Abstract
Acute HIV-1 infection is characterized by high levels of immune activation. Immunomodulation with Cyclosporin A combined with antiretroviral therapy (ART) in the setting of acute and early HIV-1 infection has been reported to result in enhanced immune reconstitution. Fifty-four individuals with acute and early infection were randomized to receive ART with 4 weeks of Cyclosporine A versus ART alone. In 48 subjects who completed the study, there were no significant differences between treatment arms in levels of proviral DNA or CD4+ T cell counts. Adjunctive therapy with Cyclosporine A in this setting does not provide apparent virologic or immunologic benefit.
Introduction
Acute Human Immunodeficiency Virus type 1 (HIV-1) infection is characterized by high levels of viremia resulting from rapid rounds of viral replication. Destruction of CD4+ T cells in massive numbers, particularly in the gastrointestinal tract, is associated with significant immune activation with high levels of circulating inflammatory cytokines [1-3]. The initiation of antiviral therapy (ART) during acute infection results in prompt and highly effective suppression of viral replication, increases in levels of circulating CD4+ T cells and partial reconstitution of the mucosal pathology that characterizes acute infection [4-6]. Moreover, treatment during acute infection also results in amelioration of symptoms, shortened duration of the acute illness, and may provide some long term virologic and immunologic benefit [7-9].
Cyclosporine A suppresses the cellular immune response by inhibiting IL-2 transcription after T-cell receptor-mediated signal transduction thus inhibiting IL-2 induced activation and proliferation [10, 11]. Since highly activated CD4+ T cells are the preferred target for HIV-1 replication, inhibiting activation during primary infection may reduce the numbers of susceptible viral targets. This would in turn preserve immune function, particularly HIV-1 specific immune responses, as it has been shown that HIV-1-specific CD4+ T cells are preferentially infected during HIV-1 infection [12]. Rizzardi and coworkers proposed the use of low dose Cyclosporine A during acute HIV infection and reported that 8-weeks of low dose Cyclosporine A as an adjunct to ART in 9 newly infected individuals resulted in dramatically improved and sustained levels of CD4+ T cells and HIV-1 specific responses when compared to historical controls [13].
Based on these results we performed a randomized, open-label study of Cyclosporine A for 4-weeks in combination with ART in subjects identified and treated during acute and early HIV-1 infection. We hypothesized that we would confirm higher CD4+ T cell levels, a sustained reduction in levels of immune activation, and a reduction in the number of cells harboring proviral DNA in the cyclosporine A-treated patients.
Methods
Patients 18 or older were enrolled in the trial with acute and early infection based on laboratory criteria within 28 days of screening. The presence of plasma viremia above 50,000 copies/mL in the presence of an absent or indeterminate antibody response was considered acute infection. Patients with a positive serologic response were classified as early and separated into two groups- those with 3 bands or less on a Western Blot and those with 5 bands or less on a Western Blot. All study sites received approval from local institutional review boards and all participants gave written informed consent.
Patients were assigned to one of 2 arms using a 2:1 randomization scheme in this open label study. Subjects assigned to Arm A received ART including 1 tablet of fixed dose zidovudine (ZDV) 300mg/lamivudine (3TC) 150mg/abacavir (ABC) 300mg (Trizivir) in combination with 3 soft-gel capsules of fixed dose combination lopinavir 133.3mg /33.3 ritonavir (LPV/rtv) taken twice daily with food with a 4-week course of liquid Cyclosporine A 0.3 mg/kg based on ideal body weight. Subjects assigned to Arm B received the same ART alone. Cyclosporine A levels were monitored at day 3, weeks 1, 2 and 3 with target trough concentrations of 250 to 450 ng/mL.
Genotypic testing on baseline viruses for resistance was performed in all patients on pretreatment plasma samples. Those with resistance to LPV/rit or to two additional components of the treatment regimen were removed from study. Study visits were conducted at day 3, weeks 1, 2, 3, 4 and every 4 weeks through weeks 48. All participants were required to remain on LPV/rit throughout the first 4 weeks of study after which substitutions were allowed based on patient or investigator preference if treatments with at least 3 fully active agents were maintained based on baseline resistance testing and treatment regimens conformed to preferred treatment guidelines.
HIV-1 RNA levels were measured using the Roche COBAS Amplicor Assay with a lower limit of detection of 50 copies/mL plasma. Proviral DNA levels were determined at a single site using a modification of published real-time PCR methods [14]. Routine (CD4+ and CD8+ T cell quantitation) and advanced T cell subsets were measured locally. Advanced flow studies were performed by flow cytometry incorporating the following markers: CD45RA, CD38, HLA-DR, and Ki67.
