Abstract
The recognition of small interfering RNAs (siRNAs) by the RNA induced silencing complex (RISC) and its precursor, the RISC loading complex (RLC), is a key step in the RNA interference pathway that controls the subsequent sequence-specific mRNA degradation. In Drosophila, selection of the guide strand has been shown to be mediated by the RLC protein R2D2, which senses the relative hybridization stability between the two ends of the siRNA. A protein with similar function has yet to be conclusively identified in humans. We show here that human TAR RNA binding protein (TRBP) alone can bind siRNAs in vitro and sense their asymmetry. We also show that TRBP can bind 21 nucleotide single-stranded RNAs, though with far lower affinity than for double-stranded siRNA, and that TRBP crosslinks preferentially to the 3′ ends of the guide strands of siRNAs. This suggests that TRBP binding depends both on the sequences of the siRNA strands as well as the relative hybridization stability of the ends of the duplex. Together these results demonstrate the importance of the siRNA-TRBP interaction in the selection of the siRNA guide strand in RNAi.
RNA interference (RNAi) is a means of enacting specific silencing of the expression of a target gene (1, 2). The pathway can be initiated by long double-stranded RNAs (dsRNAs) or pre-microRNAs (pre-miRNAs) processed by the ribonuclease III family enzyme Dicer into 21–27 nucleotide (nt) long, double-stranded small interfering RNAs (siRNAs) or miRNAs with 5′-phosphates and 3′-dinucleotide overhangs (3). Alternatively, siRNAs with the proper structure can be added directly to cells for initiation of the pathway (4, 5). In both cases, silencing is initiated through the RNA induced silencing complex (RISC) and its predecessor, the RISC loading complex (RLC) (6, 7). The RLC is minimally comprised of Dicer, the TAR RNA binding protein (TRBP), and Argonaute 2 (Ago2), although several other proteins have been shown to associate with the RLC and RISC in vivo (8–14). Ago2 is the catalytic component of RISC responsible for degradation of the complementary passenger siRNA strand for generation of active RISC and cleavage of the target mRNA for silencing (15–20). Though it has no enzymatic contribution to siRNA-initiated RNAi, Dicer has been shown to contribute to siRNA processing through coupling dsRNA processing to guide strand loading onto Ago2 (21–23), a role supported by the structure of the RLC in vitro (24). While the same structural studies suggested that TRBP can stabilize the RLC (24), a precise functional contribution of TRBP to siRNA processing has yet to be confirmed.
TRBP was first identified by its ability to bind the TAR RNA structure present in human immunodeficiency virus (HIV-1) transcripts (25). Later, it was shown that TRBP can inhibit Protein Kinase R (PKR), an important contributor to the innate response to viral infection (26). Most recently, it was found that TRBP interacts directly with Dicer, as well as the activator of PKR (PACT), through the Medipal domain present at the C-terminus of TRBP (27), and that the interaction is not mediated solely by dsRNA (9, 28). A similar interaction is detected in Drosophila, where Dicer-2 associates with the dsRNA-binding protein (dsRBP) R2D2 (29). Together, the Dicer-2/R2D2 complex senses the relative thermodynamic asymmetry within an siRNA (7). R2D2 binds to the more stable (dsRNA-like) end, leaving Dicer-2 to bind the opposite end. Furthermore, binding is enhanced by the presence of a 5′-phosphate on the passenger strand; hydroxyl groups, such as those present during chemical siRNA synthesis, inhibit binding (7). As a result of the asymmetric binding, the guide strand is preferentially loaded into active RISC, and the passenger strand is degraded (17, 20).
Extensive data have shown that human RISC senses asymmetry between the ends of the siRNA or miRNA:miRNA* duplexes, leading to the preferential incorporation of one strand as the guide strand of active RISC (6, 13, 30, 31). This has been accomplished with crude cell extracts and immunopurified proteins (6, 13), and more recently using only recombinant Dicer, TRBP, and Ago2 in a 1:1:1 stoichiometry (10). Asymmetric strand loading by human RISC is also demonstrated in analyses of large siRNA or miRNA datasets (30, 31) and is currently the cornerstone for predicting siRNA efficacy (32, 33). However, unlike in Drosophila, no studies using human cells or proteins have conclusively shown how siRNA asymmetry is detected. Since Ago2 alone cannot bind and select the siRNA guide strand (19), it suggests that its partners in the RLC, Dicer and/or TRBP, contribute to initial siRNA loading, even though other factors such as RNA helicase A (RHA) or PACT may also have an effect in vivo (11, 12, 14).
We were interested in characterizing the binding of siRNAs by recombinant TRBP in vitro to determine whether TRBP could sense asymmetry in siRNAs. Our results show that TRBP binds siRNAs and not the analogous siDNAs or siDNA/RNA hybrids. Furthermore, we demonstrate that TRBP alone can sense the asymmetry of an siRNA duplex, thus establishing a key functional role for TRBP in the human RNAi pathway. Additionally, TRBP bound 21 nt ssRNAs in a manner that reflected the asymmetry detected in their corresponding siRNA duplex, providing an indication that TRBP-RNA binding may also be sequence dependent.
Materials and Methods
Protein expression and purification
Plasmids encoding TRBP as a fusion product with maltose binding protein (MBP-TRBP) and MBP alone were kindly provided by Professor Anne Gatignol and expressed essentially as described (27). Briefly, plasmids were transformed into Rosetta2(DE3) competent cells (Novagen). An overnight culture from a single colony was diluted 1:50 (v/v) and grown for 3–4 hrs at 37°C, or until A600 = 0.6–1.0, and then induced with 0.3 mM IPTG. After 2 hrs of expression at 37°C, cells were pelleted (4000 rpm for 10 min), resuspended in Column Buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA), lysed via sonication, and then clarified by high speed centrifugation (15,000 rpm for 25 min). The supernatant was purified with an AKTA FPLC (GE Healthcare). MBP-TRBP and MBP were eluted from a MBPTrap Column (GE Healthcare) with 5 column volumes of Elution Buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, 10 mM maltose) and stored in Elution Buffer at −80°C until use.
Protein concentration was measured by Bradford assay (BioRad) or Bicinchoninic acid (BCA) assay (Pierce; Thermo Scientific). Products were assessed by separating approximately equal amounts of protein per lane on denaturing linear 4–20% Tris-HCl gels. Total protein was visualized by staining with Gel Code Blue (Pierce; Thermo Scientific), with final purity estimated at >90% by band quantification, and specific proteins were detected via western blot. For western blot, the separated proteins were transferred to nitrocellulose membranes and then incubated overnight at 4°C with the appropriate antibodies (MBP (New England Biolabs), TRBP (Abnova)). Blots were washed with TBS-Tween, incubated for an additional 1 hr with HRP-linked secondary antibody (Pierce; Thermo Scientific), and developed using the SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce; Thermo Scientific). Images were obtained on a BioRad ChemiDoc XRS imager using Quantity One software.
