Abstract
Staphylococcal enterotoxins (SEs) are major cause of foodborne diseases, so sensitive detection (<1 ng/ml) methods are needed for SE detection in food. The surface area, geometric and physical properties of gold nanoparticles make them well-suited for enhancing interactions with biological molecules in assays. To take advantage of the properties of gold nanoparticles for immunodetection, we have developed a gold nanoparticle-based enhanced chemiluminescence (ECL) immunosensor for detection of Staphylococcal Enterotoxin B (SEB) in food. Anti-SEB primary antibodies were immobilized onto a gold nanoparticle surface through physical adsorption and then the antibody–gold nanoparticle mixture was immobilized onto a polycarbonate surface. SEB was detected by a “sandwich-type” ELISA assay on the polycarbonate surface with a secondary antibody and ECL detection. The signal from ECL was read using a point-of-care detector based on a cooled charge-coupled device (CCD) sensor or a plate reader. The system was used to test for SEB in buffer and various foods (mushrooms, tomatoes, and baby food meat). The limit of detection was found to be ~0.01 ng/mL, which is ~10 times more sensitive than traditional ELISA. The gold nanoparticles were relatively easy to use for antibody immobilization because of their physical adsorption mechanism; no other reagents were required for immobilization. The use of our simple and inexpensive detector combined with the gold nanoparticle-based ECL method described here is adaptable to simplify and increase sensitivity of any immunological assay and for point-of-care diagnostics.
Keywords: Staphylococcal enterotoxins, Enhanced chemiluminescence, Food safety, CCD point-of-care detector, Gold nanoparticles
1. Introduction
Staphylococcal enterotoxins (SEs) are a group of twenty-one heat stable toxins implicated in foodborne diseases resulting from consumption of contaminated foods (Archer and Young, 1988; Bean et al., 1996; Bunning et al., 1997; Garthright et al., 1988; Olsen et al., 2000). SEs food poisoning causes gastrointestinal symptoms even at exposure levels as low as. 20–100 ng per person (Asao et al., 2003). In addition, SEs have been implicated in diseases such as atopic eczema (Breuer et al., 2000; Bunikowski et al., 1999; Mempel et al., 2003), rheumatoid arthritis (Howell et al., 1991; Uematsu et al., 1991), and toxic shock syndrome (Herz et al., 1999), and are recognized as potential bioweapons (Henghold, 2004; Ler et al., 2006; Rosenbloom et al., 2002; Wiener, 1996).
SEs are traditionally assayed immunologically with enzyme-linked immunosorbent assays (ELISA) (Bennett, 2005), which generally use optical detection. Other immunological assays for SE detection have also been described, including several biosensors (Homola et al., 2002; Nedelkov et al., 2000; Rasooly, 2001; Rasooly and Herold, 2006; Rasooly and Rasooly, 1999; Sapsford et al., 2005; Shriver-Lake et al., 2003; Soelberg et al., 2005; Yu et al., 2005). Although biosensor-based assays offer advantages such as speed and high-throughput, they are not sufficiently sensitive for every application.
Nanoparticles have the potential to enhance the sensitivity of biodetection because of their large surface area and electrical conductivity. In our previous work, carbon nanotubes (CNTs) were used to improve detection of SEB at concentrations as low as 0.1 ng/mL (Yang et al., 2008b). Although effective, CNT are toxic and difficult to manipulate, so other types of nanoparticles were investigated for their ability to enhance the sensitivity of SEB immunoassays.
Gold nanoparticles have been used extensively for electron microscopy (EM) because of their size, density and electrical properties, which are ideal for enhancing the contrast of images and for electrochemical biosensors (Chen et al., 2008; Fu et al., 2005; Huang et al., 2006; Liu and Wong, 2009; Tang et al., 2006b, 2005b) but are not widely used for optical sensors, Gold nanoparticles are especially attractive for biodetection because their high surface area (Du et al., 2009; Khalavka et al., 2009; Lai et al., 2009; Liu and Wong, 2009). Moreover, their optical properties make them well-suited for optical detection. We investigated gold nanoparticles for biodetection because they have a large surface area and are readily bioconjugated to various ligands, such as antibodies, DNA, and aptamers. There are several previously described detection assays that take advantage of these properties of gold nanoparticles, including lateral flow immunoassays (LFIA), ELISA and various biosensors. Gold nanoparticles were used for electrochemical biosensors, based on potentiometric electrochemical detection of gold nanoparticle-based immunoassays (Tang et al., 2005a,b, 2004). Other have used a metalloimmunoassay with silver-enhanced gold nanoparticle labeling detected by anodic stripping voltammetry (Chu et al., 2005) and a carbon fiber microelectrode covered with a magnetic gold nanospheres-antibody mix (Tang et al., 2008).
Amperometric responses have also been used for a label-free detection method based on core/shell (Tang et al., 2006a). Similarly, a label-free amperometric immunosensor that had an antibody immobilized with electro-deposition of gold nanoparticles and Prussian Blue was used for detection of hepatitis B surface antigen (He et al., 2007). In addition to electrochemical methods, gold nanoparticles were used for optical detection approaches including silver enhancement measured by densitometry with a microplate reader (Li et al., 2007), gold coated polystyrene beads (Au@PS) using fluorescence imaging (Cao et al., 2006), and a method based on gold nanoparticles coated with single-stranded oligonucleotides, released and hybridized to a microarray and detected with silver-amplified gold nanoparticle probes (Bao et al., 2006). However, all of these gold nanoparticle-based methods require new and complex detection systems and cannot be used with a regular plate reader. This limits their practical utility.
