptor binding was blocked with 2 μg/ml 2.4G2, and cells were stained with anti-CD44 microbeads and CD44hi cells were depleted and then the remaining CD8+ CD44lo T cells were isolated by negative selection using a CD8+ T cell kit (Miltenyi Biotech, Auburn, CA) according to the manufacturer’s protocol. OVA-specific T cells subsets, Tc1 (22) and Tc17 (24) were generated by culturing naive CD8 T cells (2 × 105/ml) with B cell blasts (6 × 105/ml) as previously described. Cells were cultured in presence of IL-2 (20 U/ml, X63. IL-2 supernatants), IL-12 (2 ng/ml), and anti–IL-4 mAb (10 μg/ml, 11B11) for Tc1 or IL-1β (10 ng/ml), IL-6 (20 ng/ml), IL-21 (80 ng/ml), IL-23 (50 ng/ml), and TGF-β (3 ng/ml), anti–IL-4mAb and anti–IFN-γ mAb (10 μg/ml, XMG1.2) for Tc17. It should be noted that we subsequently established that a higher percentage of IL-17–secreting cells is obtained if anti–IL-2 is substituted for IL-2 (H. Hamada and R.W. Dutton, unpublished observations, this protocol was not used in these studies). After 2 d of incubation, an equal volume of complete media containing IL-2 (20 U/ml) was added to the Tc1 cultures. Effectors CD8 T cells subsets were harvested at day 4. The resulting Tc17 and Tc1 effector populations were restimulated in vitro and extensively analyzed to confirm that they expressed the properties established for Tc17 and Tc1 effectors in our previous publication (24).
Adoptive T cell immunotherapy model
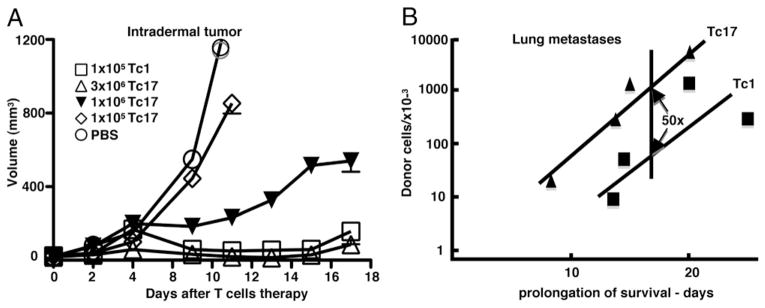
Female CD45.2 or CD45.1 C57BL/6 mice received an intradermal injection containing 2 × 105 B16-OVA melanoma cells resuspended in PBS. At day 7 or 12 after tumor injection, mice that had developed a palpable tumor (110 and 532 mm3, respectively) received an i.v. injection that contained varying doses of Tc1 or Tc17 in vitro generated effector cells. Control groups of mice were injected with Tc17 prepared from LCMV-specific P14 mice or with PBS. Tumor volume was calculated by using the following formula: tumor volume (mm3) = (length) × (width)2 × 0.4 (45). Mice with tumor volumes that equaled or exceeded 1000 mm3 were humanely sacrificed. In other experiments (Fig. 9), the tumor cells were injected i.v. and the tumor grew as lung metastases and mice were monitored for survival.
FIGURE 9.
The relative effectiveness of Tc17 and Tc1 effectors in controlling tumor growth. A, Tumor growth at an intradermal site. Mice with 7 d established intradermal tumors were injected i.v. with graded numbers of Tc17 effectors, 105 Tc1, or left untreated. Tumor growth was measured three times at week using an engineer caliper. Results are representative of three experiments. B, Tumor growth as lung metastases 2 × 105 B16-OVA were injected i.v. on day −7 to develop lung metastases and varying numbers of Tc17 or Tc1 effectors were injected i.v. on day 0. A control group was left untreated. Mouse survival was followed for 50 d. n = 5. The log of the number injected cells was plotted against the prolongation of survival calculated as actual time of death minus the mean time of death of the untreated group (single experiment).
Isolation of tumor-infiltrating lymphocytes and flow cytometry
Tumor-infiltrating lymphocytes (TILs) and leukocytes were isolated from individual intradermal melanoma tumors as described previously (46). 106 TILs were incubated in staining medium containing Abs against Fc receptor, 2.4G2, for 10 min on ice and stained with APC-conjugated MHC class I tetramers (OVA Kb/SIINFEKL) for 1 h at 20°C, followed by the addition of fluorochrome-conjugated Abs (FITC–anti-CD45.2, CD45.1, PECy7-Thy1.2, Pacific orange-CD8, BD Pharmingen, San Diego, CA) for 20 min at 4°C. Additional samples were stained with Pacific blue-F4/80, PECy7-CD11c, APC-AlexaFluor750-CD11b, PE-I-Ab, biotin-CD19 (eBiosciences, San Diego, CA) and APC-CCR3 (R&D Systems, Minneapolis, MN) or FITC-7/4 (Serotec, Oxford, U.K.), PE-Ly6C, biotin-Ly6G, and APC-CD11b. For biotinylated mAb, streptavidin-Pacific orange (Invitrogen) or PE-Cy7 was used as a second-step reagent. Cells were washed twice and then resuspended in 200 μl FACs media containing 1 μg/ml propidium iodide to select viable cells. Analyses were performed on a multiparameter flow cytometer CyAn ADP LX 9 Color (Dakocytomation. Carpinteria, CA) and data were analyzed with FlowJo software (Treestar, Ashland, OR). The total number of cells per gram of tumor was calculated by multiplying the percentage of positive cells described previously by the total number of live lymphocytes and dividing that number by 100. These numbers were divided by the mass of tumor to calculate the number of those TILs per gram of tumor. The OVA Kb/SIINFEKL tetramer was generated by The Trudeau Institute Molecular Biology Core Facility.
Intracellular staining
Lymph node cells, splenocytes, TILs or effector T cells subsets were restimulated by incubation with PMA (10 ng/ml), and ionomycin (500 ng/ml) and were maintained in media with brefeldin A (10 μg/ml, Sigma-Aldrich) for 5 h. Cells were first stained with surface markers, fixed with 4% para-formaldehyde for 20 min at room temperature, and then permeabilized with 0.1% triton X-100 (Sigma-Aldrich). Intracellular cytokine staining was performed using Abs specific for mouse IFN-γ, IL-17, and TNF. Flow cytometry analysis was performed as described previously.
RNA and quantitative PCR
RNA was extracted and purified from tumors, CD8 T cells, or neutrophils by using TRIzol (Invitrogen) and RNeasy kit (Qiagen, La Jolla, CA), sequentially. DNase-treated RNA (2 μg) was reverse transcribed with Oligo dT and SuperScript II (Invitrogen). Quantitative PCR was performed using TaqMan Universal PCR Master Mix, following the Applied Biosystems (Foster City, CA) protocol. Probes for GAPDH, CXCL2, myeloperoxidase (MPO), iNOS2, arginase, IL-17, and COX2 were obtained from Applied Biosystems. Quantitative PCR was performed using a PRISM 7700 instrument (Applied Biosystems). The relative level of mRNA expression for each gene in each tumor was first normalized to the expression of GAPDH in that tumor and normalized to the level of mRNA expression in tumor from control mice and then normalized per gram of tumor.
Immunofluorescent staining of tumor infiltrating neutrophils
Cytospin preparations of neutrophils purified from melanoma tumors were blocked with horse serum (Vector Laborarories, Burlingame, CA) and 2.4G2 for 30 min at room temperature. Endogenous biotin activity was blocked with a commercial biotin-avidin kit (Vector Laboratories). Neutrophils were incubated overnight with biotin-conjugated rat anti-mouse GR-1 and FITC anti-mouse 7/4 (Serotec). Primary Abs were visualized with streptavidin-conjugated to AlexaFluor 594 and anti-FITC conjugated to AlexaFluor 488 (Molecular Probes, Carlsbad, CA). Cytospins were mounted with medium for fluorescence with 4′,6-diamidino-2-phenylindole (Vector Laboratories).