Nucleic acids
The nucleic acid sequences are described in Tables 1–5 of the Supporting Information. The pp-luc and sod1 sequences are identical to siRNA ‘1’ and siRNA ‘a’ from (7) and had also been used previously by the Zamore group (34). DNA oligonucleotides were purchased from Integrated DNA Technologies. Chemically synthesized RNAs were purchased from Dharmacon as either ready-to-use siRNAs or ssRNAs. HIV-1 TAR RNA was prepared by in vitro transcription from a plasmid kindly provided by Dr. K-T Jeang. Briefly, 1 μg of linearized plasmid DNA was transcribed with the Megashortscript Kit (Ambion) following the manufacturer’s instructions. Transcripts were resolved in 8M urea-10% polyacrylamide gels, visualized by UV shadowing, and eluted from gel slices by crushing and soaking in TE buffer (10 mM Tris, 1 mM EDTA) for 10 min at 75°C. RNA was then concentrated by ethanol precipitation, washed with 70% ethanol, and quantitated spectrophotometrically (NanoDrop). In order to remove the 5′-triphosphate, RNAs were treated with calf intestinal phosphatase (New England Biolabs). Treated RNAs were purified first by phenol/chloroform extraction, then ethanol precipitated, gel purified (as above), and concentrated. The concentration of the product containing 5′-OH was again measured prior to end-labeling with [γ-32P]-ATP (see below).
Duplex preparation and end-labeling
For Figure 2, oligonucleotides were hybridized by mixing equal amounts of both top and bottom strands in 1× STE buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA) and then heated to 90°C for 3 min, followed by incubation at 37°C for 60 min. Products were verified on native TBE gels stained with SYBR Gold (Invitrogen). Duplexes for Figure 2 and ssRNAs (Figure 6) (3 pmol) were directly 5′-radiolabeled with 10 pmol of [γ-32P]-ATP using T4 polynucleotide kinase (New England Biolabs); unincorporated label was removed using G-25 Sephadex columns (Roche Applied Science). TAR RNA (3 pmol) was similarly labeled following CIP treatment.
FIGURE 2.
TRBP binding preferences for nucleic acids. Native gel shift assay of 32P-labeled EGFP-targeting siRNA (lanes 1&2), ssRNA (3&4), siDNA (5&6), ssDNA (7&8), and DNA/RNA (9&10) or RNA/DNA (11&12) hybrids with (+) or without (−) 2000 nM MBP-TRBP. Shifted MBP-TRBP-siRNA complexes are indicated by A (approximately monomer of MBP-TRBP with siRNA), B (dimer of MBP-TRBP), C (tetramer of MBP-TRBP), and D (larger order structure of MBP-TRBP). Note that the gel shift of the second ssRNA, RNAss, is shown in Figure 6B.
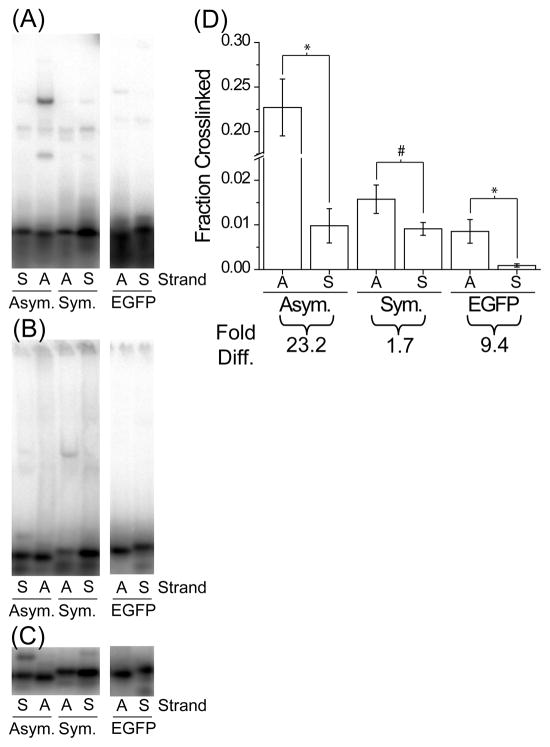
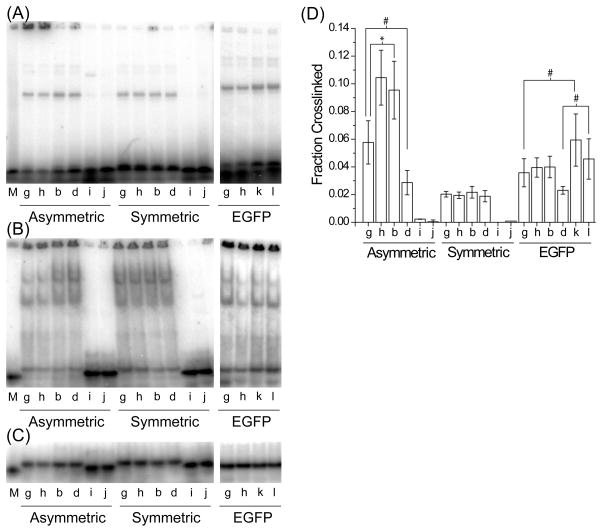
FIGURE 6.
TRBP recognition of 21 nt single-stranded RNAs. The ssRNAs from each siRNA duplex were tested by gel shift assay for (A) crosslinking, (B) native binding, or (C) native loading control, and (D) the fraction of siRNA crosslinked by MBP-TRBP was quantified within each lane, as in Figure 4. Values are average ± standard deviation; n ≥4. Note the break in the ordinate indicated by the cross-hatch. The ‘Fold Diff.’ values listed below the graph are the ratios between the AS fraction crosslinked and the SS fraction crosslinked. Symbols indicate statistically significant difference for: *, p < 1×10−3 and #, p < 2×10−2.
For the remaining figures where strands were alternately hot and cold labeled, individual single- strands (3 pmol) were labeled as described above (10 pmol of ATP) in 25 μl reactions, where nonisotopic ATP was used for cold labeling. The 5′-O-methyl modified strands were not end-labeled. The two strands comprising the siRNA duplex were then mixed (50 μl), 5.56 μl of 10x STE was added for 1.0× STE final concentration, and samples were heated to 90°C for 3 min and then 37°C for 60 min. Unincorporated label was then removed from the annealed siRNA using the Sephadex columns. Final products were verified on native TBE gels.