Statistical methods and analysis
The study was designed to enroll 50 subjects anticipating a drop out rate of 10% resulting in 45 evaluable patients with a primary endpoint being the level of proviral DNA at week 48.This resulted in 84% power to detect a difference of 0.5 log10 between treatment arms. The Fisher's exact test was used for comparisons of sex/race/ethnicity and entry criteria. The Wilcoxon rank test was used to compare age, CD4+ T cell and subset levels, and proviral DNA levels at weeks 12, 24, 48. The Gehan-Wilcoxon rank test was used for comparisons between arms for HIV-1 RNA levels and time to virologic suppression, adjusted for censoring below the limit of detection of 50 copies/mL. The interquartile ranges (IQR) were reported for the various parameters when appropriate. The geometric mean and 95% confidence intervals (CI) of proviral DNA levels were computed for each arm at weeks 12, 24, and 48. In addition, the mean and 95% CI of CD4+ T cell values were computed. The square root transformation was used in order to improve the symmetry of the CD4+ T cell distribution. A similar analysis was done for additional T cell subsets.
Results
Fifty-four individuals were randomized to treatment- 36 into Arm A and 18 into Arm B. Forty-one of 54 completed 44-48 weeks of therapy and were included in the analysis and are described in Table 1. Thirteen subjects failed to complete the study, 8 subjects (22%) in Arm A and 5 (28%) in Arm B (p=1.0). Reasons for patient discontinuation included non-adherence with study medications/procedures (N=4), lost to follow up (N=3), protocol defined virologic failure due to non-adherence (N=2), adverse events (N=1), baseline resistance to LPV/rit (N=1), suicide (N=1), and investigator preference (N=1).
Table 1.
Baseline characteristics of 41 subjects completing the trial
| Arm A (N=28) | Arm B (N=13) | |
|---|---|---|
| Age (in years) - median (IQR) | 36 (30, 40) | 35 (30, 43) |
| Sex – N (%) | ||
| Male | 27 (97%) | 13 (100%) |
| Female | 1 (3%) | 0 (0%) |
| Race/Ethnicity | ||
| Caucasian | 20 (71%) | 10 (77%) |
| Hispanic | 8 (29%) | 1 (8%) |
| African-American | 0 (0%) | 1 (8%) |
| Other | 0 (0%) | 1 (8%) |
| Baseline CD4+T cells/ mm3 - median (IQR) | 407 (340, 587) | 490 (399, 623) |
| Baseline log10 HIV RNA (copies/mL) – median (IQR) | 5.0 (4.9, 5.8) | 4.9 (4.5, 5.8) |
| Serologic entry criteria | ||
| EIA negative | 7 (25%) | 3 (23%) |
| EIA positive and 0-3 bands on Western Blot | 16 (57%) | 7 (54%) |
| EIA psotive and 4-5 bands on Western Blot | 5 (18%) | 3 (23%) |
Arm A= ART plus Cyclosporine A; Arm B= ART; EIA = HIV enzyme immunoassay; IQR = interquartile range
Of the 41 patients completing the trial, 15 remained on their originally prescribed ARV regimen. Of the 26 changing therapy all remained on a potent ART regimen consisting of at least agents anchored by either efavirenz or a ritonavir-enhanced PI. There were no significant differences in changes between arms. Of 28 completing subjects in Arm A, 27 competed Cyclosporine A treatment. One subject terminated Cyclosporine A prior to completing week 1 due to nausea. Therapeutic levels were documented in 20 of 27 (74.0%) subjects by week 2 and 23 of 27 (85.2%) by week 3. Four subjects did not achieve target concentrations with 3 above target and 1 below. Mean Cyclosporine A levels in these 27 subjects in Arm A were 321, 431, 363, and 362 ng/mL at day 3, weeks 1, 2, 3 respectively.