Chemotaxis assays
Lymphocyte chemotaxis was assayed using 24-transwell units (Corning Glass, Corning, NY) with 5 μm (lymphocytes) or 3 μm (neutrophils) pore polycarbonate filter. For lymphocyte chemotaxis, filters were soaked in distilled water containing 10 μg/ml mouse fibronectin during 1 h at 37°C. Then, transwell units were washed with 500 μl distilled water and dried out for 2 h at 37°C. Four-day cultured Th1 or Tc17 cells were harvested and dead cells were removed by density gradient centrifugation with Lympholyte M (Cedarlane). The 2 × 106 Tc17 cells/600 μl RPMI 1640 plus 2% BSA (2% RPMI) were placed in the lower chamber and 106 Th1 cells or bone marrow-derived neutrophils/100 μl 2% RPMI were added to the upper chamber. As a positive control of neutrophil migration some wells were filled with media containing 250 ng/ml CXCL2. To determine the participation of specific chemokines in the attraction of neutrophils or Th1 cells, blocking Abs were added to the lower chamber. Plates were incubated for 1 h at 37°C in a 5% CO2 atmosphere. Transmigrated cells were recovered and the number of neutrophils or Th1 cells was counted on a LSR II flow cytometer (BD Biosciences, San Jose, CA). The results are expressed as the mean ± SD of the chemotactic index (CI). The CI represents the fold-change in the number of cells that migrated in response to the chemoattractant secreted by Tc17 cells divided by the number of cells that migrated in response to control medium.
Statistical analysis
Tumor growth, mRNA levels, absolute number of TILs, and CI were analyzed by two-tailed, paired Student t test or one-way ANOVA.
Results
Adoptive transfer Tc17 effectors treatment leads to regression of melanoma tumors
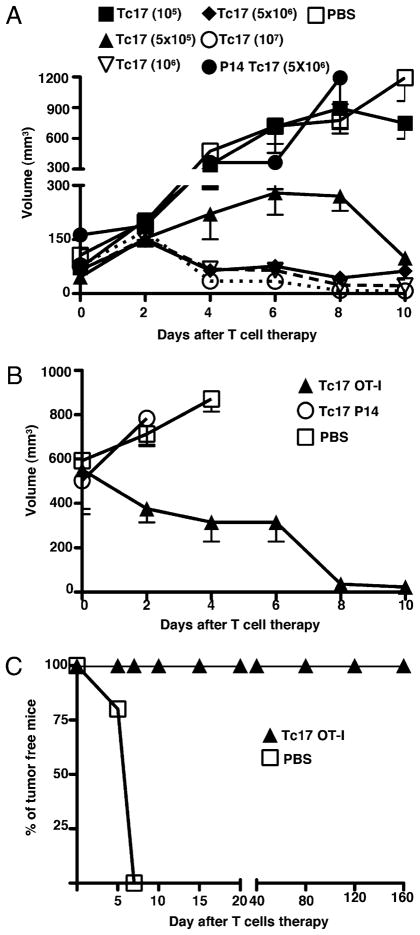
We assessed the therapeutic efficacy of in vitro generated OVA-specific Tc17 effector T cells to control the growth of small (110 mm3) and large (532 mm3) well-established OVA-expressing melanoma tumors. Mice with small tumors received varying numbers of Tc17 cells at day 7 after tumor implantation. One hundred percent of the mice treated with 1 × 106 (open inverted triangles p = 0.0006, two tailed), 5 × 106 (closed diamonds p = 0.0465 two tailed), and 107 (open circles p = 0.0426, two tailed) Tc17 cells completely suppressed tumor growth (Fig. 1A), whereas 5 × 105 effectors (closed triangles p = 0.0003, two tailed) were still suppressive and 105 (closed squares p = 0.145 two tailed) had no effect. As expected, control groups treated with LCMV-specific 5 × 106 Tc17 cells from P14 mice or PBS (Fig. 1A) also developed large tumor masses.
FIGURE 1.
A, Tc17 cells control the growth of established melanoma tumor. Seven groups of C57BL/6 mice were injected with 2 × 105 OVA-expressing B16 cells at day −7. At day 0 five groups of mice were treated with different numbers of Tc17 generated from OT-1 mice. As controls, the other two groups were treated with Tc17 cell generated from P14 mice (closed circles) or PBS (open squares). Tumor growth was measured three times a week using an engineer caliper. Results represent the mean ± SE of five mice from three separate experiments with similar results. B, Tc17 cells control growth of large tumors. The 5 × 106 Tc17 OT-1 (closed triangles) cells were adoptively transferred at day 0 into 12 d tumor-bearing C57BL/6 mice. As controls, two groups of mice were treated with LCMV-specific Tc17 (open circles) or with PBS (open squares). Tumor growth was measured three times at week using an engineer caliper. Results represent the mean ± SE of five mice. C, Tc17 cells prevent tumor implantation and induce long-lasting tumor immunity. The 5 × 106 Tc17 (closed triangles) cells were adoptively transferred at day −1 into C57BL/6 mice, whereas control mice received PBS (open squares). At day 0 mice were challenged with 2 × 105 B16-OVA cells and tumor growth was monitored. Mice that did not develop tumor were rechallenged twice (day 45 and 120). Mean ± SE, five mice.
We also tested whether transferred Tc17 effector cells have the capacity to control larger melanoma tumors. 5 × 106 Tc17 cells (closed triangles) were adoptively transferred into tumor-bearing mice at day 12, rather than day 7. The injected mice still showed an impressive tumor reduction at day 4 after T cell transfer (Fig. 1B) when compared with mice treated with LCMV-specific Tc17 cells (open circles) or with PBS (p = 0.018, two tailed).
The 5 × 106 Tc17 cells were also able to prevent tumor growth in a prophylactic setting. Tc17 cells were injected into recipient mice at day −1 and challenged with 2 × 105 B16-OVA tumor cells at day 0. One hundred percent of mice that received Tc17 cells were tumor-free at day 160, whereas some of the mice from the control group had a palpable tumor at day 5 and all had tumors by day 6 (Fig. 1C) and were sacrificed at day 15 for humane reasons. Mice preinjected with Tc17 effectors that had prevented tumor growth were subsequently rechallenged twice with 2 × 105 B16-OVA tumor cells and there was no evidence of tumor growth during 5 mo. Thus, the prophylactic transfer of OVA-specific Tc17 effector cells before tumor challenge induced a strong protective and long-lasting memory response that efficiently killed melanoma cells.
In other studies, we could find no evidence of epitope spreading in that the parent tumor grew at the same rate on mice actively rejecting B16-OVA as on control mice (data not shown).
Control of tumor growth is partially dependent on IL-17
Tc17 cells are so named because they produce IL-17A and F, but they also secrete many other potent cytokines, including TNF, IL-21, and IL-22. To evaluate the role of IL-17 derived from Tc17 cells, OVA-specific, Tc17 cells were transferred into tumor-bearing, IL-17RA–deficient mice and the control of tumor growth was compared with that in wild-type hosts that had normal expression of IL-17RA on hematopoietic and stromal cells as shown in Table I. To make a quantitative comparison, we compared the percent reduction in tumor volume at day 6. The capacity to control tumor growth by transferred Tc17 cells in mice lacking IL-17RA was modestly impaired, inducing a 54–66% reduction in tumor growth, contrasting with a more extensive reduction in the tumor mass by Tc17 cells transferred to B6 mice (82–84% reduction) (p = 0.016, two tailed). The control of tumor growth by Tc1 effectors was not significantly affected by the absence of IL-17RA (p = 0.717, two tailed) and a similar protection was observed in wild-type and IL-17RA–deficient recipients after Tc1 transfer. The tumors grew at different rates in the wild-type and IL-17RA mice and we therefore compared the tumor growth in the Tc17-treated wild-type mice with that in a wild-type control and the tumor growth in the Tc17-treated IL-17RA−/− mouse with the IL-17RA control. IL-17RA is the receptor for both IL-17A and F and the ability of the Tc17 cells to still induce a significant reduction in the control of tumor growth, in the absence of IL-17RA, suggests that other cytokines are crucial for reducing tumor growth and likely candidates include TNF and IFN-γ, as addressed in experiments below.
Table I.