Native gel shift assay
TRBP binding was determined by gel shift assays using radiolabeled oligonucleotides present at limiting concentrations relative to protein concentrations. Binding reactions (10 μl) were prepared with ~1–3×104 cpm of nucleic acid and 350 nM MBP-TRBP (unless noted otherwise) in Elution Buffer supplemented with 20 mM HEPES, 40 mM KCl, 1.5 mM MgCl2, 0.1% Nonidet P40, and 10 U SUPERase-In (Ambion). Samples were incubated at room temperature for 30 min, mixed with 2 μl of 5× Nucleic Acid Sample Loading Buffer (BioRad), and 10 μl were loaded onto a pre-run native TBE gel. Mini-gels were prepared where the top half of the gel was 4% (37:1 acrylamide:bisacrylamide) and the bottom half was a gradient between 4–20%. Gels were run at 150 V for ~40 min, dried on filter paper under vacuum at 80°C for 50 min, exposed to a storage phosphor screen overnight (~12–16 hrs), and then imaged on a Storm 860 imager (GE Healthcare). Relative signal intensities were quantified using Quantity One software and normalized within a single lane. Native gels for loading control were similarly prepared but with the volume of MBP-TRBP replaced with an equal volume of Elution Buffer.
UV crosslinking, denaturing gel shift assay
MBP-TRBP crosslinking to nucleic acids was measured as in the native gel shift assay except that after the initial 30 min binding, the samples were subsequently exposed to 312 nm UV light (Fisher Scientific Electrophoresis Systems Transilluminator) for 10 min while on an ice cold aluminum block (4°C). In order to minimize nonspecific crosslinking, the samples were covered by a Petri dish to block shorter wavelengths. Samples were then mixed with 5 μl of 3× SDS Sample Buffer (New England Biolabs), boiled at 95°C for 3 min, collected by brief centrifugation, and 12 μl were loaded onto a pre-run non-linear denaturing 4–20% Tris-HCl gel. Gels were run, dried, imaged, and analyzed as described above. Based on comparison of native binding on native gels and crosslinked sequences run on denaturing gels, we estimate roughly 5–10% crosslinking efficiency (Figure 4, compare A and B). Statistical analyses are reported as p-values from unpaired, two-tailed Student’s t-test assuming unequal variance performed using Microsoft Excel.
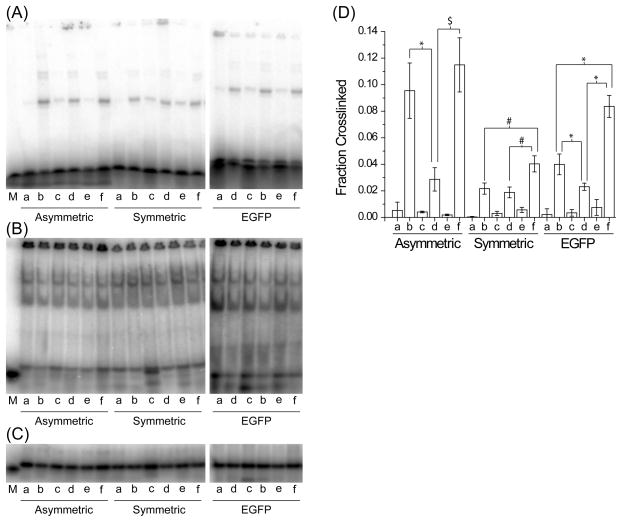
FIGURE 4.
TRBP asymmetry sensing. The siRNAs in Figure 3 were tested by gel shift assay with recombinant MBP-TRBP for (A) crosslinking, (B) native binding, or (C) native loading control, as described in the text and Materials and Methods. (D) The fraction of siRNA crosslinked by MBP-TRBP was quantified within each lane (fraction crosslinked = crosslinked signal/(crosslinked signal + uncrosslinked signal)). Values are average ± standard deviation; the number of measurements, n, were n ≥ 5 for ‘A’ and ‘G’; n ≥ 3 for ‘S’. M denotes 21 nt single-stranded RNA used as denaturing size marker. Note that EGFP ‘ b’ and ‘d’ siRNAs were interchanged on the gels as compared to the asymmetric or symmetric gel loading. Symbols indicate statistically significant difference for: *, p < 1×10−4; $, p < 1×10−3; and #, p < 1×10−2. For complete statistical comparisons, see Table 6 in the Supporting Information.
Results
Production and characterization of recombinant MBP-TRBP
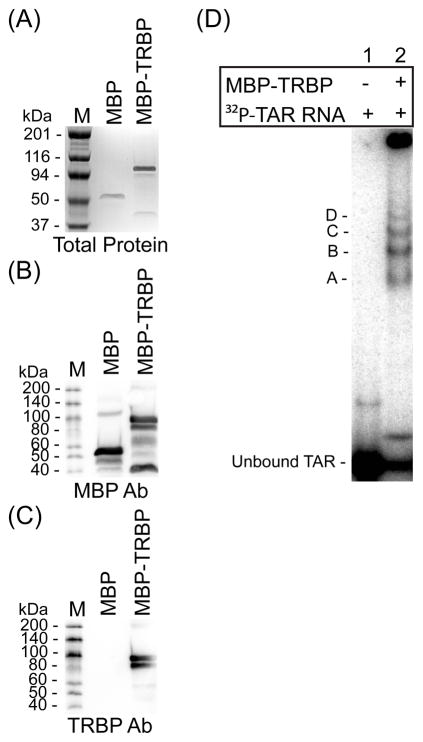
To study the contribution of TRBP to siRNA asymmetry sensing in humans, we expressed and purified recombinant human TRBP as a fusion product with maltose binding protein (MBP), as well as MBP alone for control (Figure 1). Both plasmids were generously provided by Professor Anne Gatignol (27, 35). Purified MBP and MBP-TRBP products were resolved by denaturing gel electrophoresis and visualized by protein staining or western blotting (Figure 1A–C). Proper function of the MBP-TRBP was then characterized by its ability to bind TAR RNA using a native gel shift assay (Figure 1D). Multiple shifted complexes were visible, consistent with previously observed multimerization of TRBP (35, 36).
FIGURE 1.
Characterization of recombinant TAR RNA binding protein (TRBP). Recombinant TRBP was prepared as a fusion product with maltose binding protein (MBP) as described (Materials and Methods). (A) Purified MBP or MBP-TRBP proteins were resolved by SDS/PAGE and visualized by staining with Gel Code Blue. (B and C) Western blots of gels as shown in (A) were performed with antibodies specific for (B) MBP or (C) TRBP. (D) Gel mobility-shift analysis of MBP-TRBP with 32P-labeled TAR RNA was achieved by incubating 1000 nM of protein with a limiting amount of TAR RNA (104 cpm; <1.0 nM) and resolving protein-RNA complexes by native PAGE. Four distinct complexes were visible (bands A–D). M = protein size marker (kDa).