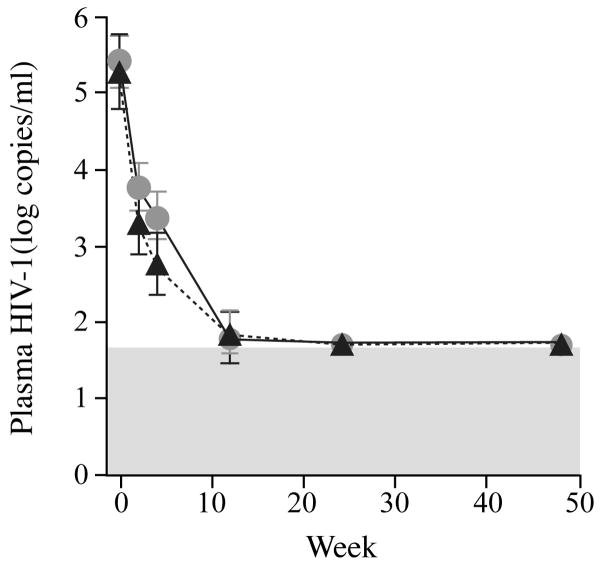
Levels of plasma HIV-1 RNA were measured longitudinally in all patients (Fig 1a). A rapid and sustained reduction in plasma viremia was seen in all patients analyzed. The median time to HIV-1 RNA levels below detection (50 copies/mL) was 15 weeks in Arm A (IQR 12, 20) and 16 weeks in Arm B (IQR 12, 20) (p=0.58). Interestingly at week 4 there was a statistically significant higher viral load in the patients receiving Cyclosporine A, 3.37 log copies/mL versus 2.75 log copies/mL (p=0.041). This difference was not sustained and its significance is unclear. Proviral DNA levels were measured in CD4+ T cells in patients at weeks 12, 24 and 48 (Fig. 1b). Levels could be quantified in 36 of 41 subjects. Quantification failed in 2 subjects and inadequate cells were available for analysis in 3 subjects. Among the 36 subjects in whom proviral DNA levels were analyzed, 24 were in Arm A and 12 in Arm B consistent with the 2:1 randomization scheme. There were no significant differences between levels of proviral DNA at weeks 12, 24, and 48 between treatment arms either expressed as median log10 copies per 106 CD4+ T cells (1.88 versus 1.92 p=0.84, 2.12 versus 1.96 p=0.34, 2.22 versus 2.13 p=0.70) or mean copies per 106 CD4+ T cells (130 versus 115 p=0.70, 105 versus 92 p=0.34, 95 versus 80 p=0.84). The mean differences (and 95% confidence intervals) between arms A and B in the change from baseline to weeks 12, 24, and 48, in log10 copies per 106 CD4+ T cells were: 0.05 (-0.33, 0.44), 0.08 (-0.24, 0.36), and 0.07 (-0.22, 0.36). When comparing Arms A to B there were no differences in proviral DNA levels at all time points. There was also no significant difference in the decay of proviral DNA from weeks 12 to 48 between arms, -0.14 versus -0.04 (p=0.99).
Fig. 1.
Fig. 1a. Longitudinal mean log10 HIV-1 RNA levels in patients completing the study.
Fig. 1b. Mean proviral DNA levels expressed as copy number per 106 CD4+ T cells at weeks 12, 24, and 48.
Fig. 1c. Longitudinal mean absolute CD4+Tcell counts (cells/mm3).
In all panels grey solid lines and circles represent values in patients on Arm A; dashed black lines and black triangles represent values for patients on Arm B. The error bars represent 95% confidence intervals for the mean.
Mean absolute CD4+ T cells counts did not differ between Arms A and B at baseline, weeks 2, 4, 12, 24, or 48 (p=0.24, 0.28, 0.43, 0.78, 0.75, 0.73 respectively) (Fig. 1c). The increase in CD4+ T cell counts at week 48 were 301 and 295 in Arms A and B respectively (p=0.95). The levels of activation in the memory (CD45RA-) CD4+ T cell population were determined with both CD38 and HLA DR staining. There were no differences noted between arms. One measurable effect of Cyclosporine A was a marked and statistically significant reduction in absolute numbers of cells positive for Ki67 staining in the memory CD4+ T cell population at weeks 2 and 4 (11 versus 24 p=0.04 and 9 versus 25 p=0.01) consistent with the ability of Cyclosporine A to inhibit IL-2 induced T cell proliferation. A more striking difference was seen between treatment arms in Ki67 staining in the CD8+CD45RO+ population at weeks 2 (15 versus 66 p<0.001) and 4, (12 versus 49, p<0.001).