Tc17 effectors are less able to control tumor growth in IL-17RA–deficient host but show considerable residual antitumor activity
| Mice | C57BL/6 + Tc17 | C57BL6 + Tc1 | IL-17R KO + Tc17 | IL-17R KO + Tc1 |
|---|---|---|---|---|
| Exp 1 | 82.08 ± 0.328 | 82 ± 0.64 | 65.63 ± 7.56 | 90 ± 3.39 |
| Exp 2 | 84.05 ± 15.4 | 78.6 ± 7.6 | 54.16 ± 15.7 | 78.8 ± 8.6 |
The 2.5 × 106 Tc17 or 5 × 105 Tc1 OT-1 cells were adoptively transferred into two groups of 7-d tumor-bearing IL-17RA–deficient and/or C57BL/6 mice. Additional two groups of mice did not receive any treatment. Tumor growth was monitored three times a week and % of tumor reduction in treated compared with untreated mice was calculated at day 6. Mean ± SE, 5 mice. Tc17 (C57BL/6) versus (IL-17RA KO) p = 0.016. Tc1 (IL-17RA KO) versus Tc17 (C57BL/6) p = 0.0717. Tc1 versus Tc17 (IL-17RA KO) p = 0.0045. Tc1 (C57BL/6) versus Tc1 (IL-17RA KO) p = 0.520
Control of tumor growth by Tc17 effectors is independent of perforin expression but is diminished in IFN-γ– and TNF-deficient Tc17 effectors
CTL activity in Tc1 cells is shown by with the capacity to kill target cells in vitro through perforin- and granzyme-dependent mechanisms but we showed previously that this activity is not important for control of tumor growth in vivo (14, 19, 22), whereas IFN-γ was essential. The Tc17 effector cells lack the usual CD8 effector mechanisms but they produce several cytokines in addition to IL-17, including IL-21 and IL-22 (not examined in this study), and proinflammatory cytokines that help to control tumor growth by activating IFN-γ– and TNF-responsive populations, including macrophages, neutrophils, and T cells.
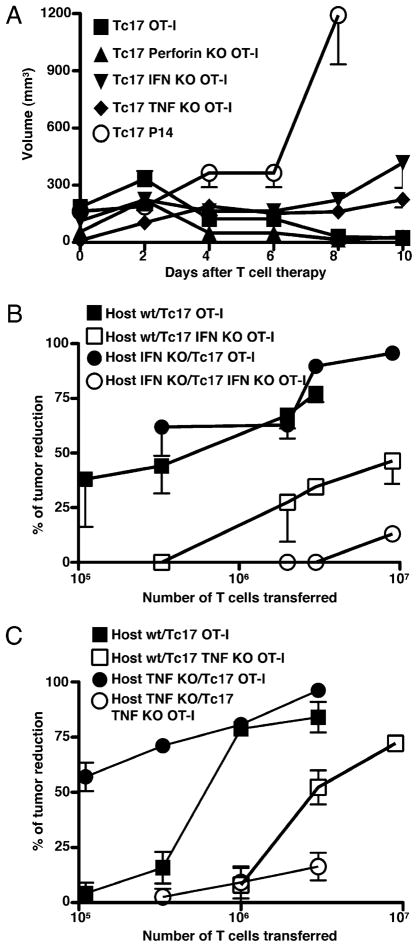
To test the participation of some of these different CD8 effector molecules in Tc17-mediated effects, we generated Tc17 cells using mice deficient in TNF, IFN-γ, or perforin and tested whether they could still control tumor growth. It was clear that Tc17 cells do not need perforin to reduce tumor growth (Fig. 2A, p = 0.08, two tailed); however, IFN-γ (Fig. 2A, p = 0.006, two tailed; Fig. 2B, p = 0.019, two tailed) and TNF (Fig. 2A, p = 0.028, two tailed; Fig. 2C, p = 0.05, two tailed) production by Tc17 CD8 T cells are partially required for inducing a complete tumor rejection. In Fig. 2A, the tumor growth was plotted against time for a single dose (5 × 106) of transferred cells, but in Fig. 2B and 2C, we determined the percent reduction in tumor volume at day 6 and plotted it against the log of the number of adoptively transferred wild-type and cytokine-deficient cells as described previously (14). To test whether cytokine produced by host cells is also important for controlling tumor growth, we transferred different numbers of wild-type or cytokine-deficient Tc17 cells into wild-type C57BL/6 and into TNF- or IFN-γ–deficient C57BL/6 mice and calculated the percentage of reduction of tumor growth at day 6 after T cell transfer. It was clear that the IFN-γ derived from host cells is not important because IFN-γ produced by Tc17 cells was sufficient to control tumor growth, even in the absence of a host source (Fig. 2B). Tumor reduction was dependent on donor TNF and there appeared to be some dependence on host TNF, to efficiently control tumor growth (Fig. 2C). The need for TNF and IFN-γ production by donor Tc17 cells was demonstrated by the fact that ~10-fold more cytokine-deficient donor cells were needed to achieve the same level of tumor control as seen with wild-type donor cells (Fig. 2B, 2C).
FIGURE 2.
A, IFN-γ and TNF, but not perforin, are required for complete control of tumor growth. Groups of tumor-bearing mice were treated with 5 × 106 Tc17 cells generated from OT-1 (closed squares), perforin- (closed triangles), IFN-γ– (close inverted triangles) and TNF- (closed diamonds) deficient OT-1 mice. A control group was treated with LCMV-specific P14 Tc17 (open circles). Tumor growth was monitored three times a week. Data represent the mean ±SE of 10 mice in two separate experiments. OT-1 Tc17 versus OT-1.IFN-γ knockout (KO) Tc17 p = 0.006, two tailed. OT-1 Tc17 versus OT-1.TNF KO Tc17 p = 0.028, two tailed. OT-1 Tc17 versus P14 Tc17 p = 0.005, two tailed. B and C, Control of tumor growth by Tc17 is associated to production of TNF and IFN-γ. C57BL/6-, IFN-γ−, and TNF-deficient mice were injected with 2 × 105 B16-OVA cells. At day 7 mice were treated with different doses of wild-type, IFN-γ– (B) or TNF- (C) deficient Tc17 in all four permutations as shown in the key. The percentage of tumor volume reduction was calculated at day 6 and plotted against number of Tc17. Results represent the mean ± SE of five mice from two separate experiments with similar results.
The finding that IFN-γ was required, at first site, seemed surprising because previous studies demonstrated that Tc17 cells lack the ability to secrete IFN-γ after restimulation when generated in vitro. However, they regain the ability to produce IFN-γ some days after injection back into the animal (24) and most IL-17 secreting cells become double producers. IFN-γ is a cytokine fundamental for induction of IFN-γ induced chemokines like CXCL9, CXCL10, and CXCL11, which we hypothesized could be important in the recruitment of effector T cells to the tumor and help to eliminate malignant cells and a similar role can be envisioned for TNF.
Tc17 cell transfer induces recruitment of donor and host lymphocytes to the tumor by IFN-γ– and TNF- dependent mechanisms
Tc17 cells express a number of proinflammatory cytokines that can, by analogy with Th17, be expected to induce the formation of chemotactic factors involved in the recruitment of lymphocytes to the tumor and we had shown previously that both TNF and IFN-γ played some role in the control of tumor growth. We next analyzed the kinetics of cell migration into the tumor in mice receiving wild-type or cytokine-deficient Tc17 cells.
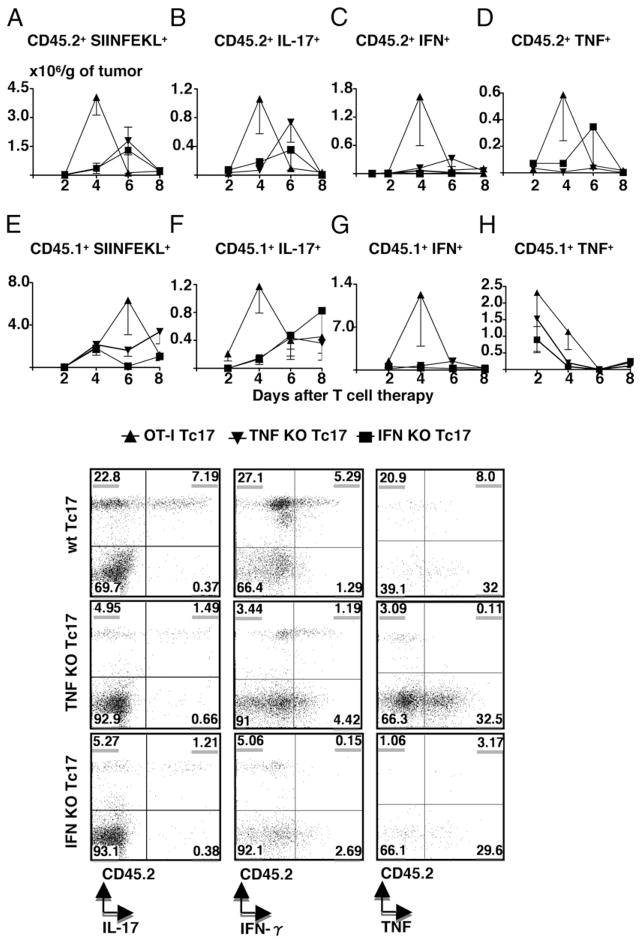
The 5 × 106 Tc17 cells derived from CD45.2+ OT-1 wild-type, or TNF- or IFN-γ–deficient OT-1 mice were injected into CD45.1. C57BL/6 mice 7 d after the injection of tumor, as before. Cohorts of mice were sacrificed at the times indicated, the tumors were removed and the number of infiltrating donor and host cells were determined by flow cytometry, as described in Materials and Methods. Mice that received wild-type Tc17 cells showed a peak in the number of tumor infiltrating tetramer+ donor T cells at day 4 after transfer (Fig. 3A). A somewhat smaller peak was seen in IL-17 secreting donor cells (Fig. 3B), donor IFN-γ–secreting cells (Fig. 3D) and donor TNF-secreting cells (Fig. 3D) at day 4. It was striking that mice injected with IFN-γ (closed squares) or TNF (closed inverted triangles) deficient donor cells showed much smaller numbers of donor infiltrating cells of all types and a delayed kinetics in each of these categories (Fig. 3A–D).