The enhanced chemiluminescence (ECL) reaction is a simple widely used detection method based on the emission of light coming from the chemical reaction. ECL is used for many biological assays because it is highly sensitive, has a low background signal, can be detected with simple instrumentation, and has a wide linear response range. ECL detection can be coupled with enzymatic reactions (e.g. glucose oxidase, horseradish peroxidase and alkaline phosphatase), combined with chemiluminescence reagents (luminol, isoluminol etc) or with metal-based labels (Fan et al., 2005).
Unlike fluorescent or colorimetric optical detection, which requires complex optical systems for illumination or light excitation of the fluorophores (light source, lenses, filters etc), ECL emits light directly. No special optical system is required, and this greatly simplifies detector design.
Taking advantage of such simplified design combined with the potential of gold nanoparticles to enhance the sensitivity of immunological assays, we present here an immunosensor-based assay for the detection of SEB in food using a point-of-care CCD detector we developed for microbial toxin analysis (Sapsford et al., 2008; Yang et al., 2008a,c).
2. Experimental
2.1. Materials and reagents
Staphylococcal Enterotoxin B (SEB), rabbit anti-SEB affinity purified IgG and peroxidase (HRP) conjugated anti-SEB IgG were purchased from Toxin Technology (Sarasota, FL). Gold nanoparticles, Poly(diallyldimethylammonium chloride) (PDDA) and carboxymethyl (CM) cellulose were purchased from Sigma-Aldrich (St. Louis, MO). The CM cellulose was used to purify the food in a centrifuge. Immun-Star HRP chemiluminescent kit was obtained from Bio-Rad (Hercutes, CA). The foods used for the analysis (mushroom, tomato, and Gerber's meat baby food) were purchased from a local store. A Spectramax M5 plate reader (Sunnyvale, CA) and regular 96 well microtiter plates were used for testing the assay with conventional plate reader.
2.2. Fabrication of the 9-well sample plates
The 9-well sample plate used in this study were designed in CorelDraw11 (Corel Corp. Ontario, Canada) and micro-machined in 1.5 mm black acrylic using a computer controlled laser cutter Epilog Legend CO2 65W cutter (Epilog, Golden, CO). Before cutting, both sides of the acrylic sheet was coated with 3 M 9770 adhesive transfer double sided tape (Piedmont Plastics, Beltsville, MD) and the polycarbonate film was bonded. The wells of the plate were then cleaned using Versa Clean® (FisherBrand, Pittsburgh, PA) and washed with MilliQ water before being dried with air. The reaction solution was added to the top of the sample wells.
2.3. Preparation of the gold nanoparticle-enhanced immunosensor
For preparation of the gold nanoparticle-enhanced immunosensor, 15 μL of a 10 nm gold nanoparticles solution at a concentration of ~0.75 A520 units/mL was dropped onto the surface of the polycarbonate wells of the immunosensor, and then dried and rinsed with 2 ml of washing buffer (20 mM phosphate buffer, pH 7.4) for 5 min to eliminate any loose or partially immobilized gold nanoparticles. Then, 10 μL of antibody solution at a concentration of 0.01 mg/mL was placed in the well for 1 h, then washed with 2 ml of washing buffer for 5 min to eliminate any loose or partially immobilized antibody. The gold nanoparticles–antibody immunosensor was blocked with 1% BSA in 15 μl buffer for 30 min.
2.4. SEB detection using gold nanoparticle-based ECL immunosensor
After washing, the gold nanoparticles–antibody immunosensor was incubated with different concentrations of SEB in 15 μl of phosphate buffer solution (20 mM, pH 8.0) buffer for 45 min. After washing (2 ml phosphate buffer), the immunosensor coated with antibody–antigen complex was exposed to HRP conjugated anti-rabbit IgG (15 μL, 0.01 mg/mL) in buffer for 1 h, followed by 3 cycles of washing with 2 ml of phosphate buffer.
For the control experiment (antibody immobilization without gold nanoparticles), 15 μL of PDDA solution (1 mg/mL PDDA containing 0.5 M NaCl) was first dropped into the wells of the plate. After 1 h, the plate was rinsed with 2 ml of washing buffer (20 mM phosphate buffer, pH 7.4). Then, 10 μL of antibody solution at a concentration of 0.01 mg/mL was placed in the well for 1 h, after which 2 ml of washing buffer was used for 5 min to eliminate any loose or partially immobilized antibody. The immunosensor was then blocked with 1% BSA in 15 μl buffer for 30 min. The capture of SEB and HRP conjugated anti-rabbit IgG was also performed according to the above procedure.
ECL was achieved by adding 7 μL of ECL buffer (formed by mixing the two solutions from the chemiluminescent kit in a 1:1 volume ratio) into each well and the ECL intensity was measured immediately with a custom-built point-of-care CCD detector (Sapsford et al., 2008; Yang et al., 2008a,c), shown schematically in Fig. 1A. The CCD-based detector consists of an enclosed Atic-16 cooled CCD camera (Adirondack Video Astronomy, Hudson Falls, NY) or SXVF-M7. Both cooled CCD cameras employ a Sony ICX-429ALL with 752x582 pixel CCD and are equipped with a 5 mm extension tube and a 12mmPentax f1.2 lens (Spytown, Utopia, NY). The luminescence was measured after 2 and 10 min of exposure. The CCD image intensities were analyzed using ImageJ software, developed and distributed freely by NIH (http://rsb.info.nih.gov/ij/download.html), and the data generated was then imported into Microsoft Excel (Microsoft, Redmond, WA) for further manipulation. To measure the signal from each well, a circle 20 pixels in radius (1250 pixel area) was used as a uniform region of interest, which covers roughly 75% of the surface of each well of the plate. The signal for the individual wells was calculated as average of the intensity values of the respective pixels. The value obtained with concentration of 0 ng/mL SEB was defined as background. The ratio of the signal-to-background (S/B) ratio was further used to quantify the SEB concentration. Similar experiments conducted with Spectramax M5 plate reader (Sunnyvale, CA).