TRBP binds dsRNA with the greatest affinity
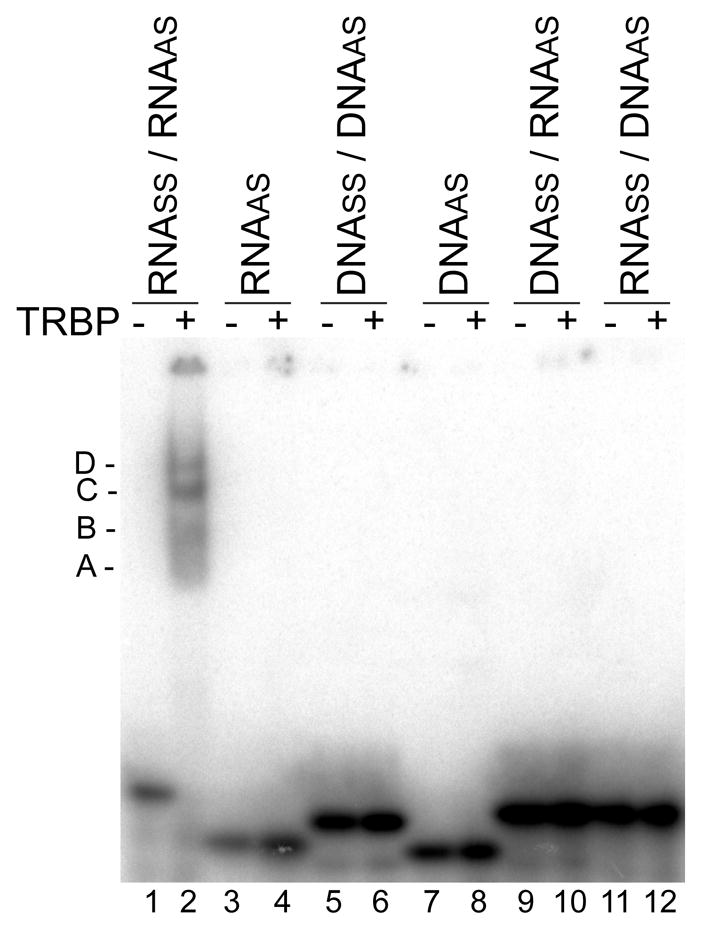
TRBP has previously been shown to bind siRNAs (37–39). We also found that to be the case in a native gel shift assay (Figure 2, lanes 1 and 2) with MBP-TRBP and an siRNA targeting EGFP used previously (40). Nucleic acid sequence information is provided in Tables 1–5 of the Supporting Information. MBP-TRBP formed at least four distinct shifted complexes with the siRNA, corresponding approximately to complexes containing at least one siRNA molecule in a complex with one, two, four, or ~six MBP-TRBP molecules (Figure S1 of the Supporting Information). As expected, larger complexes became more prominent at higher MBP-TRBP concentrations. Furthermore, although TRBP can bind long ssRNAs (41), no binding was observed for the ssRNA corresponding to the antisense (AS) (Figure 2, lanes 3 and 4) or sense (SS) (Figure 6B, EGFP S strand) strands of the duplex siRNA at this MBP-TRBP concentration. In addition, no binding was found for an siDNA or the corresponding single-stranded AS siDNA counterpart (Figure 2, lanes 5–8). We next tested DNA-RNA hybrids as these have been shown to be RNAi-competent (37, 42, 43). Somewhat surprisingly, no binding was detected with the heteroduplexes, either. Repeating the experiment with MBP alone confirmed that any observed binding was due to TRBP and not non-specific binding by the MBP portion of the fusion product (Figure S2 of the Supporting Information). These results are consistent with TRBP requiring A-form, double-stranded RNA regions for high-affinity binding, typical of dsRBPs containing dsRBDs (44).
TRBP binds preferentially to one strand of an asymmetric siRNA
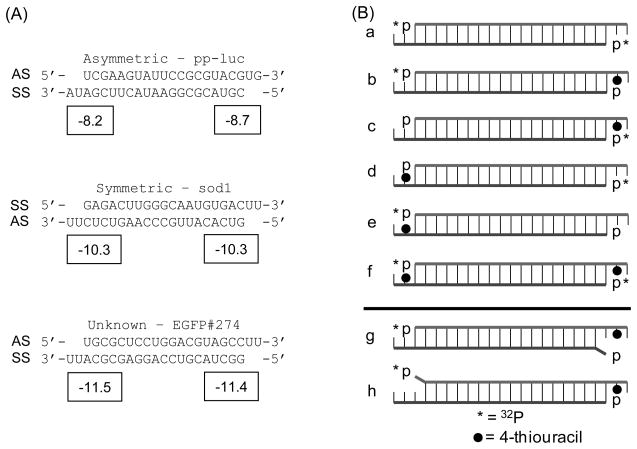
It has been hypothesized that TRBP senses siRNA asymmetry and therefore contributes to guide strand selection in RISC. In order to investigate this putative function of TRBP, we prepared three siRNAs, two of which are known to load into Drosophila RISC based on their strand thermodynamics (34) and are consistent with ΔΔG energetics calculated with existing methods (45) using current nearest- neighbor parameters and a terminal A:U penalty of 0.5 kcal/mol (46). These sequences correspond to pp-luciferase (asymmetric, ‘A’; ΔΔG = (ΔG for the AS 5′-end − ΔG for the SS 5′-end) = 0.5 kcal/mol) and human Cu, Zn-superoxide dismutase (sod1) (symmetric, ‘S’; ΔΔG = 0.0 kcal/mol). The third sequence targets EGFP and was found to be moderately active (~60% knockdown at 33 nM concentration) from our previous work in human HeLa and HepG2 cells but is predicted to be essentially symmetric (EGFP, ‘G’; ΔΔG = −0.1 kcal/mol) (Figure 3A) (40). Position 20 of each strand was chemically modified with a 4-thiouracil to allow for position-specific photocrosslinking (47), similar to the methods used for characterizing the Drosophila proteins using 5-iodouracil modified siRNAs (7). For each of these sequences, a total of six different siRNAs were created by alternately hot ([γ-32P]-ATP) or cold (ATP) end-labeling the chemically modified or unmodified single-stranded RNAs, followed by annealing with the corresponding complementary strand (Figure 3B, a–f).
FIGURE 3.
Nucleic acids used. (A) Three siRNA sequences were used in this study. Two have been documented previously for their asymmetric (pp-luc; referred to in the text as sequence ‘A’) or symmetric (sod1; sequence ‘S’) silencing activities and recognition by Dicer-2/R2D2 in Drosophila (7, 34). The third was previously used for EGFP silencing (EGFP; sequence ‘G’) and was expected to be slightly asymmetric based on silencing activity (40) and ΔΔG calculation with mfold (57). AS denotes the antisense strand and SS denotes the sense strand. Boxed numbers indicate ΔG values (in kcal/mol) calculated from the four terminal nearest-neighbor base-pairs, including the A:U penalty and 3′-overhang contribution (single base stacking energy). (B) A series of 8 different siRNAs were created for each sequence as described in the text. The strand location in (A) matches the strand location in (B). * denotes strands 5′-labeled with 32P-ATP; ● denotes 4-thiouracil modification at position 20; siRNAs ‘g’ and ‘h’ contain a single 5′-terminal mismatch as indicated by the protruding base.