FIGURE 3.
Tc17 therapy induces an increased recruitment of immune cells into tumor. Tumor-bearing mice were treated with 5 × 106 Tc17 wild-type (closed triangles), IFN-γ– (closed squares), and TNF- (inverted closed triangles) deficient OT-1 cells and then, sacrificed at day 2, 4, 6, and 8 after Tc17 transfer. TILs were recovered from fresh tumors, stained with SIINFEKL-APC and specific Abs for CD8, CD45.2, CD45.1, IFN-γ, TNF, and IL-17 and analyzed by flow cytometry for the markers indicated in panels a–h. Absolute numbers of positive cells per tumor were calculated and normalized per gram of tumor. Representative plots from Thy1.2+, CD45.2+, IL-17+, IFN-γ+, or TNF+ are shown. Results represent the mean ± SE of five mice from two separate experiments with similar results.
This same pattern was also seen for the recruitment of CD45.1 host cells into the tumor as shown in Fig. 3, panels e–g. Large numbers of host tetramer positive cells were seen at day 6 (Fig. 3, panel e), whereas smaller numbers of host IL-17–secreting cells peaked at day 4 (Fig. 3, panel f). There was an exception for the endogenous CD8+ CD45.1+ TNF-deficient cells, which had a totally different trend. They peaked at day 2 after transfer of CD45.2 Tc17 cells, began to decline at day 4 posttransfer and they were almost absent at day 6 posttransfer (Fig. 3, panel h)
It was clear that transfer of Tc17 cells, unable to produce TNF or IFN-γ, led to a delayed and less efficient recruitment of both donor and host cells, which correlated with the less effective control of tumor growth. This suggests that both cytokines are important for early recruitment of effector CD8 T cells.
Transfer of effector Tc17 cells induces recruitment of neutrophils and macrophages to the tumor by IFN-γ–and TNF-dependent mechanisms, but does not alter the recruitment of dendritic cells, eosinophils, NK cells, NKT cells, and B cells
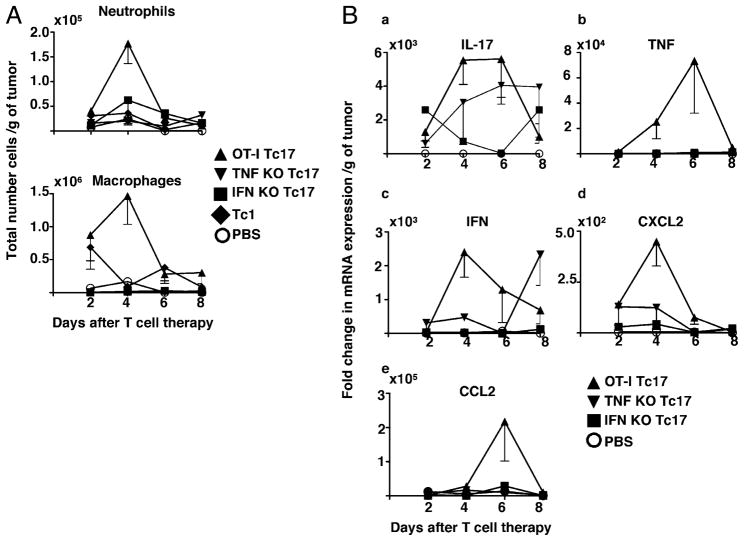
We used the same protocol as above, injecting Tc17 effectors prepared from wild type, IFN-γ and TNF deficient mice into tumor-bearing mice to determine the numbers of activated dendritic cells, NK, NKT, eosinophils, macrophages, neutrophils, and B cells recruited to the tumor site. We found no statistically significant difference in the total numbers of any of the leukocyte populations between the three groups for NK, NKT, eosinophils, and B cells (data not shown). But transfer of Tc17 effectors induced a statistically significant increase in the numbers of tumor-infiltrating neutrophils and macrophages in the treated mice. Recruitment of both neutrophils and macrophages to tumors followed the same kinetics as the T cell response, reaching its peak at day 4 in mice that received wild-type Tc17 cells (Fig. 4A). Again, it was evident that TNF and IFN-γ derived from the Tc17 donor cells were important for the attraction of these cells to the tumor site. Mice that received Tc17 cells that lack TNF and IFN-γ showed a drastic impairment of neutrophil and macrophage migration to the tumor at the peak of the response. It is noteworthy that the somewhat smaller numbers of Tc1 effectors (Fig. 4A) required for the same degree of control of tumor growth did not induce significant increase in the recruitment of either neutrophils or macrophages. It was curious that the peak in macrophage accumulation does not correlate with peak in the chemokine messages (see below) that one might have been expected to be responsible for the macrophage recruitment and this discrepancy remains unexplained.
FIGURE 4.
Tc17 therapy induces recruitment of macrophages and neutrophils into tumor. A, Tumor-bearing mice were treated with 106 Tc1 or 5 × 106 Tc17-, TNF KO and IFN-γ KO OT-1 cells. Control mice were treated with PBS. Mice were sacrificed at day 2, 4, 6, and 8 after Tc17 transfer. Tumor infiltrating cells, recovered from fresh tumors, were stained with specific Abs (F4/80, CD11b, CD11c, 7/4, Ly6G, and Ly6C) and analyzed by flow cytometry. Absolute numbers of neutrophils and macrophages per tumor were calculated and normalized per gram of tumor. Representative data of three independent experiments with similar results is shown. Mean of four mice; bars, SE. B, Elevated levels of mRNA for CXCL2, CCL2, and IL-17 correlated with neutrophil and macrophage recruitment. Tumors from the Tc17-treated mice and the control group were harvested and weighed. DNA-free RNA was isolated from tumors of each experimental group and reversed transcribed. Quantitative PCR was performed to determine relative changes in levels of mRNA. mRNA expression levels of each gene (panels a–e) were calculated as described in Materials and Methods. Data represent the mean ± SE mRNA level of five mice per group.
The relevance of TNF and IFN-γ derived from Tc17 cells in the induction of mRNA expression for TNF, IFN-γ, and IL-17 in the tumor was confirmed by quantitative PCR (Fig. 4B), which shows that IFN-γ and TNF production by Tc17 cells is important for expression of mRNA levels of IL-17 within the tumor, as demonstrated by the very low levels of IL-17 mRNA detected in the tumor of mice that received Tc17 cells lacking IFN-γ and the reduced levels seen in tumors infiltrated by effectors lacking TNF (Fig. 4B, panel a). In a similar way, absence of TNF or IFN-γ production by Tc17 cells was reflected by low levels of mRNA expression for TNF (Fig. 4B, panel b) and IFN-γ (Fig. 4B, panel c) at the tumor site suggesting that these cytokines derived from Tc17 cells are also vital for efficient intratumoral mRNA expression of IL-17, TNF, and IFN-γ.
In further analyses, we measured mRNA levels of chemokines by TaqMan PCR, at different times after transfer of Tc17 cells or Tc17 cells deficient in cytokine production and established that mRNA expression for CXCL2 (neutrophil-attracting chemokine, Fig. 4B, panel d) and CCL2 (macrophage-attracting chemokine, Fig. 4B, panel e) was reduced in tumors of mice treated with Tc17 cells from mice deficient in the production of TNF or IFN-γ as also shown in Fig. 4B, panels d and e. These results demonstrate that although IL-17 is the signature cytokine produced by Tc17 cells, the capacity to recruit inflammatory cells to the tumor and induce expression of protective cytokines is linked to their capacity to produce TNF and IFN-γ.