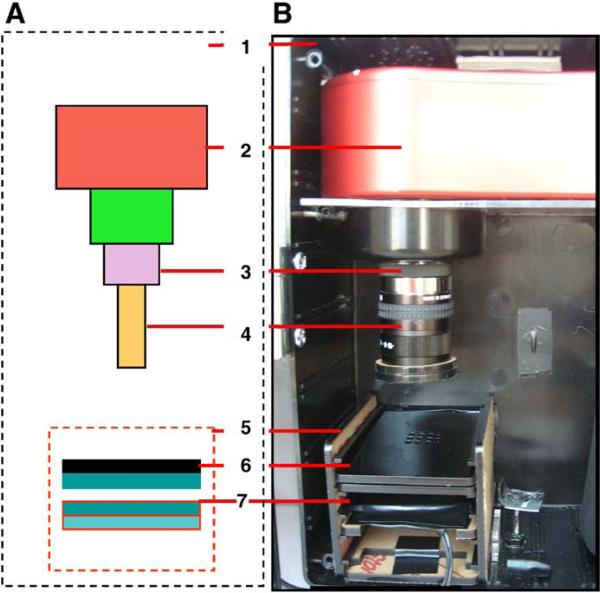
Fig. 1.
The CCD point-of-care detector. (A) a schematic configuration of the detector and (B) a photograph of the actual detection platform: plastic enclosure [1], Atic-16 CCD camera [2], 5 mm extension tube [3] attached to the 12 mm Pentax f1.2 lens [4]. A black acrylic shelf box [5] was designed to hold the sample plate [6] and a fluorescence illumination module that was not used in this work [7].
2.5. Food sample analysis
2.5.1. Food sample spiking
To spike samples of mushroom and tomato, 1 mL of sample was transferred directly to a 2 mL centrifuge tube and different concentrations of SEB were added. For the meat baby food,1 g of food sample was transferred to a centrifuge tube, and then buffer and different concentrations of SEB were added.
2.5.2. Cation exchanger carboxymethylcellulose (CM) analytical chromatography
To remove various food materials from the sample, we used our previously published method for partial SE purification from food matrices (Balaban and Rasooly, 2001). To prepare CM for approximately 100 purification assays, 1 g of CM was equilibrated with 20 mL loading buffer (loading buffer = 5 mM NaPO3, pH 5.7) until the gel was swollen and then centrifuged at 4000 rmp for 2 min. After centrifugation, the gel was allowed to settle, the buffer was removed and the CM was washed twice with 20 ml of loading buffer followed by centrifugation as above. After the removal of the buffer in the last centrifugation, loading buffer was added to a final volume of 20 mL. The equilibrated CM was stored at 4 °C before use.
2.5.3. Sample preparation
The tubes containing the spiked food samples were vortexed briefly, centrifuged at 14,000 rpm for 2 min, and the resulting supernatant transferred into a fresh tube. Loading buffer was added into the supernatant to reach a sample volume of 1 mL. The sample (400 μL) was mixed with 200 μL of equilibrated CM, and vortexed for 30 min, followed by centrifugation at 14,000 rpm for 2 min. Soluble material was removed and the remaining material in the tube was washed 3 times by repeatedly adding loading buffer and centrifuging. Finally, 100 μL elution buffer (50 mM NaPO3, pH 6.5; 50 mM NaCl) was added to the matrix, the tubes centrifuged and eluted material collected for immunoassay.
3. Results and discussion
To evaluate the potential of gold nanoparticles combined with ECL detection for point-of-care analysis of Staphylococcal Enterotoxin B (SEB), we used them in an immunosensor. The gold nanoparticles were immobilized on the polycarbonate surface of nine well plates, and then anti-SEB primary antibodies were bound through physical adsorption to the nanoparticles. SEB was detected by a “sandwich-type” ELISA assay where SEB was first bound to the immobilized anti-SEB primary antibody followed by binding of HRP-labeled secondary antibody. Bound HRP was detected by an ECL assay, in which HRP catalyzes the oxidation of luminol to an excited state in the presence of hydrogen peroxide (H2O2). The excited state luminol decays to ground state, emitting light, which was measured using a cooled charge-coupled device (CCD) sensor or a regular plate reader.
3.1. Detection of SEB using a gold nanoparticle-based ECL immunosensor
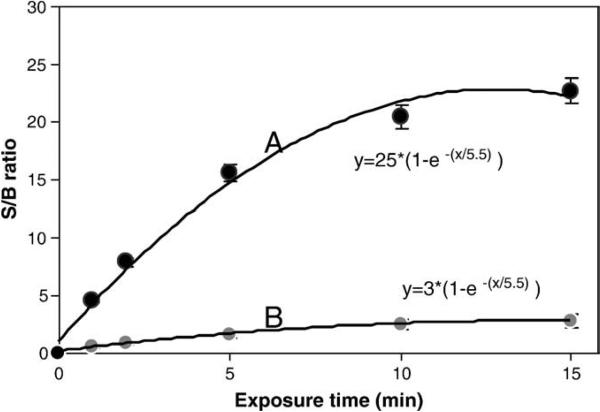
Initially, the gold nanoparticle-based ECL immunosensor was characterized for its ability to detect SEB using the point-of-care CCD detector. For this experiment, 1 ng/mL of SEB in buffer was added to wells containing gold nanoparticles and immobilized rabbit anti-SEB IgG. SEB was detected using an HRP-labeled secondary antibody, an ECL reaction and a CCD detector. The sensitivity of the detection, as measured by the signal-to-background ratio (S/B) of the CCD detector, was established as a function of exposure time (Fig. 2). The control for this experiment was the previously described anti-SEB primary antibody immobilized on the polycarbonate surface without gold nanoparticles. From an exponential fit to these results, it can be seen that gold nanoparticles (Fig. 2A) increase the sensitivity of the assay nearly 8 fold, with a coefficient of 25 for the gold nanoparticles versus 3 without gold nanoparticles (Fig. 2B). This experiment also shows that a 10 minute exposure time provided the optimal S/B for detection using the gold nanoparticles with ECL.