The relative crosslinking efficiency of each siRNA was then determined using recombinant MBP-TRBP in a denaturing gel shift assay (Figure 4A and D). MBP-TRBP showed stronger crosslinking to the asymmetric ‘b’ siRNA (Ab) relative to the ‘d’ (Ad) structure (Figure 4A and D; compare Ab to Ad) and similarly for the EGFP siRNA (Figure 4A and D; compare Gb to Gd). However, almost equal crosslinking was detected for the symmetric siRNA Sb and Sd forms (Figure 4A and D; compare Sb to Sd). As expected, little signal was returned when radiolabel and crosslinker were not present on the same strand, showing that crosslinking is specific for the 4-thiouracil positions (Figure 4A and D; compare ‘b’, ‘d’, and ‘f’ to ‘a’, ‘c’, and ‘e’). As further confirmation, for all three siRNAs, the crosslinking of ‘f’ was approximately the sum of ‘b’ and ‘d’. Since all siRNAs were bound equally in a native gel shift assay (Figure 4B) and were comparably loaded (Figure 4C), we conclude that recombinant MBP-TRBP preferentially crosslinks to the more stably hybridized end of the siRNA duplex. Similar trends were observed using HepG2 cytoplasmic extracts (Figure S3 of the Supporting Information), confirming that this behavior is not purely an in vitro artifact and that TRBP can detect siRNA asymmetry in the presence of other proteins, both competing and not.
It should be noted that the absolute level of crosslinking varies among the three sequences (compare the ‘f’ signals for each sequence). We were unable to identify one factor that determined the overall crosslinking efficiency for a particular sequence. However, because the ‘f’ signals for each sequence equal the sum of the ‘b’ and ‘d’ signals, comparisons within a particular duplex can be reliably made. It is tempting to attribute the differences in crosslinking to the differences in overhang sequence among the three siRNAs. However, the symmetric and EGFP sequences both have 3′-UU overhangs on each strand, with the crosslinking still asymmetric for the EGFP sequence. Thus, while other sequence or structural factors may alter the crosslinking efficiency of each siRNA relative to another siRNA, comparisons of ‘b’ versus ‘d’ for any single sequence reveal the presence or absence of asymmetry within a sequence and the ability of TRBP to detect the asymmetry.
Terminal mismatches can reduce binding and crosslinking by TRBP
Since the terminal base-pairs in the siRNA have been shown to be critical for establishing siRNA asymmetry, we next considered what effect the introduction of sequence mismatches would have on TRBP asymmetry sensing (analogous to (7) for R2D2). Single nucleotide mismatches were introduced at either position 1 or 19 of the bottom-strand of each siRNA (Figure 3; compare ‘g’ and ‘h’ to ‘b’). The bottom-strands were cold labeled and then annealed to the hot labeled, chemically-modified top-strand. The mismatched ends should be destabilized, reducing TRBP binding and either reducing crosslinking (‘g’) or increasing crosslinking (‘h’). Each siRNA was tested with recombinant MBP-TRBP in the gel shift assays (Figure 5, compare ‘g’ and ‘h’ to ‘b’ for each siRNA). For the asymmetric sequence, a mismatch at the 5′-end of the SS did result in reduced crosslinking (Figure 5A and D; compare Ag to Ab). However, we only detected a modest increase in crosslinking as a result of the mismatch at the AS 5′-end (Figure 5D; compare Ah to Ab). This may reflect the fact that this asymmetric sequence already has strongly biased TRBP crosslinking, so increasing the asymmetry cannot further enhance binding to the more-stable end. However, for the symmetric and EGFP sequences, neither mismatch resulted in any statistically significant change in crosslinking (Figure 5D; compare Sg and Sh to Sb; and Gg and Gh to Gb), which may be due to the ~2-fold lower crosslinking compared to the asymmetric sequence despite the fact that all native binding experiments indicated nearly complete binding. Taken together, our results show that mismatches can reduce TRBP binding in the expected fashion, but additional characterization will be required to determine why this effect is not consistent for all siRNAs in our system.
FIGURE 5.
Mismatched siRNAs alter TRBP asymmetry. The siRNAs were tested by gel shift assay for (A) crosslinking, (B) native binding, or (C) native loading control, and (D) the fraction of siRNA crosslinked by MBP-TRBP was quantified within each lane, as in Figure 4. Values are average ± standard deviation; n ≥3. M denotes single-stranded siRNA used as denaturing size marker. Symbols indicate statistically significant difference for: *, p < 1×10−2 and #, p < 3×10−2. For complete statistical comparisons, see Table 6 in the Supporting Information.
TRBP does not bind DNA/RNA heteroduplexes
As we saw similar binding but different crosslinking among the three siRNAs, we wanted to revisit MBP-TRBP binding and crosslinking of DNA/RNA hybrids (Figure 5). For all three of the sequences tested, none were appreciably bound (Figure 2, lanes 9–12, where the sequences of the heteroduplexes tested - for binding only - correspond to the EGFP sequence; also Figure 5B, ‘i’ and ‘j’, where ‘i’ and ‘j’ correspond to ‘b’ and ‘d’ in Figure 3, with the bottom- or top-strand of the siRNAs replaced with DNA, respectively) or crosslinked by MBP-TRBP (Figure 5A and D, ‘i’ and ‘j’). It has been shown recently that 5′-O-methyl modifications can alter guide strand selection and the propensity for off-target silencing (48), presumably by preventing 5′-phosphorylation by hClp (49). To examine the effect of this modification on TRBP binding and asymmetry sensing, we created EGFP siRNAs with the 5′-O-methyl modification on either end and the 4-thiouracil-modified complementary strand being radiolabeled (Gk with the bottom-strand containing the 5′-O-methyl corresponds to Gg because the 5′-O-methyl groups are only available on T nucleotides and consequently introduced a C:T mismatch at that end; Gl corresponds to Gd with the top-strand modified). We expected binding and therefore crosslinking in both cases to decrease due to the proximity of the 5′-O-methyl group and the 4-thiouracil modification. However, the methylation had no apparent effect on MBP-TRBP binding, and instead increased the overall amount of crosslinking (Figure 5A and D; compare Gk to Gg, and Gl to Gd). Thus, precluding the interaction with TRBP is likely not the means by which these modifications prevent incorporation of these strands into RISC and that the cellular effects on activity or off-target silencing are a result of additional factors associated with the RLC/RISC, such as Dicer which has lower affinity for 5′-O-methyl modified siRNAs (50).