Tc17 cell transfer induces activation of tumor-infiltrating neutrophils, accelerates death of melanoma cells, and is crucial for control of tumor growth
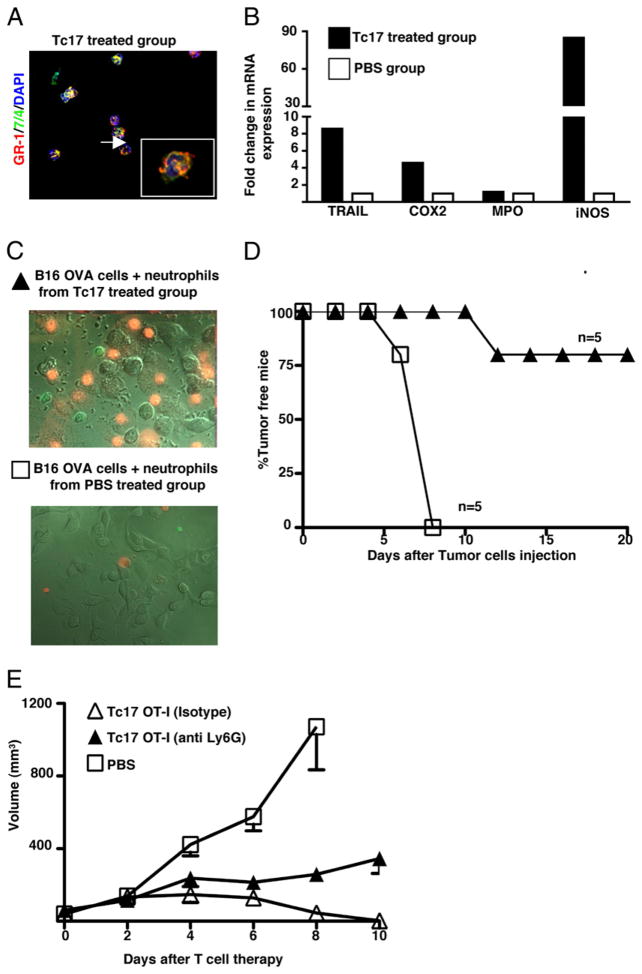
To know more about the biological activity and the activation state of tumor-infiltrating neutrophils, we isolated them from established tumors at day 4 after Tc17 injection and assessed the quality of the preparation by staining cytospin cell suspensions with Abs that recognize neutrophil surface markers, such as Gr-1 and 7/4. Only cells that are double positive for Gr-1 and 7/4 (data not shown) and displayed the characteristic multilobulated nuclei (Fig. 5A) were seen. To determine the activation state of neutrophils in the tumor, mRNA was isolated from infiltrating neutrophils from each experimental group and the relative changes in mRNA expression were determined by quantitative PCR.
FIGURE 5.
Purification and characterization of neutrophils from tumors of mice treated with Tc17. A, Mice with 7 d established tumors were treated with 5 × 106 Tc17 OT-1 cells or PBS and then, tumor infiltrating neutrophils were purified at day 4. Cytospin preparations from treated and control group were stained with anti-mouse GR-1 and 7/4. B, Elevated gene expression in neutrophils from Tc17-treated mice. DNA-free RNA was isolated from purified neutrophils of each experimental group and reversed transcribed. Quantitative PCR was performed to determine relative changes in levels of mRNA for TRAIL, COX2, MPO, and iNOS (iNOS2). The relative level of mRNA expression for each gene in each group was first normalized to the expression of GAPDH and then, normalized to the level of mRNA expression in neutrophils from control group. Data represent the mean ± SE mRNA level of a pool of 15 mice per group of two separate experiments with similar results. C, Neutrophils purified from Tc17-treated mice kill tumor cells in vitro. The 2 × 106 tumor infiltrating neutrophils from Tc17- (closed triangles) or PBS- (open squares) treated group were cocultured with 106 B16-OVA cells. A movie recorded events during a 2-h period using a Zeiss AxioVert 200M. Cells were imaged with differential interference contrast optics and the fluorescent signals were captured with the appropriate filter sets for GFP and propidium iodide. In stills from the movie, GFP+ neutrophils, isolated from treated mice, forming clusters with dying melanoma cells are shown in the upper panel. In contrast only a few neutrophils, isolated from tumors of control group were found in close proximity to live melanoma cells, lower panel. D, Neutrophils limit tumor growth in vivo. The 2 × 105 surviving tumor cells from the previously described panel (see text for more details) were injected into C57BL/6. Tumor growth was followed and the percentage of tumor-free mice was plotted. Mean ± SE, five mice per group. E, Neutrophils play an important role in the control of tumor growth. Two groups of tumor-bearing mice were treated with Tc17 OT-1 cells at day 0 (triangles). At day −1, 0, 1, 2, 4, and 6 one group of mice received intratumor injections of mouse anti-Ly6G (250 μg/mouse, closed triangles), isotype control (open triangles), or PBS (open squares). Tumor growth was monitored three times per week. Data represent the mean ± SE of five mice in one of two separate experiments.
Transfer of Tc17 cells to tumor-bearing mice was associated with a drastic activation of tumor infiltrating neutrophils, which had an enhanced expression of mRNA for TRAIL, COX2, and iNOS2 as shown in Fig. 5B. Thus, Tc17-derived soluble factors are not only important for the attraction of neutrophils to the tumor site, but also for their activation. Message for MPO was not affected.
We also evaluated the killing capacity of neutrophils isolated from Tc17-treated mice by observing neutrophil-tumor cell interactions. GFP+ neutrophils, isolated from treated mice, forming clusters with dying melanoma cells are shown in Fig. 5C, upper panel. In contrast only a few neutrophils, isolated from tumors of control group were found in close proximity to live melanoma cells, Fig. 5C, lower panel. In the movie, itself, it could be seen that tumor cells died after interaction with the activated neutrophils. To have a more quantitative idea about the killer capacity of activated neutrophils, we collected the remaining supernatants and cells from the Petri dish used for recording neutrophil-melanoma cell interactions and the frequency of dead cells was quantified by flow cytometry. We found 5% of dead tumor cells in cultures that had neutrophils isolated from Tc17-treated mice, compared with 1% of dead tumor cells in cultures containing neutrophils isolated from PBS-treated groups (data not shown). Although the frequency of dying tumor cells was higher in wells that contained neutrophils purified from tumors of Tc17-treated mice, it was not impressive. To confirm that neutrophils were inflicting damage to tumor cells, we recovered melanoma cells from the cultures incubated with neutrophils isolated from Tc17- or PBS-treated groups, injected equal numbers (2 × 105 tumor cells) into B6 mice and monitored the presence or absence of tumors. The PBS group began to develop tumors by day 5 and all had tumors by day 8, whereas in the Tc17-treated group, only one of five mice developed a tumor at day 11 and four of them (75%) remained tumor free until the end of the experiment (day 20 after tumor inoculation) as shown in Fig. 5D.
The relevance of neutrophils in the control of tumor growth was confirmed by the elimination of this cell population with the injection of a neutrophil depleting Ab. Treatment with anti-Ly6G resulted in a statistically significant reduction in the capacity of Tc17-treated mice to control tumor growth (Fig. 5E, p = 0.0016, two tailed). Similar results were obtained using a different neutrophil depleting Ab (RB6, anti-GR-1), data not shown.
These findings indicate that factors from transferred Tc17 cells are important to recruit and activate neutrophils at the tumor site, making them capable to induce damage to tumor cells and accelerate tumor eradication. Tumor infiltrating-neutrophils are thus a critical component in the induction of Tc17-dependent immunity against melanoma tumors.