Fig. 2.
Effect of exposure time on the signal of the ECL experiment for the detection of 1 ng/mL SEB: (A) anti-SEB primary antibody immobilized on gold nanoparticle, and (B) anti-SEB primary antibody immobilized on the polycarbonate surface without gold nanoparticles.
3.2. The sensitivity of the gold nanoparticle-based ECL immunosensor for detection of SEB
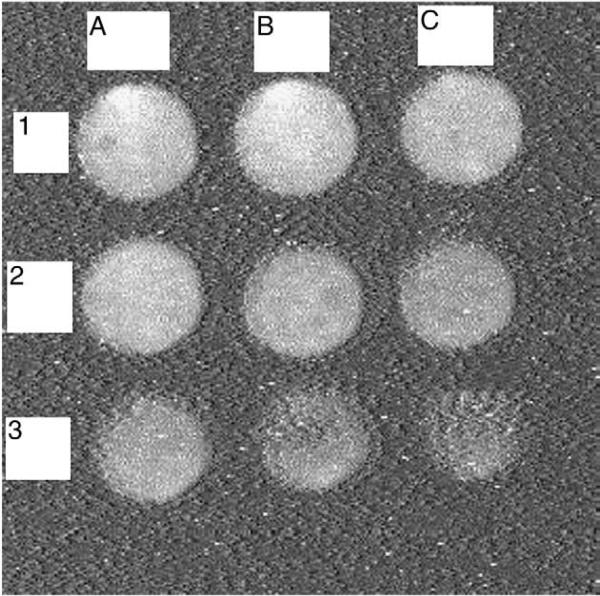
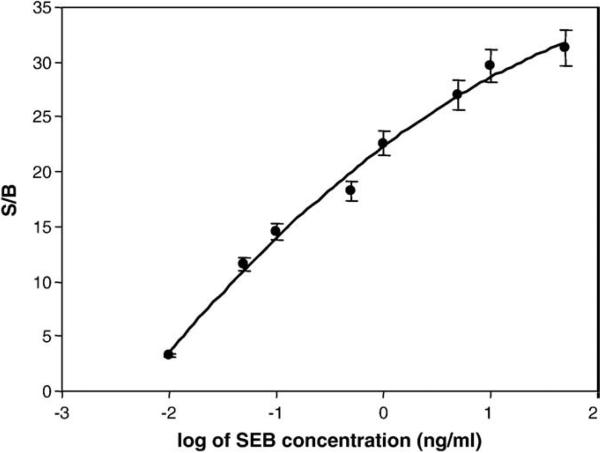
After establishing the optimal detection conditions, it was necessary to quantify the variation of the signal with the SEB concentration to establish a calibration curve. Various amounts of SEB (50 ng/mL, 10 ng/mL, 5 ng/mL, 1 ng/mL, 0.5 ng/mL, 0.1 ng/mL, 0.05 ng/mL, and 0.01 ng/mL, as well as the baseline signal at 0 ng/mL) in buffer were added to 9 well immunosensor with gold nanoparticle-immobilized rabbit anti-SEB IgG. The CCD image from the 9 well immunosensor is shown in Fig. 3, where it can be clearly seen that the intensity of the signal increased with increasing SEB concentration. The data from this experiment was quantified using imageJ and, as shown in Fig. 4, the signal increases proportionally to the amount of SEB. To produce a calibration curve, a quadratic logarithmic fit was applied and was found to be the best using the log of SEB concentration. The log fit indicates a theoretical LOD that is ~0.006 ng/mL, about 10 times better than conventional ELISA, while the measured LOD is 0.01 ng/ml. The control used for this experiment, the binding of anti-SEB IgG without gold nanoparticles, resulted in a much lower signal level (more than ten times lower). These results suggest that gold nanoparticles can be used to enhance SEB immunodetection.
Fig. 3.
Immunoassay based on gold nanoparticle–ECL detection. Different concentrations of SEB were detected by the CCD camera: (1A), 50 ng/mL; (1B), 10 ng/mL; (1C), 5 ng/mL; (2A), 1 ng/mL; (2B), 0.5 ng/mL; (2C), 0.1 ng/mL; (3A), 0.05 ng/mL; (3B), 0.01 ng/mL; and (3C), 0 ng/mL. Exposure time is 15 min.
Fig. 4.
Calibration curve of the gold nanoparticle–ECL immunoassay for SEB concentrations ranging from 0.01 to 50 ng/mL.
3.3. The performance of gold nanoparticle-based ECL assay with a plate reader
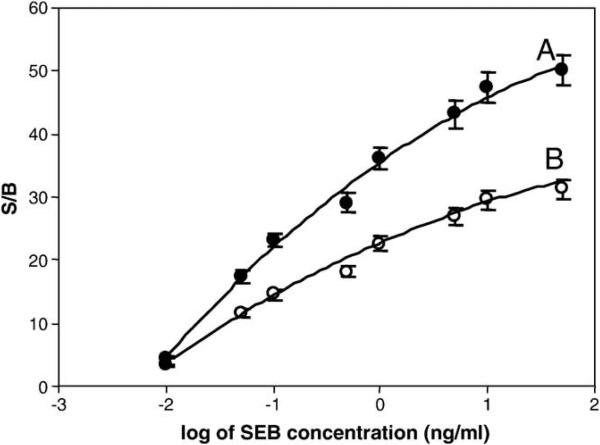
As discussed in the introduction, numerous detection methods using gold nanoparticles requiring specialized equipment have been developed. However, for an assay to be useful and practical, it is important that the assay be compatible with current detection equipment such as optical plate readers. To assess whether a conventional plate reader could be used to read the results from the gold nanoparticle-based ECL immunosensor developed here, we compared plate reader results with those from the CCD detector. As with the previous experiments, a “sandwich assay” was used. Fig. 5 shows the results from the two detection. Both the plate reader (Fig. 5A) and the CCD (Fig. 5B) detectors reached a LOD of 0.01 ng/mL, although the S/B ratio of the plate reader is higher. These results suggest that the assay can be used with either a plate reader or the more portable CCD detector.