Discussion
This clinical trial was designed to test the effectiveness of low dose Cyclosporine A as an adjunct to ART during acute and early HIV-1 infection. Based on the report by Rizzardi and colleagues we expected to see dramatic immunologic benefit and hypothesized that reductions in activation and proliferation would reduce the numbers of cells harboring proviral DNA. However, no effect of Cyclosporine A was measured, either on the quantitative immunologic response, sustained levels of immune activation or in the virologic response. Though we generated proviral DNA results on fewer than the projected 45 subjects, the difference in proviral DNA levels at week 48 between arms was small and the 95% confidence interval excluded differences of 0.4 logs or larger at this and at weeks 12 and 24, showing that the study had adequate power to detect clinically relevant treatment differences. Though we chose a 4-week treatment period as opposed to an 8-week treatment period, the Rizzardi group reported significant differences in CD4+ T cell levels as early as 1 to 2 weeks that were sustained at week 48. This was not seen in our study. Furthermore, a recent report by Miro et al using 8-weeks of low dose Cyclosporine A with ART in newly infected patients support our findings of no significant difference in CD4+ T cell levels early in the course of therapy [15]. The most likely explanation for the contradictory results would appear to be that Rizzardi et al treated 9 patients with Cyclosporine A and ART and compared these results to historical controls of patients treated with ART alone. The current study includes a more robust sample size and was randomized, resulting in a control group with matching characteristics.
In summary, this randomized clinical trial to test the effectiveness of immunosuppression with Cyclosporine A as an adjunct to ART in patients identified and treated during acute and early infection failed to document any virologic, immunologic or clinical benefit. Target measured concentrations of Cyclosporine A and evidence of reduced proliferation of T cells during the treatment rule out insufficient drug levels as an explanation for the lack of benefit. We therefore conclude that this particular modality is not indicated in the management of acute and early HIV-1 infection.
Acknowledgments
AIEDRP 501 Study Sites and Team:
ADARC: Melissa La Mar, Sandhya Vasan MD, Saurabh Mehandru MD, Anita Shet MD, Andrea Low MD
UCSD: Joanne Santangelo, Paula Potter, Tari Gilbert, Bryna Block
UCSF: Joann Volinski, RN
UAB: Karen Upton, RN
Abbott Laboratories: Michael Norton
GlaxoSmithKline: Belinda Ha
NIAID: Elizabeth Allen, Carla Petinelli, Elaine Ferguson
ACTG 5216 Study Sites:
MetroHealth: Mary Wild
Beth Israel Medical Center: Manuel Revuelta MD, Stanley Yancovitz MD
NYU/NYC HHC at Bellevue: Karen Cavanagh, RN and Margie Vasquez RN,
We thank Glaxo Smith Kline for providing Trizivir and Combivir and Abbott Laboratories for providing Kaletra.
We thank Aliza Lloyd, Brandi Davis, Kristina Rodruguez and Patrick Jean Pierre at ADARC for technical assistance.
This work was supported by the National Institutes of Health Grants AI041534 (ADARC), RR024143 (RUH), AI43638 (UCSD), AI41531 (UCSF), AI41530 (UAB), AI-69501 (MetroHealth).AI 46370 (BIMC), AI 27665, AI069532, and M01RR00096 (NYU) and AI 27661 (UM).
Footnotes
Conflict of interest statement: There are no conflicts of interest to declare.
This work was presented in part at the 15th Conference on Retroviruses and Opportunistic Infections Feb. 3-6, 2008 Boston, MA. Abstract 698c
Current affiliations: D.B. Tibotec Pharmaceuticals, Mechelen, Belguim C.H. Medical College of Wisconsin and AIDS Resource Center of Wisconsin, Milwaukee WI; J.M.K. Division of Infectious Diseases, Medical University of South Carolina, Charleston, SC
References
- 1.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–20. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hoen B, Cooper DA, Lampe FC, et al. Predictors of virological outcome and safety in primary HIV type 1-infected patients initiating quadruple antiretroviral therapy: QUEST GW PROB3005. Clin Infect Dis. 2007;45:381–90. doi: 10.1086/519428. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz M, Vesanen M, Tenner-Racz K, et al. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–37. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 6.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–33. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 8.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:3382–7. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 10.Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–20. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 11.Feutren G. The optimal use of cyclosporin A in autoimmune diseases. J Autoimmun. 1992;5 A:183–95. doi: 10.1016/0896-8411(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 12.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 13.Rizzardi GP, Harari A, Capiluppi B, et al. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J Clin Invest. 2002;109:681–8. doi: 10.1172/JCI14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohri H, Markowitz M. In vitro characterization of multidrug-resistant HIV-1 isolates from a recently infected patient associated with dual tropism and rapid disease progression. J Acquir Immune Defic Syndr. 2008;48:511–21. doi: 10.1097/QAI.0b013e31817ecb31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miro J, Lopez-Dieguez M, Plana M, et al. Randomized Clinical Trials with Immune-based Therapy in Patients with Primary HIV-1 Infection. 16th Conference on Retroviruses and Opportunistic Infections; Montreal CA. 2009. [Google Scholar]