The ability of the transferred Tc17 effector cells to attract neutrophils is associated with the production of IL17, TNF, and IFN-γ
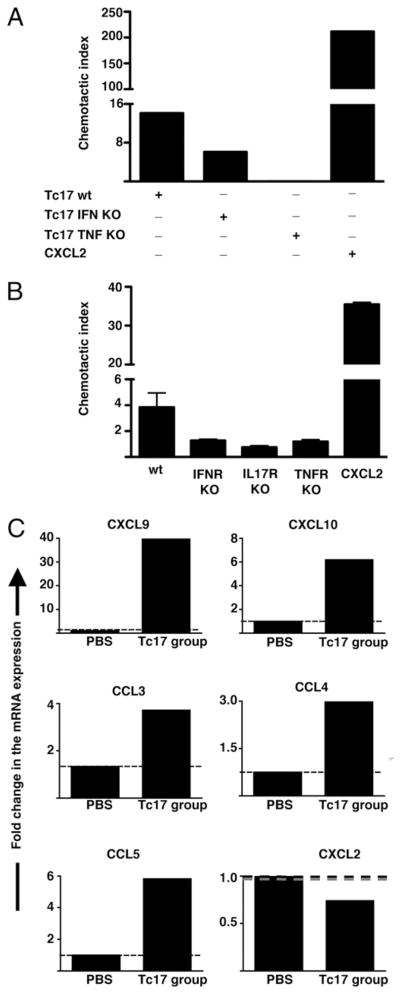
In our Tc17 transfer model, we had shown previously that proinflammatory cytokines derived from Tc17 cells induced the production of a variety of chemokines by resident stromal cells, tumor cells, or recently recruited inflammatory cells. It is also likely, however, that Tc17 cells, themselves, could be an additional source of chemokines implicated in the attraction of type I effector cells or neutrophils. To prove Tc17 cells are producing chemokines that attract neutrophils and to evaluate the role of Tc17-associated cytokines in neutrophil chemotaxis, we measured the attraction of neutrophils by cytokine deficient- and wild-type Tc17 stimulated with PMA and ionomycin. As a positive control for neutrophil chemoattraction, we loaded the low chamber with the classic neutrophil-chemoattractant CXCL2. Stimulated Tc17 cells were able to attract neutrophils, but they were less efficient than CXCL2 alone (Fig. 6A). In addition, it was clear that TNF and IFN-γ produced by Tc17 are both important for efficient neutrophil attraction, perhaps because they are involved in the autocrine induction of chemotactic factors by Tc17 cells.
FIGURE 6.
Attraction of neutrophils by Tc17 cells depends on IL-17, IFN-γ, and TNF production. A, Activated Tc17 effectors attract neutrophils. Mouse bone marrow neutrophils were placed in a transwell chamber containing 2 × 106 in vitro generated wild-type Tc17 OT-1 and IFN-γ– or TNF-deficient OT-1 cells in the bottom chamber. Neutrophils that migrated toward CXCL2, wild-type, or cytokine-deficient Tc17 effectors were collected and analyzed by FACS. The results are expressed as the mean ± SEM of the CI (see Materials and Methods) of triplicate cultures. B, Neutrophils need TNFR or IFN-γ R for activation. Tc17 cells prepared from wild-type OT-1 mice were stimulated for 3 h with PMA and ionomycin and plated on the bottom of a chemotaxis chamber. Then, 106 bone marrow neutrophils from different mouse strains, C57BL/6 Thy1.1, IL-17R, TNFR, or IFN-γ R deficient were deposited on the top chamber. As a positive control for neutrophil migration some wells were filled with media containing 250 ng/ml CXCL2. The number of transmigrated neutrophils was evaluated by FACS and the CI was calculated. C, Chemokine expression of activated neutrophils. Tumor infiltrating neutrophils were purified from tumor-bearing mice treated with Tc17 OT-1 cells or PBS. Then DNA-free RNA was isolated and reversed transcribed. Quantitative PCR was performed to determine relative changes in levels of CXCL9, CXCL10, CCL3, CCL4, CCL5, and CXCL2. The relative level of mRNA expression for each gene in each group was first normalized to the expression of GAPDH and then, normalized to the level of mRNA expression in neutrophils from PBS-treated group. Data represent the mean ± SE of a pool of 15 and 30 mice from Tc17- and PBS-treated group respectively of two separate experiments with similar results.
It is also likely that cytokines produced by Tc17 cells can activate neutrophils, inducing production of chemotactic factors that can enhance neutrophil chemotaxis. To test this latter possibility, we evaluated the migratory capacity of different cytokine receptor-deficient neutrophils, isolated from bone marrow, toward chemotactic factors produced by stimulated Tc17 cells. Again, neutrophils migrated more efficiently toward CXCL2, compared with the migration promoted by simulated Tc17 cells. We found a reduction in the migratory capacity of neutrophils from cytokine receptor-deficient mice (Fig. 6B). This was surprising but suggested that somehow the cytokines produced by Tc17 cells were responsible for improving neutrophil migration, perhaps by induction of chemokine receptors.
It is also possible that the cytokine-activated neutrophils can be a source of chemokines that are important for the attraction of more neutrophils. To test this, we isolated neutrophils from tumors of PBS- and Tc17-treated mice and evaluated the production of different chemokines at the mRNA level. Although CXCL2 was detected in neutrophils activated in vitro (data not shown), neutrophils isolated from Tc17-treated mice produced low mRNA levels for that chemokine. Interestingly, neutrophils isolated from Tc17-treated mice produced chemotactic factors that are important for the attraction of type 1 effector cells. They expressed considerable mRNA levels of CXCL9, moderate mRNA levels of CXCL10 and CCL5 and showed a poor induction of mRNA expression for CCL3 and CCL4 (Fig. 6C). These results show that activated Tc17 are able to attract neutrophils and that cytokines produced by Tc17 are somehow stimulating neutrophils to enhance their migratory abilities. In addition, activated neutrophils are a good source of chemokines implicated in the attraction of type 1 effector cells that are crucial for tumor control.
Tc17 cells produce chemokines that attract in vitro generated Th1 cells
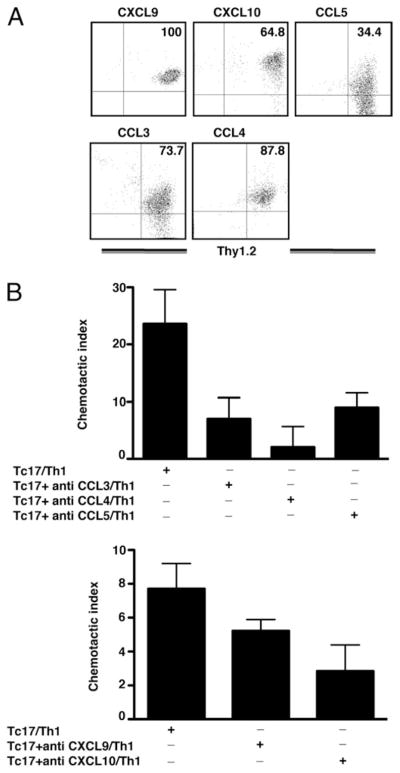
Studies have shown that infiltrating CD8 T cells can produce CXCL9, CXCL10, and CCL5 (47, 48) that can recruit type I effector cells, To demonstrate that in vitro generated Tc17 cells are a source of chemokines, we analyzed expression of CCL3, CCL4, CCL5, CXCL9, and CXCL10 by flow cytometry. We established that all Tc17 cells produced CXCL9 (100%), but only a smaller percentage of Tc17 cells produced CCL4 (87.8%), CCL3 (73.7%), CXCL10 (64.8%), or CCL5 (34.4%) as shown in Fig. 7A. To demonstrate that chemokines produced by activated Tc17 cells were functional, we set up a chemotaxis assay for in vitro generated Th1 cells. Stimulated Tc17 cells were able to attract in vitro generated Th1 cells as shown in Fig. 7B and it was clear that blocking Abs were able to reduce migration of Th1 cells. Anti-CCL3 (p = 0.050, two tailed), anti-CCL4 (p = 0.028, two tailed), and anti-CXCL10 (p = 0.047, two tailed) showed the greatest reduction in the CI suggesting that these chemokines were the most effective for recruiting Th1 cells. In contrast, Abs directed against CCL5 (p = 0.678, two tailed) and CXCL9 (p = 0.196, two tailed) had less impact on Th1 migration. These results show that activated Tc17 cells are able to recruit Th1 cells through the production of chemokines induced by proinflammatory chemokines.
FIGURE 7.
Chemokine production by Tc17 effectors. A, Tc17 cells produce chemokines involved in the attraction of Th1 cells. Four-day in vitro generated Tc17 OT-1 cells were stimulated with SIINFEKL peptide for 3 h in RPMI media containing brefeldin A. CD8 T cells were stained with Abs specific for CXCL9, CXCL10, CCL5, CCL3, and CCL4 and analyzed by FACs. B, Chemotactic activity of chemokines produced by Tc17 cells evaluated by chemotaxis assays. Tc17 cells or Tc17 and blocking Abs against the indicated chemokines were put in the bottom of a chemotaxis chamber. The 106 Th1 cells were deposited in the top chamber, and the Th1 cells that migrated to the bottom chamber were enumerated by FACS analysis after 1 h of incubation. The CI was calculated for all groups (see Materials and Methods).