Fig. 5.
SEB calibration curves for the gold nanoparticle-based ECL immunosensor obtained with two different detection methods: (A) plate reader, and (B) CCD reader. Error bar = SD (n = 3).
To evaluate the reproducibility of the immunosensor, a series of 5 surfaces with gold nanoparticles were prepared for the detection of 1 ng/mL and 0.1 ng/mL of SEB. The relative standard deviation (RSD) of measurement was 5.4% and 5.8%, respectively, suggesting that the assay is reproducible in the tested conditions.
3.4. The application of gold nanoparticles for detection of SEB in food samples
Foods are often tested for Staphylococcal enterotoxins as part of food safety efforts, since SEs are a significant cause of food poisoning. In order to evaluate the utility of the gold nanoparticle-based ECL immunosensor for food testing, we assayed various food samples spiked with SEB. Often food testing is complicated by the food matrix itself, which is highly variable and often complex, and which may contain unrelated cross-reacting materials that can affect the accuracy of antibody-based assays. Partial sample purification has been shown to help reduce assay background by reducing cross-reaction of the antibodies with other components of the food matrix (Park et al., 1992, 1993). In order to establish a widely applicable assay for different foods, the gold nanoparticles immunosensor assay was tested in food samples with and without partial toxin purification using the cation exchanger carboxymethylcellulose (CM) purification method (Balaban and Rasooly, 2001).
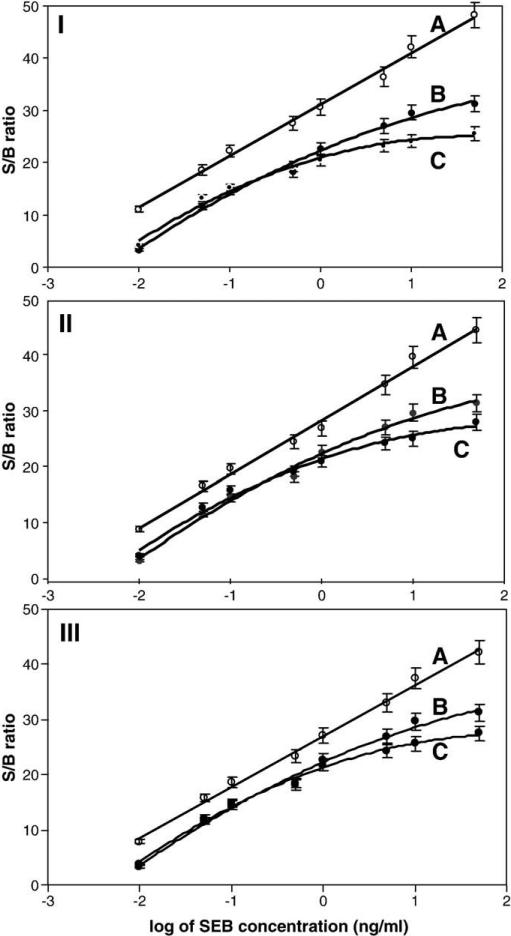
SEB spiked mushroom samples (Fig. 6I) were partially purified with CM and the eluted material was analyzed with the gold nanoparticle-based ECL immunosensor. An SEB standard solution in H2O was used as a control. As seen in Fig. 6I, at all SEB concentrations, unpurified sample (A) gave higher signals compared to the SEB control solution (B), suggesting some non-specific adsorption of other non-SEB proteins. On the other hand, with CM purification (C) the signal was lower than the SEB control solution at concentrations of SEB above 1 ng/mL (B). This suggests that SEB recovery at higher concentrations was reduced by approximately 15% by CM purification.
Fig. 6.
Detection of SEB in mushroom (I), tomato (II), and meat baby food (III) using the gold nanoparticle-based ECL immunosensor. (A) food without CM purification, (B) standard SEB solution and (C) with CM purification.
The SEB spiked tomato (Fig. 6II) and meat baby food (Fig. 6III) samples exhibited similar results. The gold nanoparticle-based ECL immunosensor was able to detect SEB at a variety of concentrations in both tomato (panel II) and meat baby food (panel II). In both cases, the purified sample (C) exhibited lower signal than the unpurified material (a), suggesting that the partial purification removed some cross-reacting materials from the sample. However, as with mushrooms, the lower signal observed after CM purification at higher SEB concentrations indicated a greater loss of SEB during CM purification.
Gold nanoparticles are attractive for biodetection, because their high surface area (Du et al., 2009; Khalavka et al., 2009; Lai et al., 2009; Liu and Wong, 2009), biocompatibility, chemical and optical properties make them well-suited for optical and electrochemical detection. The large surface area of gold nanoparticles increased immobilization of the primary antibody onto the assay surface (Du et al., 2009; Khalavka et al., 2009; Lai et al., 2009; Liu and Wong, 2009), which enhanced the sensitivity of the assay. Another explanation for the enhanced the sensitivity of the assay can be luminescence/fluorescence enhancement in close proximity to the particles and better orientation of the immobilized antibodies on the sensor surface.