TRBP differentially binds small single-stranded RNAs
Given that TRBP can sense siRNA asymmetry, and despite its apparent inability to effectively bind short ssRNA (Figure 2), we also wanted to consider whether or not any other short ssRNA sequences could be bound or crosslinked by MBP-TRBP. We repeated the gel shift assay with the individually hot labeled, 4-thiouracil modified ssRNAs from each of the three siRNA sequences, at increased MBP-TRBP concentration (~3-fold higher; 1200 nM) (Figure 6). Intriguingly, the strand favorably crosslinked when part of the asymmetric siRNAs (Figure 4A and D, ‘b’ for ‘A’ and ‘G’) was also preferentially crosslinked here as a single-strand (Figure 6A and D; compare AS to SS for ‘A’ and ‘G’), whereas the single-strands corresponding to the symmetric siRNA were crosslinked more evenly (Figure 6A and D; compare AS to SS for ‘S’). Curiously, the native binding pattern did not appear to reflect that of the crosslinking gel (Figure 6B), which might be expected for a relatively low affinity interaction that could be altered by the conditions for electrophoresis; nor are the patterns a direct result of ssRNA self-dimers being recognized by TRBP (Figure 6; compare A and B to C).
As with siRNA crosslinking, the overall crosslinking efficiency among the single-stranded sequences varies (Figure 6). This further suggests that crosslinking efficiency may be affected by the sequence of the ssRNA/siRNA. Still, if each antisense strand is compared to its complementary sense strand, there is a preference towards crosslinking the antisense strand in each case, with a more pronounced preference for the ssRNAs from the siRNAs that crosslinked asymmetrically. While this supports our contention that sequence matters in TRBP-RNA interactions, further study will certainly be required to explain this result completely and to ascertain the influence of factors such as base position and 5′-end versus 3′-end effects.
Discussion
Of the three proteins known to constitute the minimal human RLC, Dicer, Ago2, and TRBP, only the functional role of TRBP in the complex had not been concretely established. Here, we show for the first time that TRBP alone binds preferentially to one end of asymmetric siRNAs in vitro, presumably leading to asymmetry in the eventual guide strand loading onto Ago2. The capacity to bind both siRNAs and 21 nt ssRNAs suggests a potential role for TRBP prior to, during, and possibly after passenger strand cleavage by Ago2.
An important step in RLC formation is the initial binding of the double-stranded siRNA duplex. Dicer and Ago2 appear to be less capable of binding siRNA alone (19, 51), while TRBP shows a relatively higher affinity (KD ~1–100nM; data not shown and (38)). When in solution, TRBP homodimerizes (10, 14), which may contribute to TRBP forming multimeric complexes on dsRNA even as short as 21 base-pairs (e.g., siRNA; Figure 2 and Figure S1 of the Supporting Information) in an uncooperative manner (38). This suggests that TRBP is functionally distinct from dsRBPs that show improved binding with dsRNA length, such as C. elegans RDE-4 (38). Consistent with what has been observed for other dsRBPs and with dsRBD function in general (44, 52), TRBP was unable to bind double-stranded siDNA or RNA/DNA hybrids (Figure 2 and 5). Though some DNA substitutions are tolerated in siRNAs for gene silencing (37), substitutions at the 3′-end disrupted silencing, presumably by impairing TRBP binding. These results are in agreement with our observations (Figure 2 and 5), and it would be interesting to see how shorter DNA segments spread throughout the siRNA would impact binding and crosslinking by TRBP.
Though all siRNAs appear to be bound by TRBP with similar high affinity, and apparently independent of sequence (Figure 4B and 5B and data not shown), in our hands, TRBP showed a clear asymmetry in crosslinking, seemingly driven by both sequence and strand thermodynamics (Figure 4 and 6). TRBP recognized two siRNAs asymmetrically (‘A’ and ‘G’) and one symmetrically (‘S’) in our crosslinking gel shift assay (Figure 4A and D). For two of the siRNAs tested, our results are consistent with those previously shown in Drosophila RISC loading (34) and in crosslinking by Dicer-2/R2D2 (7), and agree with relative thermodynamic asymmetries calculated by established techniques (45) (Figure 3A). Interestingly, the third sequence (‘G’) is predicted to be only minimally asymmetric (ΔΔG = −0.1 kcal/mol), but favoring the opposite end from what was experimentally determined (Figure 3 and 4, Gb vs. Gd). Thus, published methods for calculating asymmetry do not satisfactorily predict all of our observations with recombinant MBP-TRBP.
We attempted to define other parameters that predicted all of our experimental results. We noticed that both the ‘A’ and ‘G’ siRNAs contained an A:U at the AS 5′-end and a G:C at the SS 5′-end, whereas the ‘S’ siRNA contained a G:C at both ends. Thus, asymmetry based solely on the first base/base-pair on each end of the siRNAs would be sufficient to explain the TRBP crosslinking. A similar conclusion was reached from a computational analysis, finding that one nearest-neighbor parameter at each end of the siRNA gave the best predictive value for silencing activity (32). In addition, recent work shows, both in vivo and using an in vitro crosslinking assay, that the terminal sequence of miRNA and miRNA* strands results in their being preferentially loaded onto either Ago1 or Ago2 in Drosophila (53). Thus, the influence of terminal sequence appears to be a general phenomenon of the RNAi pathway.
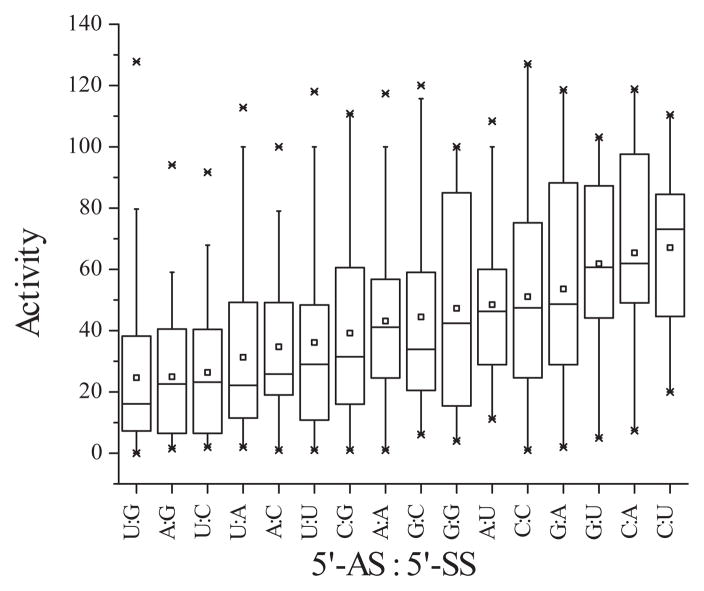
We pursued this further through analysis of a literature dataset (54), as we had in previous work investigating mRNA target structure effects (40). We sorted the tabulated siRNA activities according to the nucleotide at the 5′-end of the AS relative to the nucleotide at the 5′-end of the SS. The most active siRNAs tend to have a U at the 5′-end of the AS and a G at the 5′-end of the SS (Figure 7 and Figure S4 of the Supporting Information). Generally, the order of nucleotide preference was U > A > G > C, for the 5′-nucleotide on the AS, and G > C > A > U, for the 5′-nucleotide on the SS. These observations agree completely with those made earlier by Reynolds et al (30). This is consistent with what would be required for thermodynamic asymmetry but also implies a role for sequence, independent of stability. For instance, a U:A combination (not base-pair) is statistically more likely to be a good silencer than an A:U combination (Figure 7 and Figure S4 of the Supporting Information). While this data is for silencing efficiency, it agrees with our hypothesis that asymmetry may be better predicted from only the 5′-terminal nucleotides on each siRNA strand. Further, the agreement of our analysis of this dataset with our experimental findings gives us confidence that our crosslinking results are not an artifact of the three sequences chosen but a real and natural behavior of TRBP in identifying the eventual guide strand of the siRNA.