Rapid recruitment of CXCR3+ effector cells is key for the early control of melanoma growth
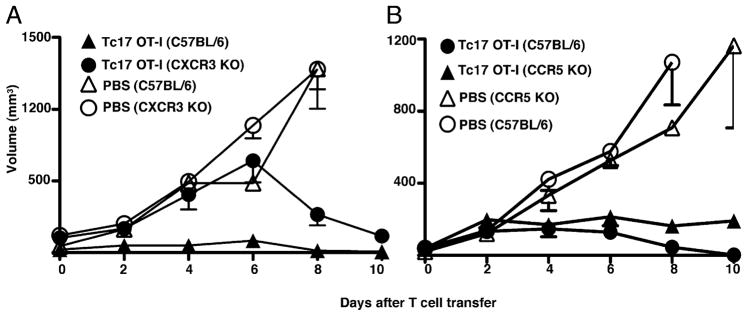
Tc17 cells and neutrophils are a considerable source of IFN-γ–induced chemokines and thus they are possibly attracting CXCR3+ T cells that can enhance tumor immunity. To confirm that CXCR3+ populations are important for the control of experimental melanoma, we transferred Tc17 cells to CXCR3-deficient mice and monitored tumor growth. Even though CXCR3-deficient mice that received Tc17 cells were still able to eliminate the tumor cells (Fig. 8A, closed circles, p = 0.035, two tailed), there was some indication of impaired immunity at early stages of tumor growth. This finding suggests that recruitment of CXCR3+ cells may be important. The control of tumor growth at later times seems to be associated to the massive numbers of infiltrating neutrophils detected by FACS at day 11 after Tc17 cell transfer (data not shown). Tc17 cells and neutrophils produce chemokines that can attract CCR5+ cells too, which can be important for generation of protective tumor immunity. To explore this alternative hypothesis, we transferred Tc17 cells to CCR5-deficient mice. In the absence of CCR5 expression, tumor volume was a slightly bigger, but significantly different from tumor volume in wild-type mice (p = 0.0032, two tailed) (Fig. 8B). This means, CCR5 is minimally important for tumor immunity in our transfer model and CCR5 absence is compensated by infiltration of tumors by neutrophils and CXCR3 effector T cells (data not shown). These results suggest that chemokines produced by Tc17 and neutrophils, specific for CXCR3 and CCR5 receptors, are required for the recruitment of effector cells that maintain optimal levels of chemokines and cytokines crucial for sustaining waves of migration of new inflammatory cells armed with antitumor cytokines.
FIGURE 8.
Early recruitment of CXCR3+ effector cells is important for rapid induction of tumor immunity. A, At day −10 groups of C57BL/6-(triangles) or CXCR3-deficient (circles) mice were injected with 2 × 105 OVA-expressing B16 cells. At day 0 two groups of mice were treated with 5 × 106 Tc17 generated from OT-1 mice (closed symbols). PBS injected wild-type (open triangles) and CXCR3 deficient (open circles) served as controls. Tumor growth was measured three times at week using an engineer caliper. Results represent the mean ± SE of nine mice in two independent experiments. B, CCR5-deficient (triangles) or wild-type (circles) mice were injected with 2 × 105 OVA-expressing B16 cells. At day 0 mice were treated with 5 × 106 Tc17 generated from OT-1 mice (closed symbols) or PBS (open symbols). Seven days later mice were injected as indicated and tumor growth was measured three times per week. Data represent the mean ± SE of four mice in one of two experiments with similar results.
Tc17 effectors are less effective than Tc1 effectors in the control of tumor growth
Finally, we sought to evaluate the relative effectiveness of Tc17 and Tc1 effectors in the control of tumor growth. We had previously shown that adoptive transfer of varying numbers of Tc1 or Tc2 effectors into tumor-bearing mice could control the growth of established tumors but that Tc1 were more effective than Tc2 in that only one twentieth of the number of Tc1 cells were required to effect the same level of control. In this paper we sought to make a similar comparison between the effectiveness of Tc17 and Tc1 effectors. Varying numbers of Tc17 and Tc1 effectors were transferred into tumor-bearing mice 7 d after tumor injection and the size of the tumors monitored for the next 20 d (data not shown). Comparisons proved rather hard to assess as the tumor mass grew more rapidly at first in the case where tumor growth was subsequently controlled so it was a problem at which point to make the comparison. Those treatments that did well at day 8 appeared to do poorly at day 3 with the crossover point around day 5. The results were somewhat variable but in an initial experiment 5 × 106 Tc17 effectors were as effective, and 5 × 105 Tc17 cells were less effective, than 5 × 105 Tc1 cells at controlling tumor growth (data not shown), suggesting that Tc17 were roughly comparable in effectiveness with Tc2. A second experiment gave comparable results with 5 × 106 Tc17 being comparable to 5 × 105 Tc1 as shown in Fig. 9A.
Our earlier studies had suggested that Tc17 homed well to lung after influenza challenge (24) and we carried out a second experiment to compare the effectiveness of Tc17 and Tc1 on the control of B16-OVA growing as lung metastases. In this model, it is not possible to follow the growth of the tumor without the sacrifice of the animal, so we determined the relative numbers of effectors required to bring about the same prolongation of survival. It can be seen (Fig. 9B) that Tc1 were again more effective than cells of the Tc17 subset in bringing about a 20 d prolongation of survival that was achieved with 50-fold fewer Tc1 effectors. Neither of these can be considered to yield a definitive number for the ratio but it is clear that Tc17 or Tc2 effectors administered alone are significantly less effective than Tc1.
Discussion
In these studies described, we have used a tumor transfected with OVA so that we may use cells from the OVA-peptide–specific OT-1 TCR transgenic mouse as source of tumor Ag-specific CD8 T cells. In other studies, not shown, we have demonstrated that naive, rather than effector, OT-1 cells behave in the same way as the host response does to intrinsic tumor Ags in that the naive cells become activated, divide many times, but are blocked at the effector stage, and do not affect the growth of the tumor. The use of CD8 T cells from transgenic mice make it possible to prepare large numbers of Tc17 and Tc1 effectors of identical Ag specificity and to track them in their response to the tumor.
We have come to realize that the control of tumor growth is influenced by multiple mechanisms and that removal of one pathway does not necessarily abolish tumor rejection but rather, other mechanisms can still slow the rate of growth but to a lesser degree. We have also come to appreciate that the complexity of the events increases as the sequence of events in the control of tumor growth proceeds and we have deliberately focused, in this study, on the early events in Tc17 adoptive therapy, which we believe can be more adequately understood in terms of the parameters we have measured.
It was clear from our studies that as few as 1 × 106 Ag-specific Tc17 effectors could bring about the complete suppression of tumor growth (Fig. 1) by an Ag-specific mechanism and 5 × 105 still manifested some level of suppression. The Tc17 were also effective against larger tumor masses. They could also completely suppress tumor growth if injected prior to the tumor innoculation and established long-term immunity. We saw no evidence of epitope spreading in that the parent tumor, B16, grew no slower on mice that were controlling the growth of the B16-OVA tumor after receiving Tc17 effectors than on the control mice that had received neither B16-OVA nor the Tc17 effectors (data not shown). Tc17 effectors secrete IL-17 (A and F), often referred to as the “signature cytokine” which can lead to a focus on this single element. But Tc17 effectors also secrete copious quantities of TNF, IFN-γ, IL-21, IL-22, and numerous chemokines and their effect on the host can be expected to have multiple components and to be very complex.
Studies with cytokine-deficient or cytokine receptor-deficient hosts established that donor derived IL-17, TNF, and IFN-γ were all involved in suppression. The involvement of IFN-γ might seem at odds with the lack of IFN-γ expression in the in vitro generated effectors but we had shown previously that Tc17 effectors regained the ability to secrete IFN-γ several days after they were injected back into compatible hosts (24).
The question of whether the phenotype of the in vitro generated effectors we have described is stable also extends to the issue of cytotoxicity. We showed, however, that Tc17 effectors derived from CD8 T cells from perforin-deficient mice were as effective as those derived from wild-type mice. The role of FasL and TRAIL in the function of the injected effectors was not examined but they lacked these killing mechanisms prior to injection.
The injection of Tc17 effectors led to a marked increase in the recruitment of both donor and host T cells into the tumor at day 4 and this recruitment was dependent on the ability of the donor cells to make both IFN-γ and TNF. The injection of Tc17 effectors also led to the recruitment of neutrophils and macrophages into the tumor mass by day 4 but other non-T cell host lineages were not affected. The neutrophils and macrophage recruitment was also dependent on both IFN-γ and TNF from the donor T cells.