4. Conclusion
In attempting to develop simple more sensitive biosensors for microbial toxins, we have worked with other nanoparticles such as carbon nanotubes (CNTs) to enhance the sensitivity of SEB detection. The assay with CNTs was able to detect a concentration of 0.1 ng/mL SEB (Yang et al., 2008b) in a fluorescent assay, ten-fold less sensitive than the ECL assay with gold nanoparticles described in this work. Although it is difficult to compare the results of the two assays directly because different detection methods were used, gold nanoparticles are generally preferable because they are not toxic as CNT and appear to more easily immobilize antibodies than CNTs. Unlike CNTs, gold nanoparticles do not require shortening and acid functionalization, and so preparation of the immunosensor is simplified.
Gold nanoparticles are attractive for biodetection; their high surface area (Du et al., 2009; Khalavka et al., 2009; Lai et al., 2009; Liu and Wong, 2009), biocompatibility, chemical and optical properties make them well-suited for optical and electrochemical biodetection. In the assay described here, gold nanoparticles were used. Gold nanoparticles have been shown to increase immobilization of the primary antibody onto sensors (Chen et al., 2008; Huang et al., 2006; Jung et al., 2007; Lai et al., 2009; Liu and Wong, 2009) and were chosen for the assay because their large surface area enhanced the assay's sensitivity. Another explanation for the enhanced sensitivity of the assay is luminescence/fluorescence enhancement in close proximity to the particles and better orientation of the immobilized antibodies on the sensor surface compared with other nanomaterials.
We demonstrated that an immunoassay based on gold nanoparticles increases the level of SEB detection using a CCD detector and conventional plate reader. The gold nanoparticle immunosensor gave a signal approximately 10 times larger than a similar assay without gold nanoparticles, and had a wider range of SEB detection (0.01 to 10 ng/mL) than the immunosensor without gold nanoparticles. The immunosensor assay has a ten-fold lower LOD (0.01 ng SEB per mL) than traditional ELISA (Schotte et al., 2002). However, many factors contribute to the level of detection (antibodies, the type of detector, the food matrix, incubation time, HRP substrate, etc.) while in this work, we looked only at the effect of a single element (gold nanoparticles) and showed that it can increase sensitivity of detection. Presumably, gold nanoparticles will increase the sensitivity of any immunological assay in a similar manner. The combined CM chromatography purification and gold nanoparticle-based immunosensor was capable of detecting SEB in three different foods, and is likely to be useful for detecting SEB in many different foods and other complex matrices.
The fact that the assay can be performed in a plate reader suggests that the it can be widely used in many labs without specialized equipment, thus broadening the usefulness of the assay. CCD detection was not only sensitive, but also portable, making it suitable for point-of-care applications.
However, the main technical advantage of the gold nanoparticle-based ECL immunosensor described here is that it is based on a simple apparatus, unlike the more complicated florescent detection systems, which require an excitation light source, excitation filter (s) and an emission filter(s). For ECL, a simple CCD camera appears to be sufficient to provide similar sensitivity of measurement to that of a more complex and expensive commercial fluorescent detector, so CCD detection appears practical for point-of-care testing.
In summary, the gold nanoparticle-based ECL immunosensor is simple, inexpensive, and can be easily mass fabricated to be used in many other types of immunological assays. Our simple, versatile, sensitive and relatively inexpensive CCD-based system can be used for a broad array of optical readouts from assays (ECL, fluorescence, colorimetric and densitometry) (Sapsford et al., 2008; Yang et al., 2008a,c) and have many potential applications, especially in the point-of-care setting, and for use in areas where sophisticated measurement are not traditionally possible.
References
- Archer DL, Young FE. Contemporary issues: diseases with a food vector. Clin. Microbiol. Rev. 1988;1:377–398. doi: 10.1128/cmr.1.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T, Kumeda Y, Kawai T, Shibata T, Oda H, Haruki K, Nakazawa H, Kozaki S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 2003;130:33–40. doi: 10.1017/s0950268802007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban N, Rasooly A. Analytical chromatography for recovery of small amounts of staphylococcal enterotoxins from food. Int. J. Food Microbiol. 2001;64:33–40. doi: 10.1016/s0168-1605(00)00439-6. [DOI] [PubMed] [Google Scholar]
- Bao YP, Wei TF, Lefebvre PA, An H, He L, Kunkel GT, Muller UR. Detection of protein analytes via nanoparticle-based bio bar code technology. Anal. Chem. 2006;78:2055–2059. doi: 10.1021/ac051798d. [DOI] [PubMed] [Google Scholar]
- Bean NH, Goulding JS, Lao C, Angulo FJ. Surveillance for foodborne-disease outbreaks—United States, 1988–1992. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 1996;45:1–66. [PubMed] [Google Scholar]
- Bennett RW. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J. Food Prot. 2005;68:1264–1270. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]
- Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB). Allergy. 2000;55:551–555. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J. Allergy Clin. Immunol. 1999;103:119–124. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- Bunning VK, Lindsay JA, Archer DL. Chronic health effects of microbial foodborne disease. World Health Stat. Q. 1997;50:51–56. [PubMed] [Google Scholar]
- Cao YC, Hua XF, Zhu XX, Wang Z, Huang ZL, Zhao YD, Chen H, Liu MX. Preparation of Au coated polystyrene beads and their application in an immunoassay. J. Immunol. Methods. 2006;317:163–170. doi: 10.1016/j.jim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang Y, Zhou J, Yan W, Li X, Zhu JJ. Electrochemical impedance immunosensor based on three-dimensionally ordered macroporous gold film. Anal. Chem. 2008;80:2133–2140. doi: 10.1021/ac7021376. [DOI] [PubMed] [Google Scholar]
- Chu X, Fu X, Chen K, Shen GL, Yu RQ. An electrochemical stripping metalloimmunoassay based on silver-enhanced gold nanoparticle label. Biosens. Bioelectron. 2005;20:1805–1812. doi: 10.1016/j.bios.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Du P, Li H, Mei Z, Liu S. Electrochemical DNA biosensor for the detection of DNA hybridization with the amplification of Au nanoparticles and CdS nanoparticles. Bioelectrochemistry. 2009;75:37–43. doi: 10.1016/j.bioelechem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fan A, Lau C, Lu J. Magnetic bead-based chemiluminescent metal immunoassay with a colloidal gold label. Anal. Chem. 2005;77:3238–3242. doi: 10.1021/ac050163b. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yuan R, Tang D, Chai Y, Xu L. Study on the immobilization of anti-IgG on Au-colloid modified gold electrode via potentiometric immunosensor, cyclic voltammetry, and electrochemical impedance techniques. Colloids Surf., B Biointerfaces. 2005;40:61–66. doi: 10.1016/j.colsurfb.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Garthright WE, Archer DL, Kvenberg JE. Estimates of incidence and costs of intestinal infectious diseases in the United States. Public Health Rep. 1988;103:107–115. [PMC free article] [PubMed] [Google Scholar]
- He X, Yuan R, Chai Y, Zhang Y, Shi Y. A new antibody immobilization strategy based on electro-deposition of gold nanoparticles and Prussian Blue for label-free amperometric immunosensor. Biotechnol. Lett. 2007;29:149–155. doi: 10.1007/s10529-006-9211-7. [DOI] [PubMed] [Google Scholar]
- Henghold WB., II Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol. Clin. 2004;22:257–262. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Herz U, Bunikowski R, Mielke M, Renz H. Contribution of bacterial superantigens to atopic dermatitis. Int. Arch. Allergy Immunol. 1999;118:240–241. doi: 10.1159/000024085. [DOI] [PubMed] [Google Scholar]
- Homola J, Dostalek J, Chen S, Rasooly A, Jiang S, Yee SS. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int. J. Food Microbiol. 2002;75:61–69. doi: 10.1016/s0168-1605(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Howell MD, Diveley JP, Lundeen KA, Esty A, Winters ST, Carlo DJ, Brostoff SW. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10,921–10,925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Liu Z, Yang X. Application of electrochemical impedance spectroscopy for monitoring allergen–antibody reactions using gold nanoparticle-based biomolecular immobilization method. Anal. Biochem. 2006;356:208–214. doi: 10.1016/j.ab.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Jung Y, Lee JM, Jung H, Chung BH. Self-directed and self-oriented immobilization of antibody by protein G-DNA conjugate. Anal. Chem. 2007;79:6534–6541. doi: 10.1021/ac070484i. [DOI] [PubMed] [Google Scholar]
- Khalavka Y, Becker J, Sonnichsen C. Synthesis of rod-shaped gold nanorattles with improved plasmon sensitivity and catalytic activity. J. Am. Chem. Soc. 2009;131:1871–1875. doi: 10.1021/ja806766w. [DOI] [PubMed] [Google Scholar]
- Lai LJ, Yang YW, Lin YK, Huang LL, Hsieh YH. Surface characterization of immunosensor conjugated with gold nanoparticles based on cyclic voltammetry and X-ray photoelectron spectroscopy. Colloids Surf., B Biointerfaces. 2009;68:130–135. doi: 10.1016/j.colsurfb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Ler SG, Lee FK, Gopalakrishnakone P. Trends in detection of warfare agents. Detection methods for ricin, staphylococcal enterotoxin B and T-2 toxin. J. Chromatogr., A. 2006;1133:1–12. doi: 10.1016/j.chroma.2006.08.078. [DOI] [PubMed] [Google Scholar]
- Li YY, Zhang C, Li BS, Zhao LF, Li XB, Yang WJ, Xu SQ. Ultrasensitive densitometry detection of cytokines with nanoparticle-modified aptamers. Clin. Chem. 2007;53:1061–1066. doi: 10.1373/clinchem.2006.082271. [DOI] [PubMed] [Google Scholar]
- Liu X, Wong DK. Picogram-detection of estradiol at an electrochemical immunosensor with a gold nanoparticle|Protein G-(LC-SPDP)-scaffold. Talanta. 2009;77:1437–1443. doi: 10.1016/j.talanta.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Mempel M, Lina G, Hojka M, Schnopp C, Seidl HP, Schafer T, Ring J, Vandenesch F, Abeck D. High prevalence of superantigens associated with the egc locus in Staphylococcus aureus isolates from patients with atopic eczema. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:306–309. doi: 10.1007/s10096-003-0928-0. [DOI] [PubMed] [Google Scholar]
- Nedelkov D, Rasooly A, Nelson RW. Multitoxin biosensor-mass spectrometry analysis: a new approach for rapid, real-time, sensitive analysis of staphylococcal toxins in food. Int. J. Food Microbiol. 2000;60:1–13. doi: 10.1016/s0168-1605(00)00328-7. [DOI] [PubMed] [Google Scholar]