FIGURE 7.
Computational analysis of first nucleotide influence on siRNA activity. siRNAs from the literature (54) were sorted according to the nucleotides at both the AS 5′-end and the SS 5′-end (5′-AS:5′-SS) and plotted in order of decreasing mean silencing activity. The small box indicates the mean; the center line, the median; the lower and upper box lines, the 25th and 75th percentiles; the outer bars, the 95th percentile; and the points, outliers.
Current siRNA selection algorithms weigh duplex asymmetry heavily in choosing siRNAs likely to be highly active (32, 33). While mismatches can dramatically alter relative asymmetry, our crosslinking studies did not show a consistent effect (Figure 5). In particular, for the ‘S’ and ‘G’ siRNAs, mismatches had no apparent effect on MBP-TRBP crosslinking, which was unexpected given that the mismatches in ‘S’ were previously shown to alter its loading in Drosophila RISC (7, 34). This may reflect a difference in the functions of the human and Drosophila proteins. TRBP alone may not entirely recapitulate the function of the Dicer-2/R2D2 heterodimer in Drosophila. Our group has recently shown that in human cytoplasmic extracts a single terminal mismatch can reduce TRBP binding while enhancing Dicer binding (55). This suggests that, while TRBP alone can sense asymmetry in siRNAs in vitro, its sensing function in vivo may result from TRBP working in conjunction with other components of the RNAi pathway. Recent evidence further supports this coordinated function, as the termini of siRNA are predicted to be primarily occupied by Dicer and Ago2 based on the structure of the human RLC (24).
In Drosophila, it was shown that the RLC (Dicer-2/R2D2/Ago2) initiates unwinding of the siRNA, with some RLCs containing ssRNAs (7). Our results showing TRBP crosslinking to ssRNAs (Figure 6) suggests that in humans TRBP may provide some stabilization of ssRNA-containing RLCs prior to loading onto Ago2. In addition, our TRBP binding results are similar to what we and others have previously shown for human Dicer (51, 56). Surprisingly, and potentially more importantly, TRBP preferentially crosslinked to the ssRNA known to be the guide strand in each pair (Figure 6B; compare AS to corresponding SS for each sequence). We examined if ssRNA secondary structure was responsible for differences in binding/crosslinking, but no patterns were discernible for structures predicted by mfold (57) (data not shown). We expect further investigation will reveal specific sequence or positional effects that impact TRBP-ssRNA binding and crosslinking.
While we have shown that TRBP can sense duplex asymmetry, other questions remain unanswered. Do other proteins enhance the sensing capacity of TRBP as Dicer-2 appears to in Drosophila (7)? This may be particularly important for better sensing of terminally-mismatched siRNAs. Quite possibly, Dicer also assists in siRNA unwinding, perhaps through its helicase domain which has been shown to be important for processing thermodynamically unstable shRNAs (58). Can other proteins sense asymmetry alone? The most likely candidate is PACT, due to its known homology to TRBP and associations with Dicer, TRBP, and Ago2 (12, 14, 27). How is asymmetry best predicted? Current methods for prediction should be coupled to in vitro crosslinking and silencing readouts to assess whether factors that are important for TRBP binding in vitro are important for guide strand selection and silencing activity. Further study on this point will provide both improved siRNA activity predictions as well as improved understanding of the RNAi mechanism.
Supplementary Material
Acknowledgments
Funding information: Financial support for this work was provided in part by Michigan State University, the National Science Foundation (#0425821), and the National Institutes of Health (#CA126136, #GM079688, #RR024439).
The authors thank Professor Gatignol for supplying TRBP/MBP plasmids and technical advice, Professor K-T Jeang for supplying TAR RNA plasmids for in vitro transcription, and members of the Cellular and Biomolecular Laboratory for helpful discussions.