The dependence of the recruitment on donor cell cytokines suggested that these must elicit chemokines at the tumor site and indeed this was found to be the case. CXCL2 and CCL2 were enhanced in the tumor site after Tc17 transfer but only in mice that had received wild-type effectors and not in mice that received either IFN-γ– or TNF-deficient effectors. The Tc17 effectors themselves secreted additional chemokines, including CCL3, 4, and 5, and CXCL9 and 10. In yet a further complication, the recruited neutrophils were the source of further chemokines and became activated as the result of the cytokines released by the Tc17 effectors. The neutrophils were shown to control of tumor growth in vitro and were active in vivo as control of tumor growth in vivo was diminished when the accumulation of neutrophils was blocked with Ab to Ly6G.
Although direct cytolytic attack on the tumor by Tc17 effectors that had regained some FasL or TRAIL-mediated cytolytic activity cannot be ruled out, other mechanisms were clearly more important. The experiments (Fig. 8) in which Tc17 effectors were transferred into tumor-bearing recipients deficient in CXCR3 or CCR5 indicated that there was an early phase of tumor control dependent on the recruitment of CXCR3+ cells, followed by a later phase dependent on the recruitment of CCR5+ lymphocytes.
When we titrated Tc17 and Tc1 effectors to determine the relative numbers needed to bring about the same level of control over tumor growth we found that ~3 × 106 was the smallest number of Tc17 effectors to completely suppress tumor growth, whereas only Tc1 1 × 105 effectors were equally effective. We had found that Tc17 homed very effectively to the lung after influenza challenge (data not shown) and hypothesized that they might be more effective at controlling tumor growth in the lung. We were not able to monitor tumor growth in the same way as when the tumor was visible at the intradermal site but we could plot the relative numbers of Tc17 and Tc1 cells to achieve the same prolongation of survival. Again, ~50-fold less Tc1 than Tc17 effectors were required to achieve the same 20-d prolongation of survival.
These comparisons raise the issue as to whether the activity of the Tc17 effectors is due to a small number of Tc17 effectors that revert to Tc1 or a Tc1/17 hybrid phenotype. At first it seemed that a difference in the mechanism of action of the two populations might argue against this interpretation. However, suppression of tumor growth by Tc1 and Tc17 effectors are both dependent on the ability to make IFN-γ and Tc1 were previously shown to be also dependent on the ability to make TNF and/or LT-α (23). Neither population is dependent on perforin-mediated lysis.
In contrast, we found that very few neutrophils were recruited when Tc1 effector were used to control tumor growth, as shown in Fig. 4. In contrast, many neutrophils were recruited when Tc17 effectors were injected and blocking the recruitment reduced the suppression of tumor growth. The lack of discernable neutrophil recruitment after Tc1 injection may result from the smaller number of Tc1 cells needed for effective control of tumor growth but still implies that Tc1 must exert control by some mechanism not involving neutrophil recruitment.
We conclude that Tc17 effectors can control tumor growth by a complex series of mechanisms of which it is likely that only some have been identified in this study. The Tc17 effectors enter the tumor, secrete chemokines, and cytokines. The cytokines induce the production of additional chemokines. These chemokines collectively lead to an early recruitment of host CD4 and CD8 T cells, and a later recruitment of and activation of neutrophils and macrophages. Some of these mechanisms may be shared with Tc1 elicited mechanisms and some of which may differ but that there is no clear advantage in the use of Tc17 effectors rather than Tc1 effectors in this experimental model.
Acknowledgments
We are indebted to Drs. Kai McKinstry, Tara Strutt, and Susan Swain for helpful discussions.
This work was supported by National Institutes of Health Grant CA71833 and by the Trudeau Institute.
Abbreviations used in this paper
- CI
chemotactic index
- COX
cyclooxygenase
- KO
knockout
- LCMV
lymphocytic choriomeningitis virus
- MPO
myeloperoxidase
- iNOS
inducible NO synthase
- Tc1
Type I CD8+ cytotoxic T cells
- Tc2
Type II CD8+ cytotoxic T cells
- Tc17
IL-17–producing CD8 T cells
- TIL
tumor-infiltrating lymphocyte
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Chang AE, Aruga A, Cameron MJ, Sondak VK, Normolle DP, Fox BA, Shu S. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J Clin Oncol. 1997;15:796–807. doi: 10.1200/JCO.1997.15.2.796. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Yoshizawa H, Sakai K, Cameron MJ, Sondak VK, Shu S. Clinical observations on adoptive immunotherapy with vaccine-primed T-lymphocytes secondarily sensitized to tumor in vitro. Cancer Res. 1993;53:1043–1050. [PubMed] [Google Scholar]
- 3.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm EA, Robb RJ, Roth JA, Neckers LM, Lachman LB, Wilson DJ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983;158:1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulé JJ, Shu S, Schwarz SL, Rosenberg SA. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984;225:1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 9.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 10.Smyth MJ, Kershaw MH, Darcy PK, Trapani JA. Adoptive transfer: the role of perforin in mouse cytotoxic T lymphocyte rejection of human tumor xenografts in vivo. Xenotransplantation. 1998;5:146–153. doi: 10.1111/j.1399-3089.1998.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402–2412. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Krauss JC, Plautz GE, Mukai S, Shu S, Cohen PA. T cell-mediated tumor rejection displays diverse dependence upon perforin and IFN-gamma mechanisms that cannot be predicted from in vitro T cell characteristics. J Immunol. 2000;165:7116–7124. doi: 10.4049/jimmunol.165.12.7116. [DOI] [PubMed] [Google Scholar]
- 13.Dobrzanski MJ, Reome JB, Dutton RW. Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection. J Immunol. 2001;167:424–434. doi: 10.4049/jimmunol.167.1.424. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW. The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol. 2004;173:1738–1743. doi: 10.4049/jimmunol.173.3.1738. [DOI] [PubMed] [Google Scholar]
- 15.Lurquin C, Lethé B, De Plaen E, Corbière V, Théate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meunier MC, Delisle JS, Bergeron J, Rineau V, Baron C, Perreault C. T cells targeted against a single minor histocompatibility antigen can cure solid tumors. Nat Med. 2005;11:1222–1229. doi: 10.1038/nm1311. [DOI] [PubMed] [Google Scholar]
- 17.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 19.Helmich BK, Dutton RW. The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and homing properties of Tc1 and Tc2 effectors. J Immunol. 2001;166:6500–6508. doi: 10.4049/jimmunol.166.11.6500. [DOI] [PubMed] [Google Scholar]
- 20.Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA. CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer. J Immunol. 2006;177:8191–8201. doi: 10.4049/jimmunol.177.11.8191. [DOI] [PubMed] [Google Scholar]
- 21.Ye Z, Tang C, Xu S, Zhang B, Zhang X, Moyana T, Yang J, Xiang J. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell Mol Immunol. 2007;4:277–285. [PubMed] [Google Scholar]
- 22.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Dutton RW. Tc1 and Tc2 effector cell therapy elicit long-term tumor immunity by contrasting mechanisms that result in complementary endogenous type 1 antitumor responses. J Immunol. 2004;172:1380–1390. doi: 10.4049/jimmunol.172.3.1380. [DOI] [PubMed] [Google Scholar]
- 23.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Hylind JC, Dutton RW. Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res. 2004;64:406–414. doi: 10.1158/0008-5472.can-03-2580. [DOI] [PubMed] [Google Scholar]
- 24.Hamada H, Garcia-Hernandez MdeL, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 27.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 30.Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 31.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16:541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 34.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 35.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 36.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 38.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 39.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 41.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 42.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 43.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 44.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 45.Ohta S, Tsukamoto H, Watanabe K, Makino K, Kuge S, Hanai N, Habu S, Nishimura T. Tumor-associated glycoantigen, sialyl Lewis(a) as a target for bispecific antibody-directed adoptive tumor immunotherapy. Immunol Lett. 1995;44:35–40. doi: 10.1016/0165-2478(94)00177-s. [DOI] [PubMed] [Google Scholar]
- 46.García-Hernández ML, Hernández-Pando R, Gariglio P, Berumen J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology. 2002;105:231–243. doi: 10.1046/j.1365-2567.2002.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catalfamo M, Karpova T, McNally J, Costes SV, Lockett SJ, Bos E, Peters PJ, Henkart PA. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity. 2004;20:219–230. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- 48.Pharoah DS, Varsani H, Tatham RW, Newton KR, de Jager W, Prakken BJ, Klein N, Wedderburn LR. Expression of the inflammatory chemokines CCL5, CCL3 and CXCL10 in juvenile idiopathic arthritis, and demonstration of CCL5 production by an atypical subset of CD8+ T cells. Arthritis Res Ther. 2006;8:R50. doi: 10.1186/ar1913. [DOI] [PMC free article] [PubMed] [Google Scholar]