- Olsen SJ, MacKinnon LC, Goulding JS, Bean NH, Slutsker L. Surveillance for foodborne-disease outbreaks—United States, 1993–1997. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 2000;49:1–62. [PubMed] [Google Scholar]
- Park CE, Akhtar M, Rayman MK. Nonspecific reactions of a commercial enzyme-linked immunosorbent assay kit (TECRA) for detection of staphylococcal enterotoxins in foods. Appl. Environ. Microbiol. 1992;58:2509–2512. doi: 10.1128/aem.58.8.2509-2512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CE, Akhtar M, Rayman MK. Simple solutions to false-positive staphylococcal enterotoxin assays with seafood tested with an enzyme-linked immunosorbent assay kit (TECRA). Appl. Environ. Microbiol. 1993;59:2210–2213. doi: 10.1128/aem.59.7.2210-2213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooly A. Surface plasmon resonance analysis of staphylococcal enterotoxin B in food. J. Food Prot. 2001;64:37–43. doi: 10.4315/0362-028x-64.1.37. [DOI] [PubMed] [Google Scholar]
- Rasooly L, Rasooly A. Real time biosensor analysis of staphylococcal enterotoxin A in food. Int. J. Food Microbiol. 1999;49:119–127. doi: 10.1016/s0168-1605(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Rasooly A, Herold KE. Biosensors for the analysis of food- and waterborne pathogens and their toxins. J. AOAC Int. 2006;89:873–883. [PubMed] [Google Scholar]
- Rosenbloom M, Leikin JB, Vogel SN, Chaudry ZA. Biological and chemical agents: a brief synopsis. Am. J. Ther. 2002;9:5–14. doi: 10.1097/00045391-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Sapsford KE, Taitt CR, Loo N, Ligler FS. Biosensor detection of botulinum toxoid A and staphylococcal enterotoxin B in food. Appl. Environ. Microbiol. 2005;71:5590–5592. doi: 10.1128/AEM.71.9.5590-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapsford KE, Sun S, Francis J, Sharma S, Kostov Y, Rasooly A. A fluorescence detection platform using spatial electroluminescent excitation for measuring botulinum neurotoxin A activity. Biosens. Bioelectron. 2008;24:618–625. doi: 10.1016/j.bios.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte U, Langfeldt N, Peruski AH, Meyer H. Detection of staphylococcal enterotoxin B (SEB) by enzyme-linked immunosorbent assay and by a rapid hand-held assay. Clin. Lab. 2002;48:395–400. [PubMed] [Google Scholar]
- Shriver-Lake LC, Shubin YS, Ligler FS. Detection of staphylococcal enterotoxin B in spiked food samples. J. Food Prot. 2003;66:1851–1856. doi: 10.4315/0362-028x-66.10.1851. [DOI] [PubMed] [Google Scholar]
- Soelberg SD, Chinowsky T, Geiss G, Spinelli CB, Stevens R, Near S, Kauffman P, Yee S, Furlong CE. A portable surface plasmon resonance sensor system for real-time monitoring of small to large analytes. J. Ind. Microbiol. Biotechnol. 2005;32:669–674. doi: 10.1007/s10295-005-0044-5. [DOI] [PubMed] [Google Scholar]
- Tang DP, Yuan R, Chai YQ, Zhong X, Liu Y, Dai JY, Zhang LY. Novel potentiometric immunosensor for hepatitis B surface antigen using a gold nanoparticle-based biomolecular immobilization method. Anal. Biochem. 2004;333:345–350. doi: 10.1016/j.ab.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Tang D, Yuan R, Chai Y, Liu Y, Dai J, Zhong X. Novel potentiometric immunosensor for determination of diphtheria antigen based on compound nanoparticles and bilayer two-dimensional sol–gel as matrices. Anal. Bioanal. Chem. 2005a;381:674–680. doi: 10.1007/s00216-004-2916-3. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Tang DY, Tang DP. Construction of a novel immunoassay for the relationship between anxiety and the development of a primary immune response to adrenal cortical hormone. Bioprocess Biosyst. Eng. 2005b;27:135–141. doi: 10.1007/s00449-004-0394-9. [DOI] [PubMed] [Google Scholar]
- Tang D, Yuan R, Chai Y. Ligand-functionalized core/shell Ag@Au nanoparticles label-free amperometric immun-biosensor. Biotechnol. Bioeng. 2006a;94:996–1004. doi: 10.1002/bit.20922. [DOI] [PubMed] [Google Scholar]
- Tang D, Yuan R, Chai Y, Zhong X, Liu Y, Dai J. Electrochemical detection of hepatitis B surface antigen using colloidal gold nanoparticles modified by a sol–gel network interface. Clin. Biochem. 2006b;39:309–314. doi: 10.1016/j.clinbiochem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Tang D, Yuan R, Chai Y. Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal. Chem. 2008;80:1582–1588. doi: 10.1021/ac702217m. [DOI] [PubMed] [Google Scholar]
- Uematsu Y, Wege H, Straus A, Ott M, Bannwarth W, Lanchbury J, Panayi G, Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SL. Strategies for the prevention of a successful biological warfare aerosol attack. Mil. Med. 1996;161:251–256. [PubMed] [Google Scholar]
- Yang M, Kostov Y, Bruck HA, Rasooly A. Carbon nanotubes with enhanced chemiluminescence immunoassay for CCD-based detection of staphylococcal enterotoxin B in food. Anal. Chem. 2008a;80:8532–8537. doi: 10.1021/ac801418n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kostov Y, Bruck HA, Rasooly A. Carbon nanotubes with enhanced chemiluminescence immunoassay for CCD-based detection of Staphylococcal Enterotoxin B in food. Anal. Chem. 2008b;80:8532–8537. doi: 10.1021/ac801418n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kostov Y, Rasooly A. Carbon nanotubes based optical immunodetection of Staphylococcal Enterotoxin B (SEB) in food. Int. J. Food Microbiol. 2008c;127:78–83. doi: 10.1016/j.ijfoodmicro.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Yu X, Kim SN, Papadimitrakopoulos F, Rusling JF. Protein immunosensor using single-wall carbon nanotube forests with electrochemical detection of enzyme labels. Mol. Biosyst. 2005;1:70–78. doi: 10.1039/b502124c. [DOI] [PubMed] [Google Scholar]