Abbreviations
- AS
antisense strand
- dsRNA
double-stranded RNA
- dsRBD
dsRNA binding domain
- dsRBP
dsRNA binding protein
- miRNA
microRNA
- nt
nucleotide
- RISC
RNA induced silencing complex
- RLC
RISC loading complex
- siRNA
small interfering RNA
- SS
sense strand
- TRBP
TAR RNA binding protein
Footnotes
Supporting Information Available
The supporting information provides siRNA sequence information and additional binding and statistical results. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev. 2005;19:2979–2990. doi: 10.1101/gad.1384005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 8.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human RISC-loading complex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:512–517. doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robb GB, Rana TM. RNA Helicase A Interacts with RISC in Human Cells and Functions in RISC Loading. Mol Cell. 2007;26:523–537. doi: 10.1016/j.molcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. The EMBO Journal. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC Couples MicroRNA Biogenesis and Posttranscriptional Gene Silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT Directly Interact with Each Other and Associate with Dicer to Facilitate the Production of Small Interfering RNA. J Biol Chem. 2007;282:17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 Is the Catalytic Engine of Mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 16.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 Mediates RNA Cleavage Targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Rand TA, Petersen S, Du F, Wang X. Argonaute2 Cleaves the Anti-Guide Strand of siRNA during RISC Activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal Structure of Argonaute and Its Implications for RISC Slicer Activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 19.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 20.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-Strand Cleavage Facilitates Assembly of siRNA into Ago2-Containing RNAi Enzyme Complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 22.Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23:227–231. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- 23.Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, Rossi JJ, Behlke MA. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic acids research. 2005;33:4140–4156. doi: 10.1093/nar/gki732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HW, Noland C, Siridechadilok B, Taylor DW, Ma E, Felderer K, Doudna JA, Nogales E. Structural insights into RNA processing by the human RISC-loading complex. Nat Struct Mol Biol. 2009;16:1148–1153. doi: 10.1038/nsmb.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatignol A, Buckler-White A, Berkhout B, Jeang KT. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991;251:1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- 26.Benkirane M, Neuveut C, Chun RF, Smith SM, Samuel CE, Gatignol A, Jeang KT. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. The EMBO Journal. 1997;16:611–624. doi: 10.1093/emboj/16.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laraki G, Clerzius G, Daher A, Melendez-Pena C, Daniels S, Gatignol A. Interactions between the double-stranded RNA-binding proteins TRBP and PACT define the Medipal domain that mediates protein-protein interactions. RNA biology. 2008;5:92–103. doi: 10.4161/rna.5.2.6069. [DOI] [PubMed] [Google Scholar]
- 28.Daniels SM, Melendez-Pena CE, Scarborough RJ, Daher A, Christensen HS, El Far M, Purcell DF, Laine S, Gatignol A. Characterization of the TRBP domain required for dicer interaction and function in RNA interference. BMC Mol Biol. 2009;10:38. doi: 10.1186/1471-2199-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Rand TA, Kalidas S, Du F, Kim HE, Smith DP, Wang X. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science. 2003;301:1921–1925. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 31.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 32.Lu ZJ, Mathews DH. Efficient siRNA selection using hybridization thermodynamics. Nucleic acids research. 2007;36:640–647. doi: 10.1093/nar/gkm920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao Y, Chan CY, Maliyekkel A, Lawrence CE, Roninson IB, Ding Y. Effect of target secondary structure on RNAi efficiency. RNA. 2007;13:1631–1640. doi: 10.1261/rna.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 35.Daviet L, Erard M, Dorin D, Duarte M, Vaquero C, Gatignol A. Analysis of a binding difference between the two dsRNA-binding domains in TRBP reveals the modular function of a KR-helix motif. Eur J Biochem. 2000;267:2419–2431. doi: 10.1046/j.1432-1327.2000.01256.x. [DOI] [PubMed] [Google Scholar]
- 36.Cosentino GP, Venkatesan S, Serluca FC, Green SR, Mathews MB, Sonenberg N. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9445–9449. doi: 10.1073/pnas.92.21.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ui-Tei K, Naito Y, Zenno S, Nishi K, Yamato K, Takahashi F, Juni A, Saigo K. Functional dissection of siRNA sequence by systematic DNA substitution: modified siRNA with a DNA seed arm is a powerful tool for mammalian gene silencing with significantly reduced off-target effect. Nucleic acids research. 2008;36:2136–2151. doi: 10.1093/nar/gkn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker GS, Maity TS, Bass BL. dsRNA binding properties of RDE-4 and TRBP reflect their distinct roles in RNAi. Journal of Molecular Biology. 2008;384:967–979. doi: 10.1016/j.jmb.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh T, Suzuki T. Specific residues at every third position of siRNA shape its efficient RNAi activity. Nucleic acids research. 2007:e27. doi: 10.1093/nar/gkl1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gredell JA, Berger AK, Walton SP. Impact of target mRNA structure on siRNA silencing efficiency: A large-scale study. Biotechnol Bioeng. 2008;100:744–755. doi: 10.1002/bit.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatignol A, Buckler C, Jeang KT. Relatedness of an RNA-binding motif in human immunodeficiency virus type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and Drosophila staufen. Molecular and cellular biology. 1993;13:2193–2202. doi: 10.1128/mcb.13.4.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamberton JS, Christian AT. Varying the nucleic acid composition of siRNA molecules dramatically varies the duration and degree of gene silencing. Molecular biotechnology. 2003;24:111–120. doi: 10.1385/MB:24:2:111. [DOI] [PubMed] [Google Scholar]
- 43.Hohjoh H. RNA interference (RNA(i)) induction with various types of synthetic oligonucleotide duplexes in cultured human cells. FEBS Lett. 2002;521:195–199. doi: 10.1016/s0014-5793(02)02860-0. [DOI] [PubMed] [Google Scholar]
- 44.Saunders LR, Barber GN. The dsRNA binding protein family: critical roles, diverse cellular functions. Faseb J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- 45.Hutvagner G. Small RNA asymmetry in RNAi: Function in RISC assembly and gene regulation. FEBS Letters. 2005;579:5850–5857. doi: 10.1016/j.febslet.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 46.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 47.Sontheimer EJ. Site-specific RNA crosslinking with 4-thiouridine. Molecular biology reports. 1994;20:35–44. doi: 10.1007/BF00999853. [DOI] [PubMed] [Google Scholar]
- 48.Chen PY, Weinmann L, Gaidatzis D, Pei Y, Zavolan M, Tuschl T, Meister G. Strand-specific 5′-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA. 2008;14:263–274. doi: 10.1261/rna.789808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weitzer S, Martinez J. The human RNA kinase hClp1 is active on 3′ transfer RNA exons and short interfering RNAs. Nature. 2007;447:222–226. doi: 10.1038/nature05777. [DOI] [PubMed] [Google Scholar]
- 50.Pellino JL, Jaskiewicz L, Filipowicz W, Sontheimer EJ. ATP modulates siRNA interactions with an endogenous human Dicer complex. RNA. 2005;11:1719–1724. doi: 10.1261/rna.2102805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kini HK, Walton SP. In vitro binding of single-stranded RNA by human Dicer. FEBS Lett. 2007;581:5611–5616. doi: 10.1016/j.febslet.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 53.Ghildiyal M, Xu J, Seitz H, Weng Z, Zamore PD. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA. 2010;16:43–56. doi: 10.1261/rna.1972910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shabalina SA, Spiridonov AN, Ogurtsov AY. Computational models with thermodynamic and composition features improve siRNA design. BMC Bioinformatics. 2006;7:65. doi: 10.1186/1471-2105-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kini HK, Walton SP. Effect of siRNA terminal mismatches on TRBP and Dicer binding and silencing efficacy. FEBS J. 2009;276:6576–6585. doi: 10.1111/j.1742-4658.2009.07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima WF, Murray H, Nichols JG, Wu H, Sun H, Prakash TP, Berdeja AR, Gaus HJ, Crooke ST. Human Dicer Binds Short Single-strand and Double-strand RNA with High Affinity and Interacts with Different Regions of the Nucleic Acids. J Biol Chem. 2009;284:2535–2548. doi: 10.1074/jbc.M803748200. [DOI] [PubMed] [Google Scholar]
- 57.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic acids research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soifer HS, Sano M, Sakurai K, Chomchan P, Saetrom P, Sherman MA, Collingwood MA, Behlke MA, Rossi JJ. A role for the Dicer helicase domain in the processing of thermodynamically unstable hairpin RNAs. Nucleic acids research. 2008;36:6511–6522. doi: 10.1093/nar/gkn687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.